Baseline predictors for visual acuity loss during observation in diabetic macular oedema with good baseline visual acuity
Abstract
Purpose
To investigate clinical baseline characteristics and optical coherence tomography biomarkers predicting visual loss during observation in eyes with diabetic macular oedema (DMO) and good baseline visual acuity (VA).
Methods
A sub-analysis of a 12-month, retrospective study, including patients with baseline VA ≤0.1 logMAR (≥20/25 Snellen) and centre-involving DMO. The primary outcome measure was the correlation between baseline characteristics and VA loss ≥10 letters during follow-up.
Results
A total of 249 eyes were included in the initial study, of which 147 eyes were observed and 80 eyes received anti-vascular endothelial growth factor (VEGF) treatment at baseline. Visual acuity (VA) loss ≥10 letters occurred in 21.8% (observed cohort) and in 24.3% (treated cohort), respectively. Within observed eyes, presence of hyperreflective foci [HRF; odds ratio (OR): 3.18, p = 0.046], and disorganization of inner retina layers (DRIL; OR: 2.71, p = 0.026) were associated with a higher risk of VA loss ≥10 letters. In observed eyes with a combined presence of HRF, DRIL and ellipsoid zone (EZ) disruption, the risk of VA loss was further increased (OR: 3.86, p = 0.034). In eyes with combined presence of DRIL, HRF and EZ disruption, risk of VA loss was 46.7% (7/15 eyes) in the observed cohort, and 26.3% (5/19 eyes) in the treated cohort (p = 0.26).
Conclusion
Patients with DMO and good baseline VA, managed by observation, are of increased risk for VA loss if DRIL, HRF and EZ disruption are present at baseline. Earlier treatment with anti-VEGF in these patients may potentially decrease the risk of VA loss at 12 months.
Introduction
Diabetic macular oedema (DMO) affects around 21 million people worldwide and is the main cause of vision loss among diabetic patients (Yau et al. 2012; Arroba & Valverde 2017). Several treatment options are currently available, including intravitreal therapy [anti-vascular endothelial growth factor (VEGF) agents and steroids], as well as macular laser. Previous randomized clinical trials (RCT) have demonstrated these can improve the outcome of DMO significantly (Figueira et al. 2009; Nguyen et al. 2012; Boyer et al. 2014; Gillies et al. 2014; Wells et al. 2016). Most RCTs, however, excluded DMO patients with very good baseline visual acuity (VA). Thus, for a long time, little was known about the appropriate management and outcome of these patients. Recently, Protocol V by DRCR.net was the first RCT on DMO patients with very good baseline VA, demonstrating that most eyes maintained vision over a period of 2 years (Baker et al. 2019). Eyes that were treated immediately at baseline did not fare better than eyes that were observed with treatment initiation only when VA loss developed (Baker et al. 2019). Treatment was required in about one-third of the observed cohort during the 2-year study period. The authors concluded that DMO eyes with good baseline VA are managed reasonably by observation without treatment until VA loss occurs (Baker et al. 2019). Recently, our group reported similar findings in a real-world setting (Busch et al. 2019). Over a 12-month period, most eyes had stable vision regardless of whether DMO was treated or not. However, in this real-world context, 43% of the non-treated eyes experienced significant VA loss during the first 6 months. Treatment initiation in those eyes led to better results than further observation (Busch et al. 2019).
Even though most DMO eyes with good baseline VA can be managed appropriately with observation, a significant proportion of those eyes will experience VA loss requiring treatment initiation. Thus, it is of great interest to identify patients with increased risk for VA loss. The purpose of this study was to investigate baseline characteristics and optical coherence tomography (OCT) features that predict a subsequent VA loss during observation in DMO patients with very good baseline VA.
Methods
The study procedures have been reported previously and are summarized briefly (Busch et al. 2019). Institutional review board (IRB) approval was obtained through the individual IRBs at the participating institutes for a retrospective consecutive chart review. This research adhered to the tenets of the Declaration of Helsinki.
Study participants
Sixteen study sites included 249 eyes from 210 diabetic patients with centre-involving DMO, defined by retinal thickness of >250 µm in the central subfield (CST) and intra- ± subretinal fluid on spectral-domain (SD)-OCT within 500 µm of the fovea and best-corrected VA ≤0.1 logMAR (≥0.8 decimal acuity, ≥20/25 Snellen equivalent or ≥80 EDTRS letters).
Medical records were initially reviewed for demographic data (i.e. age, sex); duration of diabetes; known comorbidities (i.e. hypertension, dyslipidaemia), stage of diabetic retinopathy (DR), previous DMO treatment, previous laser panretinal photocoagulation (PRP), lens status, VA and CST at baseline, 3, 6, 9 and 12 months; conduction of DMO treatment during follow-up (including macular laser, intravitreal anti-VEGF injections, triamcinolone acetonide, dexamethasone implant), laser PRP and cataract surgery.
OCT analysis
All eyes were imaged with SD-OCT (Heidelberg Spectralis, Heidelberg, Germany; Optovue Avanti, Fremont, CA, USA; Topcon 3D OCT-2000, Tokyo; Japan; or Cirrus, Zeiss, Oberkochen, Germany, Canon-OCT HS100, Tokyo, Japan). Three horizontal OCT scans at baseline: one b-scan encompassing the fovea, and two b-scans 500 µm superior and 500 µm inferior to the fovea, respectively, were exported and checked for intra- and subretinal fluid in order to confirm diagnosis of centre-involving DMO. Baseline scan through the fovea were evaluated for presence of subretinal fluid, hyperreflective foci (HRF), disorganization of inner retina layers (DRIL) and disruption to the ellipsoid zone (EZ). HRF were defined as small (<30 µm), punctiform spots, located in the inner and/or outer retina layers, with a reflectivity similar to the retinal nerve fibre layer, presenting without back shadowing (Vujosevic et al. 2017a; Vujosevic et al. 2017b). For evaluation of DRIL, the boundaries between the ganglion cell/inner plexiform layer complex, inner nuclear layer and outer plexiform layer were analysed for ability to distinguish between the layers (Zur et al. 2018b). All scans were graded by two masked retina specialists (MI, CB). Grading discrepancies were adjudicated by a third retinal specialist (MR).
Outcome measures
Main outcome measures were differences in baseline characteristics and baseline OCT features between eyes with and without VA loss ≥10 letters during the period of observation. Secondary outcome measures were differences in proportion of eyes with VA loss ≥10 letters between the treated and non-treated cohort stratified for OCT characteristics.
Statistical analysis
The demographic and clinical characteristics of our study cohort were evaluated using traditional descriptive methods. To control for the correlated nature of our data, we used a generalized estimating equations (GEE) procedure. Differences in outcome measures (baseline characteristics between observed eyes with and without VA loss ≥10 letters; and proportion of VA loss between treated and observed eyes stratified for present OCT characteristics at baseline) were analysed by univariable logistic regression analysis. Kaplan–Meier analysis was used for time-to-event analysis. Statistical analysis was performed with SPSS Statistics 25 (IBM, Armonk, NY, USA).
Results
The OBTAIN study included 249 eyes of 210 patients, with a mean age of 60.1 ± 10.7 years and a mean baseline VA 0.06 ± 0.05 logMAR (Busch et al. 2019). In 147 eyes, no treatment was initiated at baseline. Of these, 94 eyes (63.9%) continued observation throughout the 12 months follow-up. During the period of observation, 21.8% (32/147 eyes) experienced a VA loss of ≥10 letters.
Baseline characteristics associated with VA loss during observation of DMO
Demographic and baseline characteristics of observed eyes stratified for VA loss during observation are shown in Table 1. There was no significant difference in the baseline demographic or clinical characteristics between observed eyes with and without VA loss.
|
Eyes without VA loss ≥10 letters (n = 115) |
Eyes with VA loss ≥10 letters (n = 32) |
p value* | |
|---|---|---|---|
| Age, years, mean (SD) | 63.0 (9.7) | 59.0 (12.0) | 0.051 |
| Male, n (%) | 66 (57.4) | 22 (68.8) | 0.245 |
| HbA1c, %, mean (SD) |
7.8 (1.5) n = 85 |
7.3 (1.3) n = 20 |
0.174 |
| Duration of diabetes, months, mean (SD) |
168.5 (133.4) n = 99 |
179.2 (124.3) n = 25 |
0.714 |
| Proliferative diabetic retinopathy, n (%) | 13 (11.3) | 7 (21.9) | 0.154 |
| Type 1 diabetes, n (%) | 7 (6.1) | 4 (12.5) | 0.147 |
| Known comorbidities, n (%) | |||
| None | 13/111 (11.3) | 1/30 (3.1) | 0.206 |
| Hypertension | 101/114 (87.8) | 27/29 (84.4) | 0.489 |
| Dyslipidemia | 37/102 (32.2) | 14/28 (50.0) | 0.186 |
| Treatment-naïve DMO, n (%) | 92 (80.0) | 22 (68.8) | 0.201 |
| Prior macular laser, n (%) | 16 (13.9) | 7 (21.9) | 0.316 |
| Prior anti-VEGF therapy, n (%) | 15 (13.0) | 6 (18.8) | 0.461 |
| No. prior anti-VEGF injections, mean (SD) | 4.5 (2.2) | 6.1 (2.1) | 0.122 |
| Prior therapy with IVTA, n (%) | 1 (0.9) | 0 (0) | 1.000 |
| Prior therapy with DEX implant, n (%) | 1 (0.9) | 0 (0) | 1.000 |
| Pseudophakia, n (%) | 18 (15.7) | 7 (21.9) | 0.405 |
| Prior PRP, n (%) | 25 (21.7) | 6 (18.8) | 0.739 |
| Baseline VA, logMAR, mean (SD) | 0.06 (0.05) | 0.04 (0.06) | 0.201 |
| Baseline CST, µm, mean (SD) | 331.2 (50.1) | 350.0 (90.1) | 0.119 |
| CST change from baseline to end of observation period, µm, mean (SD) | +14.0 (57.6) | +40.9 (65.5) | 0.062 |
- CST = central subfield thickness, DEX = dexamethasone, DMO = diabetic macular oedema, HbA1c = haemoglobin A1c, IVTA = intravitreal triamcinolone acetonide, PRP = panretinal photocoagulation, SD = standard deviation, VA = visual acuity, VEGF = vascular endothelial growth factor.
- * p value for difference between eyes without and with VA loss ≥10 letters during non-treatment, tested by generalized estimating equation model, univariable logistic regression analysis.
Subretinal fluid, HRF, DRIL and EZ disruption was present at baseline in 4.8% (7/145 eyes), 72.9% (105/144 eyes), 51.4% (71/138 eyes) and 17.0% (24/141 eyes) in observed eyes. Visual acuity (VA) outcome stratified for OCT baseline characteristics is displayed in Table 2. The odds of experiencing VA loss ≥10 letters was increased when HRF [26.7% versus 10.3%, odds ratio (OR): 3.18, 95% confidence interval (CI): 1.02–9.90, p = 0.046] or DRIL (29.6% versus 13.4%, OR: 2.71, 95% CI: 1.14–6.45, p = 0.026) were present at baseline (univariate analysis, Table 2). Time until VA loss occurs was significantly shorter in case of present HRF (10.3 versus 11.3 months, p = 0.036), DRIL (9.9 versus 11.4 months, p = 0.005) or EZ disruption (9.3 versus 11.0 months, p = 0.016, Table 2). In combined presence of HRF, DRIL and EZ disruption the odds for a significant VA loss were further increased (46.7% versus 18.5%, OR: 3.86, 95% CI: 1.11–13.42, p = 0.034, Table 2) and time until VA loss decreased (8.6 versus 10.9 months, p = 0.001, Table 2, Fig. 1).
| VA loss ≥10 letters during F/U, n (%) |
Univariable analysis p value* |
Odds ratio (95% CI) | Time until VA loss ≥10 letters, months, mean (95% CI) | p value† | |
|---|---|---|---|---|---|
| Subretinal fluid | |||||
| Present | 2/7 (28.6) | 0.674 | – | 9.4 (6.8–12.0) | 0.329 |
| Absent | 30/138 (21.7) | 10.6 (10.1–11.1) | |||
| Hyperreflective foci | |||||
| Present | 28/105 (26.7) | 0.046 | 3.18 (1.02–9.90) | 10.3 (9.7–10.9) | 0.034 |
| Absent | 4/39 (10.3) | 11.3 (10.6–12.0) | |||
| Disruption of the ellipsoid zone | |||||
| Present | 9/24 (37.5) | 0.054 | 2.74 (0.98–7.64) | 9.3 (7.8–10.7) | 0.016 |
| Absent | 21/117 (17.9) | 11.0 (10.5–11.4) | |||
| DRIL | |||||
| Present | 21/71 (29.6) | 0.026 | 2.71 (1.14–6.45) | 9.9 (9.1–10.7) | 0.005 |
| Absent | 9/67 (13.4) | 11.4 (10.9–11.9) | |||
| Simultaneous presence of HRF and EZ disruption | |||||
| Yes | 7/19 (36.8) | 0.114 | – | 9.3 (7.7–11.0) | 0.036 |
| No | 23/122 (18.9) | 10.9 (10.4–11.4) | |||
| Simultaneous presence of HRF and DRIL | |||||
| Yes | 19/56 (33.9) | 0.007 | 3.22 (1.38–7.53) | 9.6 (8.7–10.6) | 0.001 |
| No | 11/80 (13.8) | 11.3 (10.8–11.8) | |||
| Simultaneous presence of EZ damage and DRIL | |||||
| Yes | 9/19 (47.4) | 0.010 | 4.32 (1.41–13.17) | 8.5 (6.8–10.2) | 0.001 |
| No | 20/116 (17.2) | 11.0 (10.6–11.5) | |||
| Simultaneous presence of HRF, EZ damage and DRIL | |||||
| Yes | 7/15 (46.7) | 0.034 | 3.86 (1.11–13.42) | 8.6 (6.7–10.5) | 0.003 |
| No | 22/119 (18.5) | 10.9 (10.5–11.4) | |||
- Due to low scan quality, presence of hyperreflective foci, EZ damage and DRIL was not gradable in one, four and seven patients, respectively.
- DRIL = disorganization of inner retinal layers, EZ = ellipsoid zone, F/U = follow-up, HRF = hyperreflective foci.
- * p value for difference in event of VA loss during observation between present and absent OCT features as indicated in column one, tested by generalized estimating equation model, logistic regression analysis. Optical coherence tomography (OCT) scans at baseline were missing for two patients.
- † p value for difference in time until VA loss between present and absent OCT features as indicated in column one, tested by Kaplan–Meier procedure.
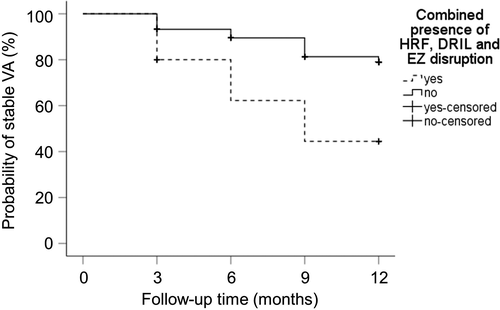
Risk of VA loss is dependent on OCT baseline characteristics in observed and treated eyes
After identifying OCT baseline characteristics associated with an increased risk for VA loss in non-treated eyes, we investigated whether the risk for VA loss was decreased in eyes with these baseline characteristics that were treated at baseline. For this purpose, eyes that were treated with anti-VEGF therapy from baseline with or without macular laser during the study period and available OCT scans (n = 74) were analysed. Baseline and treatment characteristics are shown in Table S1 (supplementary material). Visual acuity (VA) loss ≥10 letters during follow-up was observed in 24.3% (18/74 eyes). Subretinal fluid, HRF, DRIL and EZ disruption were present at baseline in 9.5% (7/74 eyes), 82.4% (61/74 eyes), 71.2% (52/73 eyes) and 34.2% (25/73 eyes) in treated eyes. After stratifying for presence of OCT risk features, the proportion of eyes with VA loss was lower in the treated cohort, compared to the non-treated cohort (Table 3) when OCT risk features were present. The difference between both groups became more evident with increasing number of OCT risk features. Eyes which simultaneously showed HRF, DRIL and EZ disruption that were treated at baseline had a lower likelihood of VA loss compared to the non-treated cohort (26.3% versus 46.7%) though this difference did not reach statistical significance (p = 0.26). Time until VA loss was 8.9 months (95% CI: 7.0–10.7 months) in observed eyes and 10.6 months (95% CI: 9.1–12.1 months) in treated eyes (p = 0.17).
| VA loss ≥10 letters during F/U | Difference between both groups, % | p value* | ||
|---|---|---|---|---|
| Non-treated eyes | Treated eyes | |||
| Present hyperreflective foci, n (%) | 28/105 (26.7) | 15/61 (24.6) | −2.1 | 0.775 |
| Present disruption of the ellipsoid zone, n (%) | 9/24 (37.5) | 7/25 (28.0) | −9.5 | 0.501 |
| Present DRIL, n (%) | 21/71 (29.6) | 13/52 (25.0) | −4.6 | 0.597 |
| Simultaneous presence of HRF and EZ disruption, n (%) | 7/19 (36.8) | 6/23 (26.1) | −10.7 | 0.483 |
| Simultaneous presence of HRF and DRIL, n (%) | 19/56 (33.9) | 10/44 (22.7) | −11.2 | 0.254 |
| Simultaneous presence of EZ damage and DRIL, n (%) | 9/19 (47.4) | 6/21 (28.6) | −18.8 | 0.251 |
| Simultaneous presence of HRF, EZ damage and DRIL, n (%) | 7/15 (46.7) | 5/19 (26.3) | −20.4 | 0.256 |
- DRIL = disorganization of inner retinal layers, EZ = ellipsoid zone, F/U = follow-up, HRF = hyperreflective foci.
- * p value for difference between treated and non-treated cohort, tested by generalized estimating equation model, logistic regression analysis.
Discussion
This study reveals that the presence of HRF, DRIL and EZ disruption on OCT is associated with an increased risk for significant VA loss in patients with DMO and very good baseline VA who are managed with initial observation in a real-life setting. Combined presence of all three features further increased the risk for VA loss. However, in eyes with all three risk factors at baseline, those that were treated immediately with anti-VEGF at baseline tended to have a lower risk for VA loss compared to eyes that were initially observed.
We identified that presence of HRF on OCT is associated with an increased risk of a VA loss in DMO with good baseline VA. These HRF are common in DMO eyes (Schreur et al. 2018) but are also seen in diabetic eyes without DMO or manifest DR (Vujosevic et al. 2013; De Benedetto et al. 2015). The number of HRF seems to increase with DR progression (Vujosevic et al. 2013). Previous studies in DMO eyes treated with intravitreal anti-VEGF and steroids demonstrated a reduction of HRF by both treatments (Vujosevic et al. 2016; Vujosevic et al. 2017a; Vujosevic et al. 2017b). The predictive value of HRF at baseline for the outcome of treated DMO eyes remains controversial. Although some studies reported a correlation of HRF with visual outcome (Schreur et al. 2018; Zur et al. 2018a), others found no association (Vujosevic et al. 2016). There are different hypotheses on the aetiology of HRF. However, growing evidence suggests that HRF seen on OCT represent aggregates of activated microglia and thus can be considered as a retinal inflammatory biomarker (Vujosevic et al. 2013; Midena et al. 2018). Thus, HRF in DMO patients with good baseline VA might indicate evident inflammatory changes and an increased risk for subsequent worsening.
Presence of DRIL was identified as an additional OCT biomarker associated with an increased risk for subsequent VA loss in our study. Santos et al. revealed that DRIL was associated with a significant increased risk of poor visual response to anti-VEGF therapy in DMO (Santos et al. 2018). Other studies reported that DRIL correlates with worse baseline VA acuity (Sun et al. 2014), impaired retinal function (Joltikov et al. 2018) and macular capillary non-perfusion (Nicholson et al. 2015) in diabetic patients. Macular perfusion was not analysed in our study. However, reduced macular perfusion might be a reason for an increased proportion of VA loss in patients with DRIL observed in our study. The combination of EZ disruption in eyes with DRIL led to a significant further increase in risk for VA loss. Disruption of the EZ represents damage to the macular photoreceptors (Maheshwary et al. 2010) and several previous studies already highlighted the importance of an intact EZ on visual outcome in DMO (Maheshwary et al. 2010; Santos et al. 2018; Zur et al. 2018a).
The predictive value of baseline subretinal fluid on visual outcome in DMO remains controversial. In DMO eyes left untreated, subretinal fluid was associated with poor visual outcome and increased risk of VA loss (Sophie et al. 2015). In eyes treated with intravitreal therapy, some studies reported better VA outcome (Sophie et al. 2015; Zur et al. 2018a), while others did not show any risk modification (Seo et al. 2016; Santos et al. 2018). In DMO eyes with very good baseline VA, we did not identify any correlation between subretinal fluid and the risk for VA loss during non-treatment.
The proportion of VA loss in eyes with HRF, DRIL and/or EZ disruption was lower in eyes treated at baseline compared to the non-treated cohort in our study. Although this difference was not statistically significant, this may be due to the small sample size and under-powering of the results. Furthermore, the treated cohort may also have been under-treated with a mean of 5.4 anti-VEGF injections over 12 months, which is less than that typically seen in RCTs. It is possible that a more intensive injection regimen might have further decreased the risk of VA loss in the treated cohort. Considering our results, the question arises whether patients with a certain OCT morphology should be treated directly and intensively when presenting with DMO and very good baseline VA. However, future studies are needed to address this.
We did not find any difference in baseline characteristics such as age, HbA1c, diabetes duration, DR severity, lens status or prior DMO treatment, between patient with and without VA loss during non-treatment in DMO with very good baseline VA. While some studies reported an association of baseline characteristics with VA outcome in DMO, others could not find any correlation or even reported conflicting results (Bressler et al. 2012; Sivaprasad et al. 2013; Channa et al. 2014; Sophie et al. 2015).
Limitations of our study are its retrospective nature and shortcomings of a real-world setting. Due to the small sample size, significant effects might have been missed. Optical coherence tomography (OCT) analysis was limited to only very few and already known OCT parameters. Furthermore, we have performed multiple statistical testing, which may have influenced the results.
In conclusion, our study emphasizes the importance of OCT morphology in patients with DMO and good VA. The integrity of inner and outer retinal layers are predictive markers of future BCVA in those patients. Early treatment despite good baseline VA in DMO eyes with HRF, DRIL and EZ damage has to potential to decrease the risk of VA loss in this selected sub-group of patients.




