Perinatal inflammation relates to early respiratory morbidity and lung function at 12 years of age in children born very preterm
Ingrid Hansen-Pupp and Ellen Tufvesson shared last authorship.
Funding: Information
This study was supported by The Swedish Heart and Lung Foundation, The Medical Faculty of Lund University, Region Skåne Council Foundation for Research and Development, and Fanny Ekdahl´s Foundation.
Abstract
Aim
Very preterm birth may be associated with lung function impairment later in life. It is not known if this is caused by prematurity per se or by associated perinatal events, such as maternal–foetal inflammation and severity of early neonatal lung disease. We assessed these factors in a prospective cohort of very preterm infants followed from birth to middle school age.
Methods
In 71 infants with a gestational age of median 27.4 (range 23.9–31.7) weeks, pro-inflammatory and modulatory cytokines were measured in umbilical cord blood and in arterial blood sampled at 6, 24 and 72 h after birth, and cumulated cytokine concentrations were calculated as area under the curve (AUC). At median 12.6 (range 12.3–13.5) years of age, pulmonary function testing was done in 53 children.
Results
There was a positive correlation between days on mechanical ventilation and AUC for IL-6 (p = 0.001), IL-8 (p = 0.015) and IL-10 (p = 0.006). Infants with bronchopulmonary dysplasia (BPD; n = 32) had higher AUC for the cytokines IL-6, IL-8 and IL-10 than those without BPD (all p < 0.01). Higher levels of AUC for IL-6 at birth correlated with lower forced expiratory volume in 1 s (p = 0.030) and lower mean expiratory flow rate between 25 and 75% of forced vital capacity (p = 0.034).
Conclusion
Perinatal inflammation, assessed by circulating cytokines in the first three days of life, was associated with BPD and with airway obstruction at 12 years of age.
Abbreviations
-
- AUC
-
- area under the curve
-
- BPD
-
- bronchopulmonary dysplasia
-
- IL
-
- interleukin
-
- IFN
-
- interferon
-
- PFT
-
- pulmonary function testing
-
- PROM
-
- premature rupture of membranes
-
- FEV1
-
- forced expiratory volume in 1 sec
-
- FVC
-
- forced vital capacity
-
- FEF25-75
-
- mean forced expiratory flow between 25% and 75% of FVC
Key notes
- An elevated circulatory inflammatory response after very preterm birth was associated with neonatal respiratory impairment and bronchopulmonary dysplasia (BPD).
- Airway obstruction at 12 years of age was a common finding and was related to increased levels of the pro-inflammatory cytokine IL-6 at birth, irrespective of a diagnosis of BPD.
- Systemic inflammation early after birth may have long-lasting consequences for the immature lung and long-term evaluation of lung function is warranted.
1 INTRODUCTION
Pulmonary inflammation plays an important role in the pathogenesis of BPD, and an imbalance between pro-inflammatory and anti-inflammatory mediators in the developing neonatal lung has been suggested to contribute to BPD development.1, 2 Several studies3-8 have shown an association between certain patterns of elevated blood cytokines early in life and a diagnosis of BPD. However, a possible persistent effect of these cytokines on lung function later in childhood has not yet been reported.
There is some evidence that, in very preterm infants, a disease pathway may start with chorioamnionitis and a foetal inflammatory response, leading via a diagnosis of BPD to obstructive airway disease in childhood.9-11 The definition of BPD has been modified several times in order to accurately predict respiratory morbidity after discharge from neonatal care and through the first two years of life.12 Yet the predictability of long-term respiratory morbidity has not been systematically studied. Children born preterm with a diagnosis of BPD have worse lung function at school age than children born at term, whereas comparisons between children born at similar gestational ages with and without a diagnosis of BPD have given conflicting results 13-17
We have now studied these relationships in a cohort of very preterm infants using blood samples at birth and in the first days of life, and clinical data throughout the neonatal period.18 Here, we present relationships between this early life sampling and pulmonary function test (PFT) completed at 12 years of age. We hypothesised that perinatal inflammation should not only be associated with BPD but also with lung function impairment at school age.
2 PATIENTS AND METHODS
2.1 Study population and neonatal data collection
The study population used here was a previously described prospective cohort of very preterm infants,18 born 2001–2003 and admitted at birth to the neonatal intensive care unit in Lund, Sweden. Inclusion criteria were gestational age <32 weeks, antenatal consent and no congenital anomalies, and acceptance rate was 86%. Clinical data were collected as described in Appendix S1.
Blood samples were obtained from the umbilical cord at birth and from an arterial line at 6, 24, and 72 h of age. Levels of pro-inflammatory (tumour necrosis factor-α, interferon (IFN)-γ, interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-12) and modulatory (IL-4, IL-10) cytokines were quantified in plasma (see Appendix S1). Plasma levels of the respective cytokines were used to calculate area under the curve (AUC) as an assessment of cytokine burden over time (0–72 h) Levels of cytokines and the calculated AUCs were logarithmically transformed to achieve a more normal distribution for the statistical calculations.18
Clinical data (presented in Tables 1 and 2) were collected as described in Appendix S1. Bronchopulmonary dysplasia (BPD) was defined as treatment with supplemental oxygen at 36 weeks postmenstrual age.
|
Survivors at 36 weeks PMA (n = 71) |
Children who did lung function testing (n = 53) | |
|---|---|---|
| Prenatal exposures | ||
| Maternal smoking at beginning of pregnancya | 6/51 (12%) | 6/39 (15%) |
| Pre-eclampsia | 18 (25%) | 14 (26%) |
| Premature rupture of membranes | 27 (38%) | 21 (40%) |
| Suspected maternal infection | 20 (28%) | 17 (32%) |
| Clinical chorioamnionitis | 3 (4%) | 2 (4%) |
| Antenatal steroids | 71 (100%) | 53 (100%) |
| Infant demographics | ||
| Gestational age, weeks +days, median (range) | 27 + 3 (23 + 6 – 31 + 5) | 27 + 5 (23 + 6 – 31 + 5) |
| Birth weight, g, median (range) | 960 (550–2025) | 1035 (550–2025) |
| Small for gestational age | 20 (28%) | 15 (28%) |
| Gender, male/female | 37/34 | 28/25 |
| Neonatal pulmonary morbidity | ||
| Surfactant treatment | 40 (56%) | 29 (55%) |
| Mechanical ventilation | 40 (56%) | 30 (57%) |
| Days on mechanical ventilation, median (range) | 2 (0–41) | 2 (0–41) |
| Supplemental oxygen at 36 weeks PMA | 32 (45%) | 24 (45%) |
| Treatment with systemic corticosteroids | 23/66 (35%) | 15/52 (29%) |
- Values are number of individuals (per cent) unless otherwise indicated.
- a Incomplete data from maternal records.
|
Infants without BPD (n = 39) |
Infants with BPD (n = 32) |
p-valuea | |
|---|---|---|---|
| Prenatal exposures | |||
| Pre-eclampsia | 9 (23%) | 9 (28%) | 0.63 |
| Premature rupture of membranes | 16 (41%) | 11 (34%) | 0.57 |
| Suspected maternal infection | 7 (18%) | 13 (41%) | 0.035 |
| Clinical chorioamnionitis | 1 (3%) | 2 (6%) | 0.59b |
| Antenatal steroids | 39 (100%) | 32 (100%) | |
| Infant demographics | |||
| Gestational age, weeks +days, median (range) | 27 + 6 (23 + 6 – 31 + 5) | 26 + 2 (24 + 0 – 30 + 0) | 0.004c |
| Birth weight, g, median (range) | 1065 (658 – 2025) | 828 (550 – 1548) | 0.002c |
| Small for gestational age | 9 (23%) | 11 (34%) | 0.29 |
| Gender, male/female | 19/20 | 18/14 | 0.53 |
| Neonatal pulmonary morbidity | |||
| Surfactant treatment | 16 (41%) | 24 (75%) | 0.004 |
| Mechanical ventilation | 17 (44%) | 23 (72%) | 0.017 |
| Days on mechanical ventilation, median (range) | 0 (0–28) | 5 (0–41) | 0.001c |
| Days on CPAP, median (range) | 6 (0–57) | 23 (0–72) | 0.003c |
| Total days on mechanical respiratory support, median (range) | 8 (0–69) | 36 (0–81) | 0.001c |
| Treatment with systemic corticosteroids | 6/36 (17%) | 17/30 (57%) | 0.001 |
| Total corticosteroid intake, mg/kgd | 0 (0 – 58) | 2.8 (0 – 185) | <0.001c |
- Values are number of infants (per cent) unless otherwise indicated.
- a Comparison of infants without vs. with BPD using two-sided chi-square statistics unless otherwise indicated.
- b Fisher's exact test.
- c Mann–Whitney U-test.
- d Accumulated intake of hydrocortisone and betamethasone from birth to discharge expressed as hydrocortisone equivalents, assuming that 1 mg of betamethasone is equal to 35 mg of hydrocortisone.
2.2 Pulmonary function testing (PFT)
Out of 74 infants enrolled at birth, 71 survived to 36 weeks postmenstrual age. In two infants, the parents declined further participation after discharge from the neonatal unit, and one child was lost to follow-up. At 12 years of age, the remaining 68 children were invited for PFT and 54 agreed to participate (a detailed flow chart is shown in Appendix Figure S1). Reasons for not coming were parental decline, family having moved outside the reach of the investigators, or that testing would be impossible because of physical or mental disability.
The 54 children were examined at median 12.6 (range 12.3–13.5) years of age. They were asked not to take bronchodilators for at least 24 h before PFT. Height and weight were measured, and a history of respiratory symptoms, medication and allergy was obtained from a questionnaire completed by the accompanying parent.
The examination consisted of four parts: measurement of exhaled nitric oxide, spirometry, measurement of static lung volumes by whole body plethysmography and measurement of the diffusion capacity of the lung for carbon monoxide. The individual tests performed and the number of children who completed the different tests are shown in Table 3. The test procedures are detailed in Appendix S1. Thirty-nine children completed all four parts of the examination, 10 children completed three, and three children only completed two parts. One child did not cooperate and generated no results.
|
All children (n = 53) |
No BPD during infancy (n = 29) | BPD during infancy (n = 24) | p-valuea | |
|---|---|---|---|---|
| Age at testing, years | 12.6 (12.3–13.5) | 12.6 (12.3–13.5) | 12.6 (12.3–13.3) | 0.96 |
| Height, cm | 156 (135–174) | 156 (142–169) | 156 (135–174) | 0.71 |
| Weight, kg | 47 (31–73) | 46 (31–73) | 49 (33–61) | 0.58 |
| Sex, n, male/female | 28/25 | 14/15 | 14/10 | 0.47b |
| Spirometry | (n = 52) | (n = 29) | (n = 23) | |
| FEV1, %pred | 92 (70–120) | 92 (70–119) | 91 (72–120) | 0.99 |
| FVC, %pred | 98 (73–124) | 97 (73–113) | 99 (81–124) | 0.19 |
| FEV1/FVC | 0.80 (0.60–0.99) | 0.82 (0.66–0.99) | 0.79 (0.60–0.93) | 0.09 |
| FEF25-75, %pred | 70 (34–130) | 76 (36–130) | 65 (34–117) | 0.17 |
| FEV1 reversibility, n | 10/50 (20%) | 4/28 (14%) | 6/22 (27%) | 0.30c |
| Static lung volumes | (n = 43) | (n = 22) | (n = 21) | |
| TLC, %pred | 96 (73–115) | 95 (82–107) | 99 (73–115) | 0.47 |
| RV, %pred | 105 (62–175) | 108 (72–175) | 104 (62–155) | 0.23 |
| RV/TLC | 0.26 (0.15–0.42) | 0.28 (0.19–0.42) | 0.26 (0.15–0.31) | 0.16 |
| Diffusing capacity | (n = 47) | (n = 27) | (n = 20) | |
| DLCO, %pred | 98 (71–121) | 99 (76–121) | 95 (71–109) | 0.29 |
| Alveolar volume, %pred | 103 (89–126) | 104 (91–120) | 102 (89–126) | 0.70 |
| KCO, %pred | 93 (75–115) | 95 (75–115) | 87 (75–106) | 0.11 |
| Airway inflammation | (n = 52d/51e) | (n = 28) | (n = 24d/23e) | |
| FeNO50, ppb | 9.3 (4.5–66.9) | 9.3 (4.8–56.8) | 9.7 (4.5–66.9) | 0.54 |
| Alveolar NO, ppb | 2.28 (0.04–4.85) | 2.29 (0.70–4.85) | 2.08 (0.04–4.09) | 0.52 |
| Bronchial flux of NO, nL/s | 0.41 (0.09–4.03) | 0.40 (0.21–3.67) | 0.51 (0.09–4.03) | 0.76 |
- Values are median (range) except where otherwise indicated. %pred indicates per cent of predicted as explained in the methods section; BPD, bronchopulmonary dysplasia defined as treatment with extra oxygen at 36 weeks postmenstrual age; FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity; FEF25-75, mean forced expiratory flow between 25% and 75% of FVC; TLC, total lung capacity; RV, residual volume; DLCO, diffusing capacity of the lung for carbon monoxide; KCO, diffusion coefficient for carbon monoxide; FeNO50, fractional exhaled nitric oxide measured at a flow rate of 50 ml/s; NO, nitric oxide. Reasons for missing measurements were lack of compliance and/or misunderstanding of instructions or machine dysfunction.
- a Comparison of children without vs. with a diagnosis of BPD using Mann–Whitney U-test unless otherwise indicated.
- b Chi2-test.
- c Fisher's exact test.
- d Number of individuals for FeNO50.
- e for alveolar NO and bronchial flux of NO.
Fifty children underwent a second spirometry 15 min after inhalation of 200 µg salbutamol. A significant airway response to salbutamol (positive reversibility) was defined as an increase in the absolute value of forced expiratory volume in 1 s (FEV1) with ≥12%. Except for this reversibility analysis, pre-bronchodilator spirometry results were used for all the statistical evaluations.
Results of spirometry and body plethysmography are reported as per cent of predicted by height and sex using reference equations from Zapletal.19 Results of diffusing capacity are reported as per cent of predicted by age, height and sex according to standards from the Global Lung Initiative (GLI).20
2.3 Ethics
Both parts of the study, in the neonatal period and at 12 years of age, were approved by the Regional Ethical Review Board in Lund. All parents and children received both oral and written information about the study, and signed parental informed consents were obtained.
2.4 Statistics
Statistical analysis was performed using IBM® SPSS® Statistics version 26. Nonparametric tests were used due to presence of asymmetric distribution in some variables even after logarithmical transformation. Spearman's rank test was used for correlation analyses, Mann–Whitney U-test was used for comparisons between two groups, and Wilcoxon's signed-rank test was used for paired comparisons. For all correlations, associations caused by outliers were considered as non-significant. Linear or logistic regression was used to adjust for gestational age (in analyses of neonatal comorbidities). Chi-squared test or Fisher's exact test was used to explore relationships between categorical variables. p-values < 0.05 were considered significant.
3 RESULTS
3.1 Population characteristics and respiratory morbidity
The perinatal characteristics of the study population are shown in Table 1 and associations with BPD in Table 2. Infants with BPD had lower gestational age and lower birth weight than infants without BPD. They had more often been exposed to suspected maternal infection, but maternal infection was also associated with a more preterm birth (median 26.3 vs. 27.9 weeks, p = 0.004), and the association with BPD did not persist after adjustment for this. A diagnosis of BPD was preceded by a more severe lung disease, as judged by a higher need for surfactant, mechanical respiratory support and postnatal corticosteroids.
3.2 Inflammatory biomarkers and neonatal respiratory morbidity
Overall, perinatal systemic inflammation quantified as a higher plasma cytokine burden (AUC from 0 to 72 h) was associated both with a more severe neonatal lung disease in terms of need for respiratory support and with a diagnosis of BPD.
Infants who were given surfactant (n = 40; within the first hour in 25 cases) had higher AUC for IL-2 (p = 0.04), IL-6 (p < 0.001) and IL-8 (p < 0.001) compared to infants not given surfactant. These associations remained significant for IL-6 and IL-8 also after adjustment for gestational age.
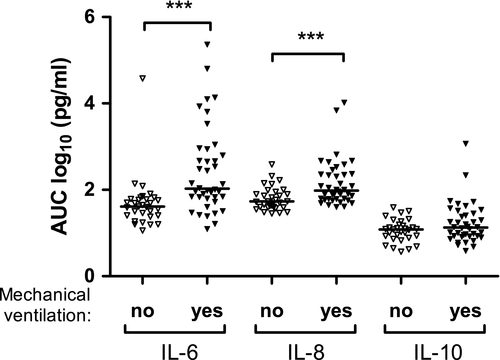
The need for mechanical ventilation was mainly dependent on a low gestational age, with 18/28 (64%) infants born before 27 weeks needing ventilation for more than 10 days in contrast to none of the 43 infants born after 27 weeks or more (p < 0.001). However, cytokine burden was a significant contributor, as AUCs for IL-2 (rs=0.33, p = 0.005), IL-6 (rs=0.55, p < 0.001), IL-8 (rs=0.49, p < 0.001) and IL-10 (rs=0.24, p = 0.042) all correlated with number of days on mechanical ventilation, and for IL-6 (p = 0.001), IL-8 (p = 0.015) and IL-10 (p = 0.006), the correlations remained significant after adjustment for gestational age (Figure 1).
Infants who were later diagnosed with BPD had higher AUCs for IL-6 (p = 0.002), IL-8 (p < 0.001) and IL-10 (p < 0.001) than infants without BPD (Figure 2A-C). Individual plasma levels of IL-6, IL-8 and IL-10 were also significantly higher in infants with BPD in cord blood and at most of the following time points when the cytokines were sampled. All these differences remained significant after adjustment for gestational age.
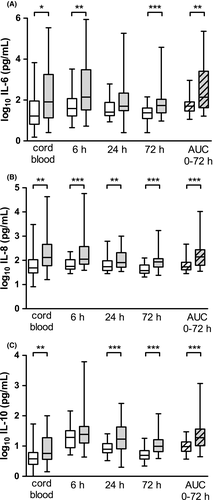
The individual cytokine patterns were as follows: In infants with BPD, the pro-inflammatory cytokine IL-6 remained equally high at 6 h as at birth, but then fell significantly to 24 h of age (p = 0.002) and remained unchanged at 72 h. Levels of IL-6 were higher in infants with BPD at all time points except at 24 h. The other pro-inflammatory cytokine IL-8 did not change significantly from birth to 72 h but was higher in infants with BPD at all time points. In contrast, the anti-inflammatory cytokine IL-10 increased significantly from birth to 6 h of age (p < 0.001) and then fell to 24 h (p < 0.001) and further to 72 h (p = 0.001), with plasma levels at 72 h still higher than at birth (p < 0.001). The size of the initial increase and then decrease in IL-10 were not significantly different between infants with or without BPD, but levels were higher in infants with BPD at all time points except at 6 h. With the exception of a higher IFN-γ in cord blood (p = 0.022) in infants with BPD, there were no differences in plasma levels or AUCs for any of the other cytokines between infants with or without BPD.
AUC for IL-8 decreased with increasing gestational age (rs = −0.37, p = 0.002) and with increasing birth weight (rs = −0.49, p < 0.001), but there was no similar correlation for the other cytokine AUCs. There was no association between preeclampsia, premature rupture of membranes (PROM), suspected maternal infection or clinical chorioamnionitis and cytokine AUCs except that AUC for IFN-γ was higher after PROM (p = 0.009).
3.3 History of airway disease and lung function at school age
Fifty per cent of the 54 children who came for PFT reported at least one of four symptoms (history of wheezing, exercise-induced wheezing, sleep disturbed by wheezing, nocturnal cough), and 63% had taken medication for airway disease at some time (Appendix Table S1). Taken medication was more common in children born after PROM (81% vs. 52%, p = 0.029) or after suspected maternal infection (83% vs. 53%, p = 0.028) and less common in children born after preeclampsia (29% vs. 75%, p = 0.002) or born small for gestational age (33% vs. 74%, p = 0.005).
More children with a previous diagnosis of BPD had a diagnosis of asthma (44% vs. 17%; p = 0.03) or a history of allergy (33% vs. 10%, p = 0.04). Seventeen per cent of children had ongoing inhalation treatment, with no difference between children with or without BPD. The proportion of children reporting at least one of the four airway symptoms mentioned above was not significantly different between those having and those not having a diagnosis of BPD (60% vs. 41%, p = 0.17; see Appendix Table S1).
The background characteristics of the children who did PFT (n = 53) were similar to those of the whole cohort of survivors (n = 71, Table 1). The 18 children who did not do PFT were born at a lower gestational age and had more often been treated with systemic corticosteroids than the 53 children who did PFT (Appendix Table S2). Physical characteristics and results of PFT are shown in Table 3. Values below the lower limit of normal were more common for tests evaluating airway obstruction than for lung volumes; FEV1/FVC was <0.75 in 21% and FEF25-75 below 70% of predicted in 48% of the children. In contrast, measurements of lung volumes showed few results lower than normal: FVC below 80% of predicted was only seen in one child, TLC below 80% of predicted was seen in three children (7%) and alveolar volume below 80% of predicted in none. DLCO below the lower limit of normal (77% of predicted) was seen in two children, and KCO below the lower limit of normal (77% of predicted) was also seen in two children.
Median values for the different lung function measures were not significantly different between children with or without a previous diagnosis of BPD. Median FEV1, FEV1/FVC and FEF25-75 were lower in boys than in girls, though not significant (p = 0.11, p = 0.10 and p = 0.07, respectively).
After inhalation of salbutamol, FEV1 increased by a median of +6% (range −10% – +23% and p < 0.001 when comparing before and after salbutamol), but a clinically significant reversibility (≥12% increase) was seen only in 10/49 children (20%). Children who reported at least one of the four symptoms of airway obstruction mentioned above had significantly lower FEV1 as a ratio of forced vital capacity (FEV1/FVC; p = 0.029) and lower FEF25-75 (p = 0.022) than children with none of these symptoms. They also had a lower diffusion coefficient for carbon monoxide (KCO, p = 0.039) and a tendency towards lower diffusing capacity of the lung for carbon monoxide (DLCO, p = 0.055).
FeNO50 was below 20 ppb in 45/52 children (87%), and above 35 ppb21 in four children only (8%), two with and two without a diagnosis of BPD; these four infants all reported a history of wheezing and had taken medication for airway disease.
3.4 Perinatal morbidity, inflammatory biomarkers and lung function at school age
There was no association between PROM, suspected maternal infection or clinical chorioamnionitis and lung function at school age.
Within the span studied, 23.9 – 31.7 weeks, there was no association between gestational age or birth weight and PFT results, except that forced vital capacity (FVC) as per cent of predicted decreased with increasing gestational age (rs = −0.28, p = 0.047).
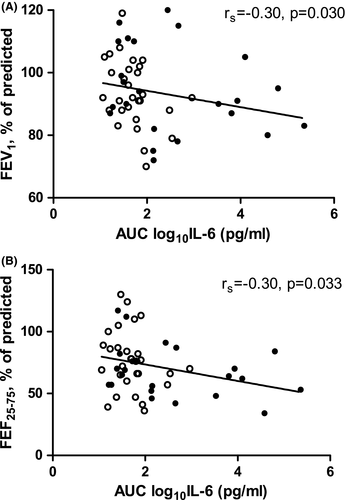
Out of the three cytokines significantly associated with development of BPD (IL-6, IL-8 and IL-10; Figure 1), IL-6 correlated with a lower FEV1 (rs = −0.30, p = 0.030) and a lower FEF25-75 (rs = −0.29, p = 0.034) as per cent of predicted (Figure 3). For the other cytokines, AUCs did not correlate with lung function at school age.
Cytokine AUCs from birth to 72 h of age were not significantly different in children with a later history of airway disease (having a diagnosis of asthma, having had at least one of the symptoms of airway obstruction mentioned above, or having ever taken medication for airway obstruction) than in children without this history. A higher AUC for IL-4 correlated with a higher FeNO50 (rs = 0.30, p = 0.033), but not with any lung function measurement. None of the other cytokines correlated with FeNO50, alveolar or bronchial flux of NO.
There were no associations between indicators of morbidity in the neonatal period (treatment with surfactant, levels of PCO2 and FiO2 at 6, 24, 48 and 72 h of age, days with mechanical ventilation, sepsis, ligation of ductus arteriosus, cumulative dose of corticosteroids) and lung function measurements at school age.
4 DISCUSSION
In a prospective cohort of children born very preterm and followed from birth to middle school age, early inflammation, quantified as plasma AUC for the cytokines IL-6, IL-8 and IL-10 over the first 72 h of age, was strongly associated with severity of early lung disease and with a later diagnosis of BPD. At 12 years of age, airway obstruction was a common finding with almost half of the children achieving forced expiratory flows (FEF25-75) less than 70% of predicted. Airway obstruction occurred equally often in children with or without a previous diagnosis of BPD, was probably not associated with a persistent airway inflammation, as 87% of children had exhaled NO less than 20 ppb, and was not reversible in the majority of cases. Our finding of an inverse correlation between AUC for the pro-inflammatory cytokine IL-6 in the first days of life and FEV1 and FEF25-75 at school age may indicate that there is a period at or early after birth when inflammation can have a long-lasting effect on the lung in terms of a persistent airflow limitation, warranting further validation and investigation.
The main strengths of our study are that it is prospective, including consecutively recruited inborn infants with a high acceptance rate, comes from a single regional centre with little variation in treatment, and has a long observation period. It includes both cytokine profiles and clinical data from the neonatal period and a relatively comprehensive lung function testing at school age, when a high proportion of the original cohort (75% of survivors) was examined within a narrow age span at around 12 years of age.
The design of the study with an antenatal informed consent may have introduced a selection bias in the original study population, since informed consent could not be obtained for infants born after unexpected deliveries. We are aware of the risks of making multiple comparisons and acknowledge that our findings need to be confirmed in larger populations.
The wide gestational age span in our cohort (23 – 31 weeks) may confound our results because of the different reasons for preterm birth at different durations of pregnancy, with maternal infection being more common at low and foetal growth restriction at higher gestational ages. However, the present study focused on systemic inflammation, quantified as cytokine burden early in life, which we identified as a risk factor for a poor early respiratory outcome, independent of gestational age and obstetric events.
Our finding of higher AUCs for several cytokines in infants given surfactant and in infants subjected to mechanical ventilation should not be taken as proof that there was a cause-and-effect relationship in any of the possible directions. It may be that early surfactant instillation, which at the time of the study always included tracheal intubation, most often followed by mechanical ventilation, started or accentuated an inflammatory response, but it may also be that a pre-existing inflammation had already predestined the lungs for a severe course in terms of respiratory support.
Because cytokine concentrations may change rapidly and often by a large magnitude between time points, we chose to mainly use AUC from birth to 72 h of age for our calculations, as this should be a more robust measure of early cytokine burden than separate values. Several earlier studies3-7 have shown an association between elevated and in some cases depressed blood cytokines in the early life of preterm infants and a later diagnosis of BPD, but the long-term outcome of the infants in these studies has not been reported. Four of the studies4, 6-8 had lower inclusion criteria for gestational age and weight than we used, and the time points for blood sampling were different, making their temporal cytokine profiles difficult to compare with ours. However, the cytokines shown to be associated with BPD (IL-6, IL-8 and IL-10) in the present study were also implicated in previous studies, with an early elevation of IL-6 and IL-10, and a more persistent elevation of IL-8 in infants developing BPD.7, 8, 22 IL-8 is a powerful attractor of neutrophils and the pro-inflammatory cytokine most often associated with BPD.5, 23 IL-6 is known to play a key role both in the acute phase response and in the transition to chronic inflammation,24 and was in the present study the only cytokine that correlated with lung function at school age.
Large cohort studies have suggested that there is a link between perinatal inflammation, usually defined as clinical or histologic chorioamnionitis, and obstructive airway disease in infancy and childhood.9 None of these studies have included measurement of biochemical markers of inflammation in the neonatal period, and lung function at school age has not been reported. In our cohort, the incidence of clinical chorioamnionitis was too low for any meaningful evaluation, but we did not observe any association between maternal infection or PROM and school-age lung function in the children.
In the present study, the lung function at 12 years of age was similar in infants with and without a diagnosis of BPD. While BPD was originally described as a distinct disease entity, later definitions of BPD have been developed to maximise the possibility to predict future respiratory morbidity in infancy and early childhood up to approximately two years of age, in a recent study through 18–26 months corrected age.12 However, the natural history of BPD later in childhood and adolescence is less well understood.14 A recent review 13 identified 34 studies that evaluated long-term effects of BPD on respiratory outcome in school-aged children. Spirometric measurements of airway patency were decreased in BPD survivors compared with term control subjects, while TLC and functional residual capacity were normal or only moderately reduced. We found a similar pattern for the whole cohort of children born very preterm in comparison with a well-established reference population.19 The above-mentioned review 13 found mixed results as to whether children with a very low birth weight and BPD had any difference in lung function as compared with those without BPD. Another review 25 found an increased risk of deficits in FEV1 as per cent of predicted in all preterm-born survivors, but the reported deficits were greater in infants with BPD. A study of 120 very preterm-born children at 8.5 years of age14 showed lower z-scores for FVC, FEV1 and FEV25-75 than in term controls but, as in the present study, no difference between children with or without a previous diagnosis of BPD. In the same study, RV/TLC was higher in preterm-born children with BPD than in those without BPD, which was not found in our study.
Though it seems reasonable that a more difficult neonatal respiratory trajectory should be associated with an increased risk of future lung function impairment, this may not always be so. In our cohort, children with a previous diagnosis of BPD had not only, as the definition of BPD implies, needed extra oxygen for a longer time than infants without BPD but had also needed more mechanical and pharmacologic respiratory support in the neonatal period. However, this did not translate to a significant difference in lung function at school age. Both groups of very preterm infants were found to have a high incidence of expiratory flow limitation, in some cases severe, but this was neither related to obstetric risk factors, nor to their neonatal morbidity. Other factors related to prematurity may be involved, and our finding of an association between an elevated IL-6 in plasma in the early neonatal period and airflow limitation at school age suggests that one such factor may be systemic inflammation.
In conclusion, this study shows that perinatal inflammation, measured as cytokine burden in the first 72 h of life in very preterm children, was associated with severity of neonatal lung disease, a diagnosis of BPD and airway obstruction at 12 years of age. Long-term evaluation of lung function in these children is warranted.
ACKNOWLEDGEMENT
We would like to thank all children who came for lung function measurement as well as their accompanying parents. We would also like to thank Ann-Cathrine Berg for excellent help in patient inclusion, Jonas Olsson and Anna Sikesjö for help with patient testing, and Nicole van der Burg for review of the English language.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.




