Intravitreal anti-vascular endothelial growth factors, panretinal photocoagulation and combined treatment for proliferative diabetic retinopathy: a systematic review and network meta-analysis
Abstract
Purpose
To conduct a systematic review with network meta-analysis (NMA) of randomized clinical trials (RCTs) comparing panretinal photocoagulation (PRP) versus anti-vascular endothelial growth factor (VEGF) treatment alone or in combination with PRP, for proliferative diabetic retinopathy (PDR).
Methods
PubMed, Medline and Embase databases were searched for RCTs comparing PRP versus intravitreal anti-VEGF therapy and/or combined PRP and intravitreal anti-VEGF for PDR. The primary outcome measures were the mean best corrected visual acuity (BCVA) change and the regression of neovascularization. Mean change of central macular thickness (CMT), the subgroup analyses of patients without diabetic macular oedema (DME) and the rate of vitreous haemorrhage and vitrectomy were secondary outcomes. Frequentist NMAs were performed.
Results
Twelve RCTs were included. For the 12-month mean BCVA change, NMA showed a better visual outcome in both the anti-VEGF group and combined group compared to PRP [anti-VEGF vs PRP, mean difference (MD) = 3.42; standard error (SE) = 1.5; combined vs PRP, MD = 3.92; SE = 1.65], with no difference between combined group and anti-VEGF (MD = −0.50; SE = 1.87). No difference in neovascularization regression was found between PRP and anti-VEGF alone or in combination with PRP, but there was significant inconsistency (p = 0.016). Subgroup analyses in patients without DME yielded no difference for the 12-month visual outcome between the three interventions, but with significant inconsistency (p = 0.005).
Conclusion
This NMA showed limited evidence of comparable efficacy in terms of neovascularization regression between PRP and anti-VEGF therapy alone or in combination with PRP, but better visual outcomes were associated with anti-VEGF use. Intravitreal anti-VEGF therapy could be a valid therapeutic option in association with PRP.
Introduction
Diabetic retinopathy represents the fifth cause of blindness globally (Leasher et al. 2016) and the commonest visual impairing condition in working-age people (Wong & Sabanayagam 2019). Proliferative diabetic retinopathy (PDR) accounts for one of the major causes of vision loss in diabetic population (Antonetti, Klein & Gardner 2012).
The mainstay treatment for PDR has been panretinal photocoagulation (PRP) since the early treatment diabetic retinopathy study showed its efficacy in 1980s (‘Photocoagulation Treatment of Proliferative Diabetic Retinopathy: Clinical Application of Diabetic Retinopathy Study (DRS) Findings, DRS Report Number 1981’ 1981). Guidelines from both the American Academy of Ophthalmology and the Royal college of Ophthalmologists recommend PRP as first-line treatment in proliferative disease (Ghanchi et al. 2013; Flaxel et al. 2020).
However, the use of intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents for the treatment of PDR has provided encouraging results in the last decade, with better functional outcomes compared to PRP (Gross et al. 2015; Sivaprasad et al. 2017).
A systematic review investigated the use of anti-VEGF therapy for PDR in 2015, but the pooled analysis comparing anti-VEGF therapy versus PRP included only two trials (Simunovic & Maberley 2015). Afterwards, new evidence from randomized clinical trials (RCTs) comparing PRP to anti-VEGF therapy or to combined anti-VEGF and PRP treatment has become available (González et al. 2009; Ernst et al. 2012; Lopez-Lopez et al. 2012; Preti et al. 2013; Ferraz et al. 2015; Gross et al. 2015; Figueira et al. 2016; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Gross et al. 2018).
The issue of whether intravitreal anti-VEGF treatments should be preferred over PRP for the treatment of PDR is of clinical relevance, in particular for patients without diabetic macular oedema (DME). Therefore, we decided to systematically review RCTs comparing PRP versus anti-VEGF treatment alone or in combination with PRP, in patients with PDR. Network meta-analyses (NMA) of data primarily aiming at evaluating the visual outcome and the effect of treatments on neovascularization regression were performed.
Materials and Methods
The methodology of the present study was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statements (Liberati et al. 2009) and the Cochrane Handbook’s guidelines (Higgins & Green 2011) (PRISMA checklist in Table S1).
Search method
Randomized clinical trials (RCTs) comparing the outcomes of PRP versus anti-VEGF treatment alone and/or in combination with PRP were systematically searched for on PubMed, Medline and Embase databases, from their inception to 18 March 2020. The search strategy included the terms ‘proliferative diabetic retinopathy’, ‘anti’, ‘vascular endothelial growth factor’, ‘bevacizumab’, ‘ranibizumab’, ‘aflibercept’, ‘pegaptanib’, ‘photocoagulation’, ‘laser’, connected by and/or in various combinations (Table S2). Reference lists of potentially eligible articles were also reviewed.
Eligibility criteria
The following inclusion criteria had to be satisfied: (1) RCT design; (2) to compare at least two of the following interventions for PDR and/or severe non-PDR: PRP alone (PRP group), intravitreal anti-VEGF therapy alone (anti-VEGF group); combined PRP and intravitral anti-VEGF therapy (combined therapy group); (3) a follow-up of 6 months or longer; (4) to provide data for the primary outcome measure of this meta-analysis. Only articles published in peer-review journals, with no limitations with regards to publication date or status, were included. Abstracts and non-English language studies were excluded. Patients in the PRP group were treated with PRP and received no intravitreal anti-VEGF therapy unless it was a rescue therapy for complications and/or macular oedema. Similarly, patients in the anti-VEGF group were treated with anti-VEGF therapy alone and received no PRP, unless it was a rescue treatment for complications. The primary outcome measures were the mean best corrected visual acuity (BCVA) change and the rate of regression of retinal and/or optic disc neovascularization in the different intervention groups. The mean change of optical coherence tomography (OCT) central macular thickness (CMT), the subgroup analyses of patients without centre involving DME at baseline and the incidence rate of vitreous haemorrhage and vitrectomy in the different interventions groups were considered as secondary outcome measures. Central macular thickness (CMT), also reported as central subfield thickness, referred to the average value of the fovea-centred circle with 1 mm diameter (Sull et al. 2010).
Data extraction and quality assessment
Titles and abstracts of all identified articles were independently screened by two authors (M.F. and A.R.), applying inclusion/exclusion criteria. All articles deemed potentially eligible were full-text reviewed to assess whether eligibility criteria were fully met. In cases of discrepancy, a third investigator (M.R.) was consulted for obtaining consensus. In case two or more articles reported data of the same cohort of patients, the one either with better quality or more recent was included in our analysis. Data extraction from included studies was performed by two investigators (M.F. and A.R.). Study year, first author, location and design, follow-up and type of intervention groups were extracted from each included studies, together with characteristics and findings of each intervention group, such as number of patients, mean age, gender, anti-VEGF agent and number of injections, BCVA change, neovascularization regression rates, OCT CMT change and complications, as rate of vitreous haemorrhage and vitrectomy. Additionally, characteristics and findings of the subgroup of patients without DME at baseline were extracted from each intervention group where reported. When clarifications or further information were needed for eligibility assessment or data extraction, the authors of the study were contacted. Risk of bias assessment was based on the Cochrane collaboration tool (Higgins & Green 2011). To detect publication bias and small study effect, we produced and investigated the comparison-adjusted funnel plots.
Statistical analysis
We performed network meta-analyses within a frequentist framework, assuming equal heterogeneity across all comparisons and accounting for correlations induced by multi-arm RCTs (Salanti 2012; Miladinovic et al. 2014). We compared primary and secondary outcomes of intravitreal anti-VEGF therapy alone (treatment C) or combined with PRP (treatment B) using the PRP treatment as reference group (treatment A). For continuous outcomes, mean difference (MD) and standard error (SE) were calculated using mean change and standard deviations (SD) if reported. Alternatively, we used methods outlined in the Cochrane Handbook to obtain mean change and SD (Higgins & Green 2011). When SD was missing, we estimated it from standard errors, range, 95% confidence intervals (CIs), or graphs as described by Wan et al. (2014). Mean BCVA change was reported in ETDRS letters. For dichotomous outcomes, we calculated odd ratios (ORs) from the number of events and total populations. We assessed statistical heterogeneity in the entire network based on between-study variance estimated from the network meta-analysis (NMA) models (Jackson et al. 2014). We used the design-by treatment and the node splitting methods to evaluate inconsistency globally and locally, respectively (Shim et al. 2017). The hierarchy of interventions was established using surface under the cumulative ranking curve (SUCRA) and mean ranks. We also calculated the ranking probabilities of being the best, the second, or the worst treatment, accordingly (Salanti, Ades & Ioannidis 2011). We also did a subgroup analysis by restricting the network meta-analyses to individuals without DME at the baseline. All the analyses were conducted with STATA (version 15, StataCorp LLC; College Station, TX, USA) using the network and network graphs packages.
Results
The study selection flow chart is displayed in Fig. 1. Electronic search identified a total of 3519 articles, of which 2862 were screened once duplicates had been removed. Fifty-five articles were full-text reviewed to evaluate whether eligibility criteria were satisfied. A total of 13 articles were included in this systematic review, of which two results of the same RCT at different follow-up (Gross et al. 2015; Gross et al. 2018).
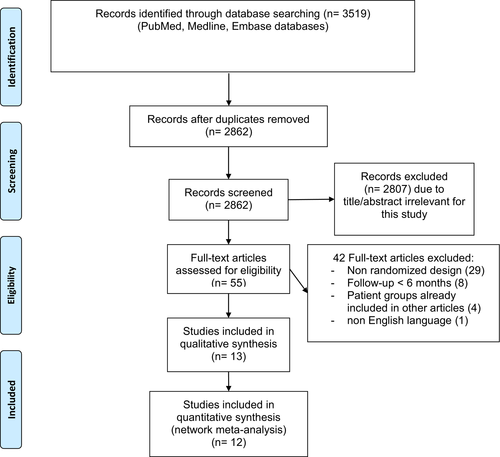
Study characteristics
A total of 12 RCTs was included in the present systematic review (González et al. 2009; Filho et al. 2011; Ernst et al. 2012; Lopez-Lopez et al. 2012; Preti et al. 2013; Ferraz et al. 2015; Gross et al. 2015; Figueira et al. 2016; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Gross et al. 2018; Lang et al. 2019). Of note, two different articles reported the results of Protocol S trial at 2- and 5-year follow-up, respectively (Gross et al. 2015; Gross et al. 2018). Consequently, the included articles were 13. Main characteristics of included studies are shown in Table 1. All RCTs compared two or more interventions, of which one was PRP in all cases: three RCTs compared PRP vs combined PRP and intravitreal bevacizumab (Lopez-Lopez et al. 2012; Preti et al. 2013; Roohipoor et al. 2016); one compared PRP vs intravitreal bevacizumab alone (Ernst et al. 2012); three compared PRP vs combined PRP and intravitreal ranibizumab (Filho et al. 2011; Ferraz et al. 2015; Figueira et al. 2018); two 2 compared PRP vs intravitreal ranibizumab alone vs combined PRP and intravitreal ranibizumab (Figueira et al. 2016; Lang et al. 2019); one compared PRP vs intravitreal ranibizumab alone (Gross et al. 2015); one compared PRP vs intravitreal aflibercept alone (Sivaprasad et al. 2017); one compared PRP vs intravitreal pegaptanib alone (González et al. 2009). Follow-up time ranged from 6 months to 5 years. Four RCTs featured a follow-up ranging from 6 to 8 months, being classified as 6-month follow-up study (González et al. 2009; Lopez-Lopez et al. 2012; Preti et al. 2013; Ferraz et al. 2015); 7 RCTs with a follow-up ranging from 10 to 12 months were classified as 12-month follow-up study (Filho et al. 2011; Ernst et al. 2012; Figueira et al. 2016; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019); only the Protocol S trial had a follow-up longer than 1 year (Gross et al. 2015; Gross et al. 2018). Data on the subgroup of patients not affected by centre involving DME at the baseline were provided by seven RCTs (Ernst et al. 2012; Ferraz et al. 2015; Gross et al. 2015; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019).
| Author, year | Location | Interventions | Patients, n | Follow-up | Patients without DME at baseline, n |
|---|---|---|---|---|---|
| Ernst et al. (2012) | Colorado, USA | Bevacizumab vs PRP |
Bevacizumab group, 10 PRP group, 10 |
12 months |
Bevacizumab group, 10 PRP group, 10 |
| Lopez-Lopez et al. (2012) | Spain | PRP vs combined bevacizumab and PRP |
PRP group, 20 Combined group, 20 |
6 months | – |
| Preti et al. (2013) | Sao Paulo, Brazil | PRP vs combined bevacizumab and PRP |
PRP group, 35 Combined group, 35 |
6 months | – |
| Roohipoor et al. (2016) | Tehran, Iran | PRP vs combined bevacizumab and PRP |
PRP group, 32 Combined group, 32 |
10 months |
PRP group, 32 Combined group, 32 |
| Filho et al. (2011) | Sao Paulo, Brazil | PRP vs combined ranibizumab and PRP |
PRP group, 20 Combined group, 19 |
11 months | – |
| Ferraz et al. (2015) | Sao Paulo, Brazil | PRP vs combined ranibizumab and PRP |
PRP group, 30 Combined group, 30 |
6 months |
Control group, 16 Combined group, 15 |
| Figueira et al. (2016) | Multicentre (Portugal) | PRP vs ranibizumab vs combined |
PRP group, 13 Ranibizumab group, 10 Combined group, 12 |
12 months | – |
| Figueira et al. (2018) | Multicentre (Europe) | PRP vs combined ranibizumab and PRP |
PRP group, 46 Combined group, 41 |
12 months |
PRP group, 46 Combined group, 41 |
| Gross et al. (2018) | Multicentre (USA) | PRP versus ranibizumab |
PRP group, 203 Ranibizumab group, 191 |
5 years |
PRP group, 141 Ranibizumab group, 136 |
| Lang et al. (2019) | Multicentre (Germany) | PRP vs ranibizumab vs combined |
PRP group, 35 Ranibizumab group, 35 Combined group, 36 |
12 months |
PRP group, 35 Ranibizumab group, 35 Combined group, 36 |
| Sivaprasad et al. (2017) | Multicentre (United Kingdom) | PRP vs aflibercept |
PRP group, 116 Aflibercept group, 116 |
12 months |
PRP group, 116 Aflibercept group, 116 |
| Gonzalez et al. (2009) | Texas, USA | PRP vs pegaptanib |
PRP group, 10 Pegaptanib group, 10 |
8 months | – |
- DME = diabetic macular oedema (centre-involving), PRP = panretinal photocoagulation.
Risk of bias assessment is shown in Figs S1 and S1. Random sequence generation was judged as low risk of bias in five studies (González et al. 2009; Gross et al. 2015; Sivaprasad et al. 2017; Figueira et al. 2018; Gross et al. 2018), while was unclear in eight (Filho et al. 2011; Ernst et al. 2012; Lopez-Lopez et al. 2012; Preti et al. 2013; Ferraz et al. 2015; Figueira et al. 2016; Roohipoor et al. 2016; Lang et al. 2019). Allocation concealment was high risk in one study (Lopez-Lopez et al. 2012), low risk in three (Gross et al. 2015; Sivaprasad et al. 2017; Gross et al. 2018) and unclear in nine (González et al. 2009; Filho et al. 2011; Ernst et al. 2012; Preti et al. 2013; Ferraz et al. 2015; Figueira et al. 2016; Roohipoor et al. 2016; Figueira et al. 2018; Lang et al. 2019). All studies featured a high risk with respect to performance bias because masking of participants and personnel was unfeasible given the nature of the treatments. Detection bias was high risk in two studies (Lopez-Lopez et al. 2012; Figueira et al. 2018), unclear in six (González et al. 2009; Ernst et al. 2012; Preti et al. 2013; Figueira et al. 2016; Roohipoor et al. 2016; Lang et al. 2019) and low in five (Filho et al. 2011; Ferraz et al. 2015; Gross et al. 2015; Sivaprasad et al. 2017; Gross et al. 2018). Attrition bias and reporting bias were unclear in two (Lopez-Lopez et al. 2012; Ferraz et al. 2015) and three studies (Gross et al. 2015; Roohipoor et al. 2016; Gross et al. 2018), and low risk in 11(González et al. 2009; Filho et al. 2011; Ernst et al. 2012; Preti et al. 2013; Gross et al. 2015; Figueira et al. 2016; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Gross et al. 2018; Lang et al. 2019) and 10 (González et al. 2009; Filho et al. 2011; Ernst et al. 2012; Lopez-Lopez et al. 2012; Preti et al. 2013; Ferraz et al. 2015; Figueira et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019), respectively. Risk of other bias was unclear in six studies (González et al. 2009; Filho et al. 2011; Ernst et al. 2012; Lopez-Lopez et al. 2012; Figueira et al. 2016; Roohipoor et al. 2016) and low in the remaining seven (Preti et al. 2013; Ferraz et al. 2015; Gross et al. 2015; Sivaprasad et al. 2017; Figueira et al. 2018; Gross et al. 2018; Lang et al. 2019). The funnel plots for each outcome of interest revealed no evidence of publication bias, featuring a nearly symmetrical shape (Fig. S3–S5).
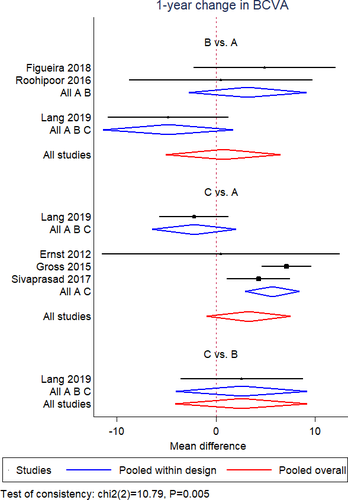
Mean BCVA change
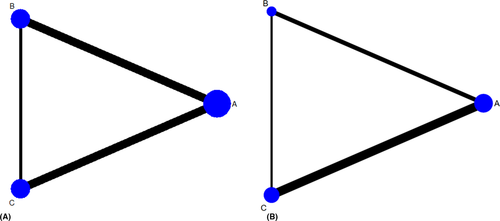
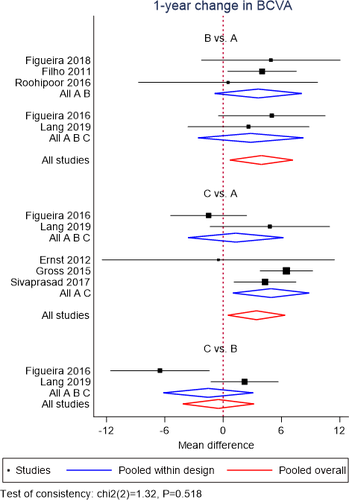
A NMA of data from 8 RCTs (Filho et al. 2011; Ernst et al. 2012; Gross et al. 2015; Figueira et al. 2016; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019) was performed for mean BCVA change at 12 months. Geometry of the three-node network for BCVA outcome is reported in Fig. 2A. Overall, a total of 475 eyes, 362 eyes and 140 eyes were pooled in the PRP group, anti-VEGF group and combined group, respectively. The NMA comparing the combined group versus the PRP group yielded a greater visual gain in the combined group (MD = 3.92; SE = 1.65). Likewise, anti-VEGF group showed a better visual change compared to PRP group (MD = 3.42; SE = 1.5). No difference was found between anti-VEGF group and combined group (MD = −0.50; SE = 1.87; Fig. 3). No inconsistency was found among included studies (p = 0.52). According to SUCRA analysis, the probability of being the best treatment was higher for the combined treatment (60.1%) (Fig. S6).
No NMA was conducted for mean BCVA change at 6 months because of the limited number of RCTs reporting these data.
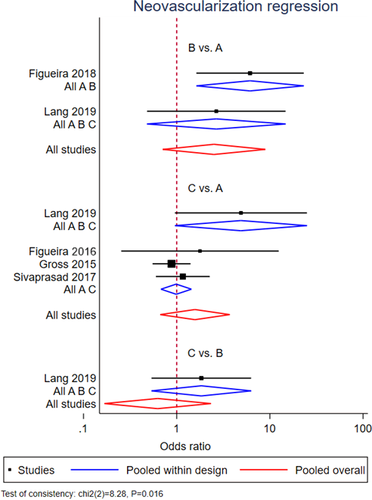
Regression of neovascularization
A NMA was performed for regression of neovascularization, pooling data from five RCTs with 12- and 24-month follow-up (Gross et al. 2015; Figueira et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019). Geometry of the three-node network for this outcome is reported in Fig. 2B. Overall, a total of 346 eyes, 294 eyes and 89 eyes were pooled in the PRP group, anti-VEGF group and combined group, respectively. The NMA comparing the combined group versus the PRP group showed no significant difference in the proportion of eyes which regressed between the two groups (OR = 2.51; 95% CI = 0.71–8.92). Similarly, the anti-VEGF group had not a significantly better outcome than PRP group (OR = 1.57; 95% CI = 0.67–3.71) (Fig. 4). However, significant inconsistency was found among included studies (p = 0.016). According to SUCRA analysis, the probability of being the best treatment was higher for the combined treatment (99.5%) (Fig. S7).
Mean OCT central macular thickness change
A NMA of data from six RCTs (Filho et al. 2011; Figueira et al. 2016; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019) was performed for mean CMT change at 12 months. Geometry of the three-node network for CMT outcome is reported in Fig. S8. Overall, a total of 262 eyes, 161 eyes and 140 eyes were pooled in the PRP group, anti-VEGF group and combined group, respectively. The NMA comparing the combined group versus the PRP group showed no difference in mean CMT change between the two groups (MD = −10.05; SE = 6.17). Anti-VEGF group had a better outcome with a significant change in terms of reduced CMT, compared both to PRP group (MD = −36.93; SE = 5.11) and combined group (MD = −26.88; SE = 6.22) (Fig. S9). No inconsistency was found among included studies (p = 0.88). According to SUCRA analysis, the probability of being the best treatment was higher for the anti-VEGF treatment (100%) (Fig. S10).
No NMA was conducted for mean BCVA change at 6 months because of the limited number of RCTs reporting these data.
Subgroup analyses in patients without DME at baseline
A NMA of data from six RCTs (Ernst et al. 2012; Gross et al. 2015; Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019) was conducted for mean BCVA change at 12 months in patients without DME at baseline. Geometry of the three-node network for BCVA outcome among patients without DME is reported in Fig. S11A. Overall, a total of 380 eyes, 297 eyes and 109 eyes were pooled in the PRP group, anti-VEGF group and combined group, respectively. The NMA revealed no difference among the three interventions in terms of visual outcome (combined group vs PRP, MD = 0.23, SE = 3.41; anti-VEGF group vs PRP, MD = 3.3, SE = 2.2; anti-VEGF vs combined group, MD = 0.60, SE = 3.96) (Fig. 5). However, significant inconsistency was found among included studies (p = 0.005). Indeed, anti-VEGF group showed a better visual change compared to PRP group, if pooling only two-arm studies (MD = 5.7, SE = 1.4). According to SUCRA analysis, the probability of being the best treatment was higher for the anti-VEGF therapy alone (75.9%) compared to the PRP treatment (2.5%) and combined treatment (21.6%) (Fig. S12).
Data from four RCTs (Roohipoor et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019) were pooled for NMA of mean CMT change at 12 months in patients without DME at baseline. Geometry of the three-node network for CMT outcome among patients without DME is reported in Fig. S11B. Overall, a total of 229 eyes, 151 eyes and 109 eyes were included in the PRP group, anti-VEGF group and combined group, respectively. The NMA comparing the combined group versus the PRP group showed a reduced CMT in the combined group (MD = −13.82; SE = 6.94). The anti-VEGF group yielded a reduced CMT compared to both the PRP and combined group (anti-VEGF vs PRP group, MD = −38.98, SE = 5.4; anti-VEGF vs combined group, MD = −25.15, SE = 6.73) (Fig. S13). No evidence of inconsistency among the included studies was demonstrated (p = 0.91). The anti-VEGF intervention had a 100% probability of being the best treatment according to SUCRA analysis (Fig. S14).
Rate of vitreous haemorrhage and vitrectomy
Network meta-analyses of rate of vitreous haemorrhage and vitrectomy were conducted pooling data from six RCTs with 12- and 24-month follow-up (Filho et al. 2011; Ernst et al. 2012; Gross et al. 2015; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019) and five RCTs with 12- and 24-month follow-up (Gross et al. 2015; Figueira et al. 2016; Sivaprasad et al. 2017; Figueira et al. 2018; Lang et al. 2019), respectively. Geometry of the three-node networks for the risk of vitreous haemorrhage and vitrectomy is reported in Fig. S15. Overall, vitreous haemorrhage occurred in 103 out of 430 eyes, 63 out of 352 eyes and 12 out of 96 eyes of the PRP group, anti-VEGF group and combined group, respectively. Vitrectomy was performed in 48 out of 413 eyes, 10 out of 352 eyes and five out of 89 eyes in the PRP group, anti-VEGF group and combined group, respectively. Network meta-analyses comparing the proportion of vitreous haemorrhage and vitrectomy revealed no difference between the combined group and the PRP group (vitreous haemorrhage: OR = 0.89, 95% CI = 0.20–3.78; vitrectomy: OR = 0.53, 95% CI = 0.17–1.66) and no difference between combined group and anti-VEGF group (vitreous haemorrhage: OR = 0.27, 95% CI = 0.09–1.36; vitrectomy: OR = 0.42, 95% CI = 0.20–1.21). The proportion of both vitreous haemorrhage and vitrectomy was lower in the anti-VEGF group compared to the PRP group (vitreous haemorrhage: OR = 0.24, 95% CI = 0.08–0.70; vitrectomy: OR = 0.23, 95% CI = 0.11–0.46; Figs S16 and S17). No inconsistency was found among the included studies for both the rate of vitreous haemorrhage and vitrectomy (p = 0.56 for vitreous haemorrhage; p = 0.61 for vitrectomy). Intravitreal anti-VEGF intervention had a higher probability of being the best treatment according to SUCRA analysis for both vitreous haemorrhage (96.5%) and vitrectomy (90.4%) outcomes (Figs S18 and S19).
Discussion
The present NMA compared three different interventions for PDR, showing that both intravitreal anti-VEGF therapy alone and combined PRP and anti-VEGF treatment yielded a better 12-month visual outcome compared to PRP treatment. With regard to neovascularization regression, NMA revealed no difference between PRP treatment and anti-VEGF therapy alone or in combination with PRP, but significant inconsistency was found.
Current mainstay treatment for PDR remains PRP (Ghanchi et al. 2013; Flaxel et al. 2020). However, sometimes retinal laser could prove challenging due to poor fundus view and to poor patient compliance (Muqit et al. 2010a; Muqit et al. 2010b). Additionally, PRP complications such as macular oedema and peripheral field loss can represent an issue for being allowed to drive and, in general, for the visual outcome (Maeshima et al. 2004; Muqit et al. 2010a; Muqit et al. 2010b; Gabrielle et al. 2019). These shortcomings have been partially improved following the development of new laser technology (Blumenkranz et al. 2006; Muqit et al. 2010a; Muqit et al. 2010b; Muqit et al. 2013). Semi-automated multi-spot laser improves patient compliance, reducing the level of pain, headache and anxiety compared to standard single-spot laser (Muqit et al. 2010a; Muqit et al. 2010b). Panretinal photocoagulation (PRP)-related macular thickening has been reduced as well, but can still happen (Muqit et al. 2010a; Muqit et al. 2010b; Reddy & Husain 2018).
Very recently, the use of intravitreal anti-VEGF therapy has been extended to the treatment of PDR. Two large RCTs showed the non-inferiority of anti-VEGF agents compared to PRP in terms of visual outcome and, even, the superiority of intravitreal therapy (Gross et al. 2015; Sivaprasad et al. 2017). Intravitreal anti-VEGF therapy reduced DME onset and peripheral field loss (Gross et al. 2015; Sivaprasad et al. 2017). Protocol S demonstrated a lower rate of vitrectomy in those treated with intravitreal ranibizumab compared to a PRP group, whereas no difference was found with regard to vitreous haemorrhage rate (Gross et al. 2015). Conversely, the Clarity trial showed a lower incidence of vitreous haemorrhage in the PRP group compared to intravitreal aflibercept group, whereas no difference was found with regard to vitrectomy rate (Sivaprasad et al. 2017).
Our results corroborated previous findings of better visual gain for intravitreal anti-VEGF therapy compared to PRP treatment. Of note, the visual outcome was comparable between anti-VEGF group and combined treatment. Anti-VEGF therapy resulted in less macular thickening than the PRP group and was also better than the combined treatment. Rates of vitrectomy and vitreous haemorrhage were lower in anti-VEGF group compared to PRP group, but comparable with those of the combined treatment. According to SUCRA analysis, the combined treatment could be considered the treatment of choice when looking at the best visual outcome.
When it comes to assess the efficacy of PDR treatment, the regression of neovascularization represents an outcome of primary interest. With respect to this, our results showed that PRP treatment was comparable with anti-VEGF therapy alone or combined with PRP. Even if this finding was influenced by significant inconsistency, a limited evidence of comparable efficacy in terms of neovascularization regression does not support anti-VEGF therapy as solo treatment for PDR. According to SUCRA analysis, the combined treatment could be considered the treatment of choice.
Furthermore, the beneficial effect of the anti-VEGF therapy on the visual outcome was not evident when considering the subgroup of patients without centre involving DME at the baseline. In this subgroup, the visual outcome was comparable between the three different interventions, even if the macular thickness was better in the anti-VEGF group. However, the visual outcome has been influenced by significant inconsistency, which represents a limit for the strength of the result. Nonetheless, this limited evidence of comparable visual outcome has some clinical relevance in support of PRP treatment as first-line approach for PDR without DME. It is important to point out that intravitreal aflibercept is not licensed in Europe for PDR treatment (‘Eylea 40 mg/ml solution for injection in a vial - Summary of Product Characteristics (SmPC) - (emc)’ n.d.) and intravitreal ranibizumab only recently has been approved for PDR and could be administered also in absence of DME (‘Lucentis 10 mg/ml solution for injection - Summary of Product Characteristics (SmPC) - (emc)’ n.d.).
Additionally, several meaningful drawbacks relate to intravitreal anti-VEGF therapy. Complications of intravitreal therapy can include lens damage (Meyer et al. 2010), intraocular pressure spikes (Furino et al. 2020) and endophthalmitis (Reibaldi et al. 2019). Furthermore, an increased risk of systemic adverse events has been described for intensive anti-VEGF therapy in diabetic patients (Avery & Gordon 2016; Reibaldi et al. 2020). From this point of view, PRP treatment is safe.
Panretinal photocoagulation (PRP) is a long-lasting treatment, which is another significant advantage when it comes to a chronic disease as diabetic retinopathy. On the contrary, intravitreal anti-VEGF therapy is a short-term treatment, whose effect could last up to 10 weeks or slightly longer (Stewart & Rosenfeld 2008; Niwa et al. 2015). Recent ultrawide field fluorescein angiography studies showed that anti-VEGF therapy proved unable to re-perfuse ischaemic retina in diabetic patients (Bonnin et al. 2019; Couturier et al. 2019). The absence of reperfusion of vessels or capillary network under anti-VEGF therapy could lead to a worsening of diabetic retinopathy in case of treatment discontinuation (Bonnin et al. 2019). This means that patients must be followed up closely and for a long time. A loss to follow-up can lead to irreversible visual impairment because of tractional retinal detachment and iris neovascularization (Obeid et al. 2019).
Panretinal photocoagulation (PRP) has been associated with severe visual field loss, while 2-year reports of Protocol S trial showed almost unchanged visual field condition in eyes treated with anti-VEGF therapy (Gross et al. 2015). This could have been a relevant advantage of anti-VEGF therapy compared to PRP treatment. However, post hoc analyses of 5-year visual field changes in eyes enrolled in the Protocol S trial demonstrated that anti-VEGF therapy alone does not prevent a progression of visual field loss, reporting an unexpected visual field deterioration between years 2 and 5 in eyes treated with only anti-VEGF therapy in the absence of laser treatments (Maguire et al. 2020).
Anti-VEGF therapy requires frequent treatments and follow-ups, with a subsequent significant socioeconomic burden. Panretinal photocoagulation (PRP) is less expensive than anti-VEGF therapy (Gross et al. 2015). Not surprisingly, cost analysis studies confirmed that PRP is the least expensive treatment for PRD, with an increasing cost differential on a predictive lifetime model (Lin, Chang & Smiddy 2016; Lin et al. 2018). While anti-VEGF therapy demonstrated a modest improved visual acuity outcome (about three letters), it is debatable whether the cost and inconvenience of repeated anti-VEGF injections justify this therapy over PRP.
The following limitations characterized this systematic review with NMA. First, a relatively small number of studies was included, but a meta-analysis has a higher power with more accurate CIs than an individual study (Fallico et al. 2020). Second, all included studies presented a high risk of bias for masking of participants and personnel, but this issue was related to the nature of the treatments. Randomized clinical trials (RCTs) mainly differ for inclusion/exclusion criteria which could have limited the application of reported results. In particular, a few studies considered the presence of centre involving DME as an exclusion criterion (Ernst et al. 2012; Roohipoor et al. 2016; Figueira et al. 2018; Lang et al. 2019). However, the subgroup analyses of patients without DME at baseline allowed to include a quite homogenous population. Different anti-VEGF agents were used, with different treatment protocol. Anti-VEGF drug could have been administered also in the PRP group. However, this was allowed only if representing a rescue therapy for DME. Finally, when considering neovascularization regression outcome and the rates of vitreous haemorrhage and vitrectomy, we pooled together 12- and 24-month studies. The different follow-up time could have had an influence on the occurrence of these events. A 24-month follow-up would have been more adequate to assess clinical outcomes in PDR. However, a follow-up clustering would have led to a lower number of studies and no analysis could have been performed. Moreover, no inconsistency was found among the included studies for all our analyses, except for the neovascularization regression outcome and the BCVA outcome among non-DME subgroup. This satisfies the assumption of consistency, as such the comparative effect sizes obtained through direct and indirect comparisons are consistent.
In conclusion, this NMA showed a limited evidence of comparable efficacy in terms of neovascularization regression between PRP treatment and anti-VEGF therapy alone or in combination with PRP. Even if anti-VEGF therapy provided a better visual outcome, the gain was less than one line on a visual acuity chart; thus, it might not be clinically relevant, considering also the burden of the anti-VEGF therapy. In patients without DME at baseline, the observed inconsistency for visual outcome does not allow us to clearly state benefits from anti-VEGF therapy. The comparison of only two-arm trials suggests that patients treated with anti-VEGF could exhibit better visual change than those treated with PRP. Nonetheless, we do not recommend intravitreal anti-VEGF therapy alone as the standard first-line treatment for PDR. Intravitreal anti-VEGF therapy could be a valid therapeutic option in association with PRP and it could be considered when PRP is not feasible because of poor view or other circumstances.