Non-invasive structural and metabolic retinal markers of disease activity in non-proliferative diabetic retinopathy
Abstract
Purpose
Metabolic and structural microvascular retinal alterations are essential components in diabetic retinopathy (DR). The present study aimed to measure changes at different stages of non-proliferative DR (NPDR) and to explore interactions of imaging-based metrics.
Methods
This cross-sectional, cohort study included 139 eyes from 80 diabetic patients. Each patient underwent dilated fundal examinations including colour fundus photography, retinal oximetry and optical coherence tomography angiography (OCTA), analysed by semi-automated and automated software. Diabetic retinopathy (DR) severity was classified according to the International Clinical Diabetic Retinopathy (ICDR) Severity Scale, ranging from no DR to severe NPDR (level 0–3). Retinal metabolism was evaluated by oximetry as retinal arteriolar (raSatO2) and venular oxygen saturation (rvSatO2), and macular microvascular structure was measured by OCTA as the area of foveal avascular zone (FAZ), vessel density (VD), vessel diameter index (VDI), FAZ circularity and fractal dimension (FD) in the superficial and deep retinal capillary plexus.
Results
A trend for increasing rvSatO2 was found with increasing DR severity (51.3%, 53.3%, 54.2%, 59.8%, p = 0.02). Increasing severity of DR associated with decreasing FD in the superficial and deep plexus (p < 0.001 and p = 0.014), and in the superficial plexus decreasing VD (p < 0.001), increasing VDI (p = 0.003) and decreasing FAZ circularity (p = 0.006). A few interactions were identified between raSatO2, rvSatO2 and VDI, but only in the deep capillary plexus (p < 0.01 and p < 0.01).
Conclusion
Alterations of the venular retinal vascular oxygen saturation and microvascular structural abnormities were found continuously throughout the DR-spectrum. Given the sparse correlations between metabolic and structural abnormalities, it seems that these occur independently in DR.
Introduction
Diabetic retinopathy (DR) is the most common complication in diabetes and one of the leading causes of blindness in the working-age population of developed countries (Prokofyeva & Zrenner, 2012). As a way to prevent vision loss, regular eye screening by retinal fundus photography is a cost-effective (Peters et al., 1993) and valid method (Liesenfeld et al., 2000). Eye screening aims to identify sight-threatening DR, like proliferative diabetic retinopathy (PDR) and diabetic maculopathy, prior to irreversible visual loss. Many patients are examined annually regardless of disease activity, even though individualized screening intervals may save resources (Aspelund et al., 2011). In order to identify patients at risk of disease progression, multiple risk factors that may be related to DR progression, such as duration of DM, hyperglycaemia and hypertension, were identified in previous studies. However, they are insufficient to explain the risk of DR progression (The Diabetes Control and Complications Trial Research Group 1995).
Retinal oximetry is a non-invasive method that can be used to measure the oxygen saturation in retinal vessels, and as a marker for retinal oxygen metabolism (Beach et al. 1999). The method uses a dual-wavelength technique and is based on the principle that the colour of blood depends on the oxygen saturation of haemoglobin. In a systematic review, Rilvén et al reported higher retinal venular oxygen saturation in patients with more advanced levels of DR, potentially identifying retinal oximetry as tool for screening or a biomarker of treatment outcome in patients with ischaemic retinal diseases (Rilven et al., 2017). In a group of young patients with type 1 diabetes, Veiby et al demonstrated retinal metabolic alterations in the early stages of DR (Veiby et al., 2020), but in older patients, it is not known if this increment happens gradually along the DR-spectrum, or if it is triggered at a certain level of structural damage in the retinal microvasculature.
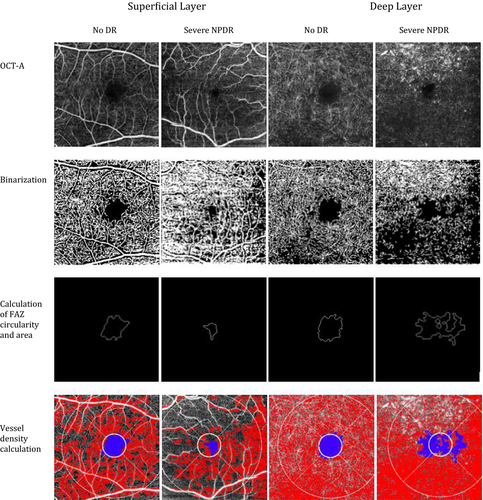
The retinal microvasculature has traditionally been visualized by fluorescein angiography, but this is an invasive, time-consuming procedure with potential side effects, which makes it unsuitable as a biomarker of treatment outcome. Hence, it is predominantly used in patients with suspected sight-threatening disease. Optical coherence tomography angiography (OCTA) is a novel, non-invasive method to visualize the retinal microvasculature. Motion-contrast images are used to image high-resolution blood flow in order to generate angiographic images in a matter of seconds. Optical coherence tomography angiography (OCTA) enables clinicians, for the first time, to visualize the separate layers of the retinal microvasculature, which have previously only been visible with histological examinations (Fig. 1) (Dimitrova et al., 2017); (Ting et al., 2017). To quantify central retinal ischaemia and remodelling, a variety of quantitative metrics can be obtained from OCTA, including different matrices of the foveal avascular zone (FAZ) dimensions, a circularity index (FAZ circularity), vessel density (VD), fractal dimension (FD) and vessel diameter index (VDI) (Tang et al., 2017). However, there is a paucity of data examining how retinal microvasculature is influenced by retinal oxygen metabolism.
Therefore, the aim of the present study was to identify at which stages metabolic and microstructural changes occurred in DR and to explore potential associations between the corresponding non-invasive retinal markers.
Methods
Cohort and recruitment
Patients were recruited from the Funen Diabetes Database (FDDB), which was established in 2003 on the island of Funen, Denmark, which has approximately 473 000 inhabitants (Danmarks Statistik 2018). The FDDB contains data of over 22 000 patients with diabetes (Larsen et al., 2017). It is continuously updated on an individual level and contains information of age, sex, type and duration of diabetes, glycaemic regulation, blood pressure, body mass index and levels of microvascular complications including level of DR.
All patients registered in the FDDB had their retinal fundus images graded by trained ophthalmic graders and the results were entered onto the relevant section of FDDB. For the current study, patients identified in FDDB with valid grading of DR (using the International Clinical Diabetic Retinopathy (ICDR) severity scale) (Wilkinson et al., 2003) were invited to participate. Patients were approached by a standard letter, and if they indicated their willingness to participate in the study, a mutually agreeable time was found for detailed examination and imaging that took place at the Department of Ophthalmology, Odense University Hospital, Odense, Denmark. Altogether, we included 100 patients with diabetes with equal distribution of the severity of DR from no DR to severe non-proliferative DR (NPDR) in at least one eye as per last screening episode. Eyes treated with panretinal photocoagulation for PDR were not included in the study.
Examination
All eyes were dilated using one drop of tropicamide 1%. For retinal oximetry, optic disc-centred fundus images were captured by the Oxymap model T1 (Oxymap, Reykjavik, Iceland) using a standard protocol as specified by the manufacturer. Two-field (optic disc and macula centred) 45-degrees colour fundus images and fovea-centred 4.5 × 4.5 mm OCTA images were taken by Topcon DRI OCT Triton (Topcon, Tokyo, Japan).
Image analysis
All colour fundus images were graded using the ICDR severity scale by the single, certified grader (GW) at the Belfast Reading Centre, Queen’s University, Royal Victoria Hospital, Belfast, Northern Ireland, UK. Categories included no DR (level 0), mild NPDR (level 1), moderate NPDR (level 2) and severe NPDR (level 3) (Wilkinson et al., 2003). Finally, PDR was categorized as level 4, classified by the presence of neovascularization and/or vitreous or pre-retinal haemorrhage.
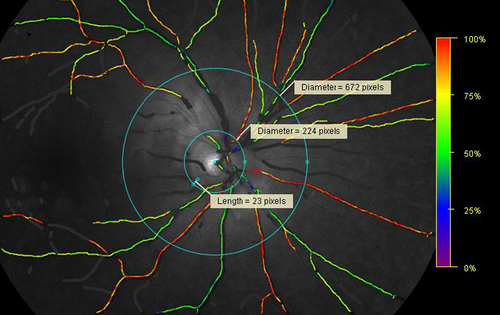
The retinal arteriolar and venular oxygen saturation (raSatO2 and rvSatO2) was determined by retinal oximetry in larger retinal arterioles and venules, measured around the optic disc by the Oxymap T1 using the Oxymap Analyzer software. A two-wavelength technique was used to estimate the oxygen saturation of haemoglobin (Beach et al. 1999), and a colour map delineated the oxygen saturation of the retinal vessels (Fig. 2) (Stefansson et al., 2019). The equipment and technique for retinal oximetry have previously been described in detail (Geirsdottir et al., 2012). All images were graded by a single trained grader (GW) according to a pre-specified protocol. The oximetry measurements were performed between two semi-automatically placed rings, around the optic disc. The first, inner ring was manually placed between 20 and 30 pixels from the edge of the optic disc and the second, outer ring was automatically placed at three times the diameter of the inner ring (Torp et al., 2017). The largest retinal arteriole and venule in each quadrant were manually traced at a length of 50-150 pixels and oxygen saturations are presented as the average saturation of the four traced arterioles (raSatO2) and venules (rvSatO2), respectively.
The OCTA images were analysed by an automated software, measuring the size of the FAZ, VD, FD, VDI and the circularity of the FAZ of the superficial and deep retinal vascular plexus according to a pre-specified protocol (Tang et al., 2017). All OCTA images were analysed at the Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong, China.
Ethical aspects
This study was carried out in accordance with the Declaration of Helsinki and good Clinical Practice. Written informed consent was obtained from all patients after thorough written and oral information about the course and possible consequences of the study. Approval for this study was obtained from the FDDB board committee, and ethical approval was obtained from the Regional Scientific Ethical Committee of Southern Denmark.
Statistical analysis
Stata Intercooled 15.1 (StataCorp, College Station, Texas) was used for all statistical analyses in this study. Continuous data are presented as mean (with 95% confidence intervals) and categorical data as percentages. Differences or trends across DR severity and, respectively, retinal oxygen saturation, FAZ size, VD, VDI, FD and FAZ circularity were tested employing cluster robust standard errors for linear regression models, as patients were allowed to participate with both eyes. Chi2 test was used for comparison of proportions.
All statistical differences were tested at the 0.05 level, and confidence intervals that did not cross 1.0 were considered statistically significant.
Results
One hundred ninety-nine eyes of 100 patients were examined (one eye of one patient could not be examined due to anophthalmia). Four eyes were known to have PDR at the time of last screening and therefore were ineligible for the study. Forty-five eyes were excluded due to poor image quality, and 11 eyes had progressed to PDR since the last screening episode, or had not been updated accordingly in the FDDB. These eyes were, consequently, also excluded from the study. This led to a final inclusion of 139 eyes of 80 patients.
The overall characteristics of the included participants are shown in Table 1. Among the included eyes were 49 (35.3%) eyes with no DR, 29 (20.9%) eyes with mild NPDR, 40 (28.8%) eyes with moderate NPDR and 21 (15.1%) eyes with severe NPDR. Additional data for the participants included type of diabetes, HbA1c and body mass index (BMI). A statistical significant difference among the four groups was identified for duration of diabetes for moderate NPDR compared to no DR (25.6 years versus 18.1 years, p = 0.02, linear regression model employing cluster robust standard errors), HbA1c for severe NPDR compared to mild NPDR (89.1 mmol/mol versus 73.8 mmol/mol, p = 0.02) and retinal oximetry image quality for severe NPDR compared to no, mild and moderate NPDR (7.9 versus 7.4, 7.3 and 7.3, p = 0.03).
| Characteristics | Level 0 (49 eyes) | Level 1 (29 eyes) | Level 2 (40 eyes) | Level 3 (21 eyes) | Total (139 eyes) | p-value |
|---|---|---|---|---|---|---|
| Age (years) | 58.5 ± 3.0 | 60.5 ± 2.3 | 60.8 ± 2.1 | 54.4 ± 3.2 | 59.0 ± 1.5 | 0.34 |
| Sex (% male) | 46.9 | 69.0 | 57.5 | 61.9 | 56.8 | 0.28 |
| Type of diabetes (% type 1) | 49.0 | 48.3 | 57.5 | 61.9 | 53.2 | 0.68 |
| Duration of diabetes (years) | 18.1 ± 2.2 | 21.4 ± 2.2 | 25.6 ± 1.6 | 22.5 ± 2.1 | 21.7 ± 1.3 | 0.02 |
| HbA1c (mmol/mol) | 94.5 ± 8.8 | 73.8 ± 2.5 | 91.3 ± 8.7 | 89.1 ± 3.5 | 88.0 ± 4.3 | 0.02 |
| BMI (kg/m2) | 28.0 ± 1.4 | 31.5 ± 1.9 | 29.4 ± 1.4 | 29.3 ± 1.4 | 29.4 ± 0.5 | 0.49 |
| Image quality (Oxymap T1) | 7.4 ± 0.1 | 7.3 ± 0.2 | 7.3 ± 0.2 | 7.9 ± 0.2 | 7.4 ± 0.1 | 0.03 |
- Continuous data presented as means ± SE, analysed using linear regression models, employing cluster robust standard errors. Categorical data presented as per cent, analysed using Chi-square test.
Metabolic and structural results are presented in Table 2. The overall raSatO2 and rvSatO2 were 90.7 ± 1.1% and 50.9 ± 1.5%. RvSatO2 increased with increasing level of DR (p = 0.02). Eyes with severe NPDR (59.8 ± 3.1%) had a higher rvSatO2 as compared to eyes with no DR (51.3 ± 1.6%), mild NPDR (53.3 ± 1.9%) and moderate NPDR (54.2 ± 2.4). There was no association between raSatO2 and severity of DR (p = 0.48). There was a tendency towards a decreased arteriolar-venular difference with increasing levels of DR (p = 0.08).
| Level 0 (95% CI) | Level 1 (95% CI) | Level 2 (95% CI) | Level 3 (95% CI) | p-trend | |
|---|---|---|---|---|---|
| Retinal vascular oxygen saturation (%) | |||||
| Arteriolar | 91.0 ± 1.3 (88.5–93.5) | 90.3 ± 1.2 (87.9–92.8) | 92.0 ± 1.2 (87.6–96.3) | 92.2 ± 1.2 (89.9–94.5) | 0.48 |
| Venular | 51.3 ± 1.6 (48.0–54.6) | 53.3 ± 1.9 (49.5–57.0) | 54.2 ± 2.4 (49.5–59.0) | 59.8 ± 3.1 (53.7–65.9) | 0.02 |
| Difference in arteriolar-venular oxygen saturation | 39.7 ± 1.4 (37.5–42.0) | 37.1 ± 1.6 (33.8–40.3) | 37.7 ± 2.3 (33.2–42.3) | 32.4 ± 2.7 (27.1–37.7) | 0.08 |
| Area of foveal avascular zone (mm2) | |||||
| Superficial layer | 0.363 ± 0.023 (0.318–0.408) | 0.311 ± 0.033 (0.246–0.376) | 0.376 ± 0.027 (0.322–0.430) | 0.405 ± 0.047 (0.310–0.499) | 0.36 |
| Deep layer | 1.253 ± 0.176 (0.904–1.603) | 1.164 ± 0.221 (0.723–1.605) | 1.704 ± 0.271 (1.163–2.244) | 1.097 ± 0.197 (0.704–1.490) | 0.60 |
| Total Vessel Density (%) | |||||
| Superficial layer | 0.695 ± 0.010 (0.675–0.714) | 0.674 ± 0.010 (0.653–0.695) | 0.650 ± 0.011 (0.627–0.673) | 0.623 ± 0.018 (0.587–0.660) | <0.001 |
| Deep layer | 0.386 ± 0.006 (0.374–0.399) | 0.389 ± 0.006 (0.378–0.401) | 0.364 ± 0.005 (0.353–0.374) | 0.379 ± 0.010 (0.358–0.399) | 0.09 |
| Vessel diameter index (mm) | |||||
| Superficial layer | 0.0164 ± 0.0002 (0.0160–0.0169) | 0.0169 ± 0.0003 (0.0163–0.0174) | 0.0173 ± 0.0003 (0.0168–0.0178) | 0.0173 ± 0.0003 (0.0168–0.0178) | 0.003 |
| Deep layer | 0.0133 ± 0.000 1(0.0131– 0.0134) | 0.0186 ± 0.0052 (0.0082–0.0290) | 0.0139 ± 0.0001 (0.0137–0.0142) | 0.0141 ± 0.0002 (0.0138–0.0144) | 0.76 |
| Foveal avascular zone circularity | |||||
| Superficial layer | 0.632 ± 0.018 (0.596–0.669) | 0.649 ± 0.032 (0.585–0.713) | 0.594 ± 0.017 (0.559–0.629) | 0.556 ± 0.018 (0.520–0.593) | 0.006 |
| Deep layer | 0.439 ± 0.016 (0.408–0.470) | 0.474 ± 0.027 (0.419–0.528) | 0.392 ± 0.018 (0.356–0.429) | 0.452 ± 0.028 (0.400–508) | 0.48 |
| Fractal dimension | |||||
| Superficial layer | 1.695 ± 0.002 (1.691– 1.699) | 1.691 ± 0.002 (1.690–1.194) | 1.685 ± 0.002 (1.681–1.689) | 1.681 ± 0.003 (1.675–1.687) | <0.001 |
| Deep layer | 1.691 ± 0.003 (1.685–1.695) | 1.691 ± 0.003 (1.686–1.696) | 1.678 ± 0.003 (1.672–1.683) | 1.683 ± 0.004 (1.674–1.692) | 0.01 |
- Numbers presented as means ± SE (95% CI). Levels of diabetic retinopathy (DR): 0 (no DR), 1 (minimal non-proliferative DR [NPDR]), 2 (moderate NPDR) and 3 (severe NPDR). The data in this table were analysed using regression models employing cluster robust standard errors.
The overall FAZ size of the superficial and deep capillary plexus was 0.345 ± 0.024 mm2 and 1.277 ± 0.172 mm2. These did not associate with level of DR (p = 0.36 and p = 0.60). Likewise, no correlations were found between superficial or deep FAZ size and raSatO2 and rvSatO2 (superficial FAZ: p = 0.90 and p = 0.76, deep FAZ: p = 0.50 and p = 0.21, for raSatO2 and rvSatO2, respectively) (Table 3).
| Retinal arteriolar oxygen saturation | Retinal venular oxygen saturation | |
|---|---|---|
| Area of foveal avascular zone (superficial layer) | 0.90 | 0.76 |
| Area of foveal avascular zone (deep layer) | 0.50 | 0.21 |
| Total vessel density (superficial layer) | 0.39 | 0.60 |
| Total vessel density (deep layer) | 0.49 | 0.52 |
| Vessel diameter index (superficial layer) | 0.76 | 0.45 |
| Vessel diameter index (deep layer) | <0.01 | <0.01 |
| Foveal avascular zone circularity (superficial layer) | 0.53 | 0.36 |
| Foveal avascular zone circularity (deep layer) | 0.76 | 0.07 |
| Fractal dimension (superficial layer) | 0.91 | 0.54 |
| Fractal dimension (deep layer) | 0.93 | 0.96 |
- Numbers presented as p-values. The data in this table were analysed using regression models employing cluster robust standard errors.
The overall VD, VDI, FD and FAZ circularity were 0.696 ± 0.009%, 0.0165 ± 0.0002 mm, 1.695 ± 0.002 and 0.644 ± 0.019 at the superficial capillary plexus, and 0.387 ± 0.006%, 0.0146 ± 0.0013 mm, 1.691 ± 0.003 and 0.444 ± 0.016 at the deep capillary plexus, respectively. A trend was identified for decreasing VD, increasing VDI, decreasing FAZ circularity at the superficial capillary plexus across ICDR levels 0–3 (p < 0.001, p = 0.0033 and 0.006), and, likewise, for decreasing FD at the superficial and deep capillary plexus (ICDR level 0–3: 1.695, 1.691, 1.685, 1.681, p < 0.001, and 1.691, 1.691, 1.678, 1.683, p = 0.014). No trend was found regarding VD, VDI or FAZ circularity across ICDR levels 0-3 (p = 0.092, p = 0.76 and p = 0.48, respectively) at the deep capillary plexus.
A correlation was identified between raSatO2 and VDI (p < 0.01) and likewise between rvSatO2 and VDI (p < 0.01), at the deep capillary plexus. No correlations were found between raSatO2 or rvSatO2 and VD, FAZ circularity and FD at the superficial or deep capillary plexus. Likewise, no correlation was found between raSatO2 or rvSatO2 and VDI at the superficial capillary plexus (Table 3).
Discussion
In the present study, we examined the connection between retinal metabolic and structural alterations in eyes with increasing severity of DR. Trends for increasing venular retinal vascular metabolism and retinal structural changes were found along the DR-spectrum, but the sparse correlations between metabolic and structural abnormalities indicates that these changes occur independently.
In a systematic review, Rilven et al., (2017) examined associations between retinal oxygen saturation and retinal ischaemic diseases, and concluded that in patients with diabetes increasing rvSatO2 was associated with increasing levels of DR. In the present study, we similarly report significant associations between retinal venular oxygen saturation and DR severity, identifying a trend of increasing rvSatO2 with increasing levels of NPDR. This indicates lower retinal oxygen consumption in eyes with more advanced stages of DR. In contrast, we did not observe differences in retinal arteriolar oxygen saturations between DR groups. The reason for this discrepancy may be the fact that the retinal arteriolar oxygen saturation, as opposed to the retinal venular oxygen saturation, does not depend upon the retinal oxygen consumption.
Foveal avascular zone (FAZ) size did not associate with level of DR in the present study. De Carlo et al., (2015) prospectively evaluated OCTA images of 61 eyes of patients with diabetes and 28 control eyes of healthy subjects with regard to FAZ size, irregularity, vessel beading and tortuosity, capillary non-perfusion and microaneurysms. In that study, FAZ size was increased in eyes of patients with diabetes as compared to eyes of healthy controls (0.35 mm2 versus 0.29 mm2, p = 0.04). Likewise, Dimitrova et al., (2017) found an increased superficial FAZ size in patients with diabetes (0.37 mm2) in comparison to healthy controls (0.31 mm2). This current study found no such trend, which might be explained by the small patient cohort, the lack of a healthy control group, or that potential changes in FAZ size does not concur with the ICDR levels tested. It was noted that size of FAZ varies among individuals, and thus, it is not easy to assess possible pathologic FAZ enlargement in the setting of retinal diseases (Mammo et al., 2015; Tang et al., 2017).
In this study, a trend for decreasing VD, increasing VDI and decreasing FAZ circularity were identified at the superficial capillary plexus across ICDR levels, while a trend for decreasing FD was observed at both superficial and deep capillary plexus, indicating increasing microvascular damage with increasing DR severity. Similar results were found by Tang et al, who found that increasing DR severity was associated with increased FAZ area, decreased FAZ circularity, lower VD, lower FD and increased VDI (Tang et al., 2017). These matrices were chosen specifically, as previous studies had identified correlations between increased FAZ area and shorter axial length and thinner central subfield macular thickness. Statistically significant correlations were also found between decreased FAZ circularity and visual function, decreased VD and thinner ganglion-cell inner plexiform layer, lower FD and increased DR severity and increased VDI and higher fasting glucose levels. Therefore, it has been suggested that the extent of microvascular damage can be quantified from OCTA (Tang et al., 2017).
In our study, no correlations were found when comparing the superficial and deep FAZ sizes, VD, FAZ circularity and FD to raSatO2 and rvSatO2 within the levels of DR severity (data not presented). This might not be such a surprise, as the OCTA images evaluate the superficial and deep capillary plexus with a 4.5 mm × 4.5 mm field in the central macular area. In contrast, retinal oximetry is based on fundus images and hence, a more uniform field of view would have been preferred and may readily become possible as the technology of OCTA develops for wide-field imaging. However, a correlation was identified, when comparing raSatO2 and rvSatO2 to VDI at the deep capillary plexus. The discrepancy between the fact that microvascular structural alteration were observed throughout the DR-spectrum in the superficial capillary plexus, but that the metabolic-structural connection occurred in the deep capillary plexus is intriguing. It may indicate that the deeper part of the retinal vascular tree plays a bigger role in retinal metabolism.
Strengths of the present study include the use of a well-characterized population of patients with diabetes with representation of all degrees of severity of NPDR. However, the findings from our study should be interpreted with caution, given its cross-sectional design and the limited number of patients. Also, the differences in age and duration between included and excluded patients may have influenced our results.
In conclusion, the present study found few correlations between retinal metabolic and microvascular structural alterations, indicating that metabolic and structural abnormalities may occur independently at increasing DR stages. Furthermore, it demonstrates trends for increased microvascular structural changes and venular retinal metabolic alterations throughout the spectrum of DR. If confirmed by prospective data, this could confirm retinal oximetry as an easily accessible, non-invasive retinal risk factor of DR progression. Such observations would imply that retinal oximetry could be used in adjunct to level of DR and OCTA imaging to identify patients at risk of PDR in diabetic eye screening.




