Nonarteritic anterior ischaemic optic neuropathy and its association with obstructive sleep apnoea: a health insurance database study
Abstract
Background
Nonarteritic anterior ischaemic optic neuropathy (NAION) is the most common acute optic neuropathy in old age. Although there are several known risk factors, the influence of obstructive sleep apnoea (OSA) has not been completely elucidated. The aim of this study was to evaluate the association between NAION and OSA.
Methods
This retrospective, longitudinal cohort study used the national health insurance database of Taiwan covering the period 1996–2013. Patients without NAION at the diagnosis of OSA or who developed NAION 1 year after the diagnosis of OSA were enrolled. The patients were followed until death or the last day of the study. Cox proportional hazard regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) to investigate the association between OSA and NAION.
Results
There were 8488 patients in the OSA group and 33 952 in the control group (without OSA), for a ratio of approximately 1:4. The percentages of NAION were 0.36% and 0.2% in the OSA and control groups, respectively, with a statistically significant difference (p < 0.01; chi-square test), and this significant difference remained in multivariate analysis (p = 0.019) with a significantly higher HR (1.66; 95% CI: 1.08–2.55). There was significant difference in the 30–39 years age group in multivariate analysis (p < 0.01, HR: 6.30; 95% CI: 2.28–17.40).
Conclusion
There was a strong association between NAION and OSA, and the patients with OSA had a higher risk of NAION. Further large-scale, prospective studies are warranted to evaluate the effect of OSA on developing NAION.
Introduction
Nonarteritic anterior ischaemic optic neuropathy (NAION) is the most common acute optic neuropathy in patients with age >50 years (Johnson & Arnold 1994; Hattenhauer et al. 1997; Biousse & Newman 2015). Patients with NAION usually present with sudden-onset painless visual loss on awakening in the morning (Hayreh et al. 1994a). Clinical findings of NAION include an acute oedematous optic disc, disc haemorrhage, altitudinal visual field loss and chronic optic atrophy (Hayreh 2009). Approximately 41–43% of patients with NAION have improved vision 6 months after the disease onset, although 15–19% may experience further deterioration (Group 1995; Hayreh & Zimmerman 2008).
The pathogenesis of NAION is ischaemic damage to the anterior part of the optic nerve around the laminar and retrolaminar regions (Arnold 2003; Hayreh 2009; Biousse & Newman 2015). As NAION is a multifactorial disease, several risk factors are been suspected to contribute to its development (Repka et al. 1983; Beck et al. 1987; Hayreh et al. 1994b; Purvin et al. 2004; Kerr et al. 2009; Hayreh 2013), and these include structurally crowded optic discs, nocturnal arterial hypotension, diabetes mellitus, hypertension, hypercholesterolaemia, atherosclerosis and the use of phosphodiesterase type 5 (PDE 5) inhibitors (Hayreh 2000; Arnold 2003; Arda et al. 2013; Chen et al. 2013; Galvez-Ruiz & Arishi 2013; Nathoo et al. 2015; Reddy et al. 2015).
Obstructive sleep apnoea (OSA), characterized by recurrent cessation of breathing during sleep, has been proposed to be an important predisposing factor for NAION in recent studies (Mojon et al. 2002; McNab 2005; Palombi et al. 2006; Arda et al. 2013; Bilgin et al. 2013; Aptel et al. 2015) due to hypoxia, autoregulation impairment and vascular endothelium disruption (Palombi et al. 2006; Archer & Pepin 2013; Arda et al. 2013; Fraser 2014). In addition, some studies have reported that not treating severe OSA with continuous positive airway pressure (CPAP) can increase the risk of a first event of NAION or second eye involvement (Stein et al. 2011; Aptel et al. 2015). Given that OSA is an important risk factor for NAION, screening for OSA in NAION patients has been recommended (McNab 2005; Kolb & Backhouse 2013).
Few nationwide studies have investigated the relationship between NAION and OSA. One study reported that NAION was more prominent in patients with OSA after analysing the i3-InVision-Data-Mart-database (Stein et al. 2011). However, that database represented patients in a large network care system in the United States, which cannot represent all groups as the data source was not the sole institution providing health insurance. Moreover, patients younger than 40 years of age were excluded, and as OSA can still occur in young patients (Chang et al. 2014), eliminating this group may have led to selection bias. Furthermore, 88.2% of the OSA patients in their study were Caucasian, and no study has reported on an Asian population. Therefore, the aim of this study was to investigate whether there was an association between NAION and OSA using data from the National Health Insurance Research Database (NHIRD) in Taiwan.
Materials and Methods
Data source
This retrospective, population-based, longitudinal study used data from the NHIRD of Taiwan, which is provided by the Taiwan National Health Research Institute for research purposes. Because the National Health Insurance programme in Taiwan is mandatory, this database represents the medical data of almost the entire population in Taiwan. In addition, the NHIRD is regularly validated by the authorities. Data are available from 1 January 1996 to 31 December 2013, with diagnoses coded using International Classification of Diseases ninth version (ICD-9) codes. Medications prescribed, interventions and examinations, and patient demographics, education level, socioeconomic status and area of residence are also included in the NHIRD. The Institutional Review Board of Chang Gung Memorial hospital approved this study.
Subject selection
Patients in the NHIRD of any age ≥30 years were regarded as having NAION if they had an ICD-9 diagnostic code of 377.41, which represents ischaemic optic neuropathy. We excluded the subjects less than 29 years old, as the diagnosis of NAION in this age group is extremely rare, and may be due to an error in coding in children with other optic neuropathies. Although posterior ischaemic optic neuropathy and arteritic anterior ischaemic optic neuropathy are also types of ischaemic optic neuropathy, the incidence of these two disorders is relatively low compared to NAION (Johnson & Arnold 1994) and any bias can be ignored.
Patients with OSA were identified using the ICD-9 diagnostic codes 327.2 (organic sleep apnoea), 327.20 (organic sleep apnoea, unspecified), 327.23 [obstructive sleep apnoea (adult) (paediatric)], 327.29 (other organic sleep apnoea), 780.51 (insomnia with sleep apnoea, unspecified), 780.53 (hypersomnia with sleep apnoea, unspecified) and 780.57 (unspecified sleep apnoea). ICD-9 code 327.23 was used to identify cases with a definitive diagnosis of OSA, but the other diagnostic codes were also used for some of the patients with OSA. As most patients with OSA in Taiwan are only diagnosed after complete polysomnography with positive results, and as the doctors who make these diagnoses are mostly either pulmonary or neurological specialists, the possibility of misdiagnosis or mistakes in the ICD-9 diagnostic codes is rare.
Only patients with OSA but without NAION and those who developed NAION 1 year after the diagnosis of OSA were included in the study group. Patients who had received a diagnosis of NAION (ICD-9 diagnostic code of 377.41) previously or developed NAION 1 year within the diagnosis of OSA were excluded. The control group consisted of patients from the NHIRD at a ratio of 4:1 compared to the OSA group.
The whole study period was divided into sections according to different years (different section read as 1996, 1997 until 2013). Every patient with OSA was matched to patients who received the first ICD-9 diagnostic code of other diseases in the same year in terms of age and gender. The diseases we selected to match the study group involved all type of diseases except for OSA, NAION, comorbidities used in multivariate analysis (the ICD-9 diagnostic codes are detailed in the following section), obesity (the ICD-9 diagnostic codes are detailed in the following section), inflammatory diseases leading to chronic upper airway narrowing (ICD-9 diagnostic codes 474, 474.1, 474.11, 474.9, 475 and 476), laryngeal neoplasm (ICD-9 diagnostic codes 161.0, 161.1, 161.2, 161.3, 161.8 and 161.9), hearing loss (ICD-9 diagnostic codes 389.0, 389.1, 389.2, 389.7, 389.8, 389.9), open-angle glaucoma (ICD-9 diagnostic codes 365.10, 365.11, 365.12, 365.13, 365.14, 365.15) and floppy eyelid syndrome (ICD-9 diagnostic code 374.89). Similarly, only patients without NAION or who developed NAION 1 year after the labelled diseases coding were recruited in the control group.
The age, gender and comorbidities were matched between the study and control groups. The comorbidities included the following: (1) hypertension (ICD-9 diagnostic code 401.9), (2) diabetes mellitus (ICD-9 diagnostic codes 250.00, 250.02, 250.10, 250.12, 250.20, 250.22, 250.30, 250.32, 250.40, 250.42, 250.50, 250.52, 250.60, 250.62, 250.70, 250.72, 250.80, 250.82, 250.90 and 250.92), (3) coronary artery disease (ICD-9 diagnostic codes 414.01, 414.2, 414.3, 414.4, 414.8 and 414.9), (4) hyperlipidaemia (ICD-9 diagnostic code 272.4), (5) angina (ICD-9 diagnostic codes 413.0, 413.1 and 413.9), (6) peripheral arterial occlusive disease (ICD-9 diagnostic code 443.9), (7) atherosclerosis (ICD-9 diagnostic codes 440.0, 440.1, 440.2, 440.3, 440.4, 440.8 and 440.9) and (8) cerebrovascular diseases (ICD-9 diagnostic codes 433.0, 433.1, 433.2, 433.3, 433.8, 433.9, 434.0, 434.1, 434.9, 435.0, 435.1, 435.2, 435.3, 435.8 and 435.9).
Obesity, an important comorbidity in OSA with ICD-9 diagnostic codes of 278.00, 278.01, 278.02 and 278.03 only accounted for about 1% (10 619 patients) of all patients in the NHIRD from 1996 to 2013. This was disproportionate to the general prevalence of obesity in Taiwan, which reached 17.0% in males in 2005 (Pan et al. 2008), probably due to the rare application of the obesity diagnostic code in practice. Thus, adjusting for diagnostic codes for obesity in multivariate analysis was not appropriate.
Nonetheless, we adjusted for several metabolic and vascular disorders such as hyperlipidaemia and atherosclerosis, which are common in obese individuals, in multivariate analysis to reduce the influence of obesity on the study results. A patient was defined as having a comorbidity if they had the ICD-9 code in 2005. If the duration of these systemic disorders was short, the effect on the patients may have been minor. Patient data were censored under two conditions: if the patient died or met the last day of the database record (December 31, 2013). The selection flow chart is shown in Fig. 1.
Statistical analysis
spss software version 19 (New York, NY, USA) was used for all data analysis in this study. The chi-square test was used for univariate analysis to evaluate any relationship between NAION and OSA. Cox proportional hazard regression was then used to compute hazard ratio (HRs) and 95% confidence intervals (CIs) within the 18-year study period to investigate the association between OSA and NAION. Adjusted factors in the multivariate Cox proportional hazard regression analysis included duration of the modified comorbidities to counter the influence of different duration, age, gender, hypertension, diabetes mellitus, coronary arterial disease, hyperlipidaemia, peripheral arterial occlusive disease, angina, atherosclerosis and cerebrovascular diseases. Although tobacco consumption is also important risk factors for NAION, a history of tobacco consumption is not available in the NHIRD. Therefore, we could not analyse this factor in this study. As most patients documented in the NHIRD are of Han ethnicity (97%) and nearly all citizens in Taiwan are Asian (more than 99%), race/ethnicity was not adjusted for due to the insignificant influence and effectiveness. Statistical significance was set at p < 0.05.
Results
There were 8488 patients in the OSA group and 33 952 in the control group. The OSA group consisted of 5417 males and 3071 females, while the control group had 21 668 males and 12 284 females. The mean ages were 48.11 ± 11.55 years in both the OSA and control groups. There was no significant difference in comorbidities between the two groups. The demographic data of both groups are shown in Table 1.
| Study group OSA patients (N = 8488) | Control group Non-OSA patients (N = 33 952) | p valuea | |
|---|---|---|---|
| Age group (years), n (%) | |||
| 30–39 | 2370 (21.91) | 9480 (21.91) | 1.00 |
| 40–49 | 2655 (24.54) | 10620 (24.54) | |
| 50–59 | 2170 (20.06) | 8680 (20.06) | |
| 60–69 | 858 (7.93) | 3432 (7.93) | |
| 70–79 | 378 (3.49) | 1512 (3.49) | |
| 80 or above | 57 (0.53) | 228 (0.53) | |
| Gender, n (%) | |||
| Male | 5417 (63.82) | 21668 (63.82) | 1.00 |
| Female | 3071 (36.18) | 12284 (36.18) | |
| Major comorbid medical diseases, n (%) | |||
| Hypertension | 244 (2.87) | 976 (2.87) | 1.00 |
| Diabetes mellitus | 537 (6.33) | 2148 (6.33) | |
| Coronary arterial disease | 193 (2.27) | 772 (2.27) | |
| Hyperlipidaemia | 718 (8.46) | 2872 (8.46) | |
| Angina | 68 (0.8) | 272 (0.8) | |
| PAOD | 2 (0.02) | 8 (0.02) | |
| Atherosclerosis | 0 (0.00) | 0 (0.00) | |
| Cerebrovascular diseases | 46 (0.54) | 184 (0.54) | |
- N and n = number, OSA = obstructive sleep apnoea, PAOD = peripheral arterial occlusive disease.
- a The p value is calculated by the Pearson's chi-square test for these categorical variables.
The overall percentages of NAION were 0.36% in the OSA group and 0.2% in the control group. In chi-square analysis, NAION was significantly more common in the OSA group (p < 0.01) (Table 2, Fig. 2A). Multivariate Cox regression analysis showed that the patients with OSA had a significantly higher risk of developing NAION than those without OSA within the 18-year study period after adjusting for hypertension, diabetes mellitus, coronary artery disease, hyperlipidaemia, angina, peripheral arterial occlusive disease, atherosclerosis, cerebrovascular disease and gender (HR: 1.66; 95% CI: 1.08–2.55; p = 0.019) (Table 2).
| OSA number | NAION in OSA (%) | Control number | NAION in control (%) | P1 | P2 | Hazard ratio (CI) | |
|---|---|---|---|---|---|---|---|
| Overall | 8488 | 0.36 | 33 952 | 0.2 | <0.01 | 0.019 | 1.66 (1.08–2.55) |
| 30–39 (years) | 2370 | 0.46 | 9480 | 0.06 | <0.01 | <0.01 | 6.30 (2.28–17.40) |
| 40–49 (years) | 2655 | 0.26 | 10620 | 0.24 | 0.79 | 0.85 | 1.09 (0.47–2.52) |
| 50–59 (years) | 2170 | 0.28 | 8680 | 0.23 | 0.69 | 0.79 | 1.14 (0.45–2.85) |
| 60–69 (years) | 858 | 0.47 | 3432 | 0.38 | 0.72 | 0.84 | 1.12 (0.36–3.47) |
| 70–79 (years) | 378 | 0.79 | 1512 | 0.26 | 0.13 | 0.18 | 2.80 (0.62–12.64) |
| 80+ (years) | 57 | 0.00 | 228 | 0.44 | 1.00 | 1.00 | 0.76 (N/A) |
- CI = confidential interval, lower limit > 1 or upper limit < 1 demonstrated significant difference between groups; N/A = not applicable due to statistical difficulty, NAION = nonarteritic anterior ischaemic optic neuropathy, OSA = obstructive sleep apnoea.
- P1: Chi-square analysis for comparing the percentage of NAION in OSA group with the percentage of NAION in control group, p < 0.05 demonstrated significant difference between groups.
- P2: Multivariate Cox regression analysis for hazard ratio of developing NAION in OSA group relative to control group after adjusting for hypertension, diabetes mellitus, coronary artery disease, hyperlipidaemia, angina, peripheral arterial occlusive disease, atherosclerosis, cerebrovascular disease and gender. p < 0.05 demonstrate significant difference between groups.
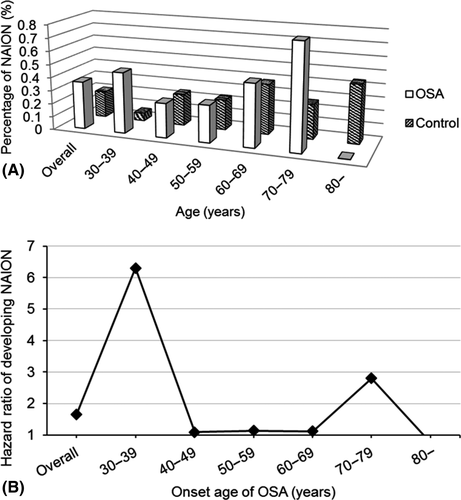
With regard to the percentage of NAION in the OSA and control groups stratified by age group (in 10-year intervals), the peak percentage of NAION in the OSA group occurred in the 70–79 years age group (Table 2, Fig. 2A). Compared to the control group, the patients with OSA aged 30–39 years had a significantly higher risk of developing NAION than the other age groups after controlling for comorbidities in multivariate Cox regression analysis (HR: 6.30, 95% CI: 2.28–17.40, p < 0.01) (Table 2, Fig. 2B). This trend was also similar in univariate Cox regression analysis (Table 3).
| Years | OSA | Hypertension | Diabetes | CAD | Hyperlipidaemia | Angina | PAOD | Atherosclerosis | CVD | Gender |
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| 30–39 | 7.38 (2.73–19.95) | 17.11 (2.27–129.1) | 2.20 (0.50–9.67) | 8.55 (1.94–37.79) | 3.32 (1.08–10.22) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 16.55 (2.18–125.6) | 3.15 (0.72–13.78) |
| 40–49 | 1.12 (0.48–2.59) | 1.39 (0.19–10.23) | 3.22 (1.48–7.01) | 0.71 (0.10–5.19) | 1.43 (0.61–3.32) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.85 (0.41–1.74) |
| 50–59 | 1.20 (0.48–2.99) | 1.98 (0.59–6.60) | 1.14 (0.45–2.86) | 1.52 (0.52–4.42) | 2.12 (0.97–4.64) | 0.63 (0.08–4.66) | 3.45 (0.47–25.5) | 4.99 (0.67–36.94) | 1.92 (0.26–14.19) | 0.77 (0.36–1.66) |
| 60–69 | 1.23 (0.40–3.78) | 0.00 (0.00–inf) | 2.07 (0.79–5.40) | 1.96 (0.68–5.66) | 1.02 (0.36–2.91) | 1.47 (0.33–6.49) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 2.34 (0.53–10.40) | 2.39 (0.84–6.80) |
| 70–79 | 3.00 (0.67–13.42) | 3.38 (0.66–17.45) | 4.47 (1.00–19.96) | 2.71 (0.61–12.15) | 5.22 (1.17–23.38) | 1.74 (0.21–14.45) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 1.98 (0.38–10.25) |
| 80 + | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) | 0.00 (0.00–inf) |
- CAD = coronary artery disease, CVD = cerebrovascular disease, Gender = male relative to female, HR = hazard ration, Inf = infinity; OSA = obstructive sleep apnoea, PAOD = peripheral arterial occlusive disease.
Discussion
Our results showed that the percentage of NAION in the OSA group was significantly higher than that in the control group and also that the hazard of developing subsequent NAION within 18 years was also higher in the patients with OSA than in those without OSA. In addition, the patients with OSA aged 30–39 years had the highest risk.
Although the relationship between OSA and NAION has been proposed in some studies (Archer & Pepin 2013; Fraser 2014), the exact mechanism by which OSA can trigger NAION is unknown. Possible mechanisms include OSA-induced hypoxia that either damages the optic nerve directly or via elevated intracranial pressure during apneic episodes that then compresses the optic nerve leading to ischaemic insults (Mojon et al. 2002). Hypoxic status may also induce a sympathetic surge and oxidative stress that can be harmful to the arterial endothelium, thereby impairing autoregulation of arteries that nourish optic nerve (Mojon et al. 2002; Woodson et al. 2004; Palombi et al. 2006; Kohler & Stradling 2010). In addition, OSA-related derangement of ocular blood flow is associated with variations in ocular perfusion pressure and imbalance between vasoconstrictive [endothelin-1 (ET-1)] and vasodilatory [nitric oxide (NO)] agents (Mentek et al. 2018). On the other side, OSA is also associated with several predisposing factors for NAION including diabetes, hypertension, atherosclerosis and endothelial dysfunction (Kato et al. 2000; Yaggi et al. 2005; Chahal & Somers 2015; Zhang et al. 2015). Taken together, OSA may lead to NAION by itself or with other concurrent systemic disorders through vascular endothelial dysfunction.
Several studies have reported an association between NAION and OSA (Mojon et al. 2002; Palombi et al. 2006; Stein et al. 2011; Archer & Pepin 2013; Bilgin et al. 2013; Kolb & Backhouse 2013; Aptel et al. 2015; Wu et al. 2015). In terms of a large, nationwide study, Stein et al. (2011) also showed a significantly greater HR (1.16) of NAION in patients with OSA who did not receive CPAP compared to individuals without OSA. However, another study suggested that OSA was not a risk factor for NAION as no significant difference was noted in the prevalence between the NAION patient group and control group (Arda et al. 2013). However, this finding could be explained by several matched risk factors between the two groups, which could also contribute to OSA.
In the current study, there was a significantly greater risk of developing NAION in the patients with OSA compared to those without OSA (HR: 1.66). Compared to a previous study (Stein et al. 2011), we enrolled patients of ages ≥30 years so it represented a wider population in Taiwan. The results were compatible to those of previous studies. The estimated prevalence of OSA has been reported to be around 4% in middle-aged men and 2% in middle-aged women (aged 30–60 years) in Western countries (Young et al. 1993) and 3.7–97.3% in Asian countries (Mirrakhimov et al. 2013). The prevalence of OSA in this study was lower (1.3%), which may be due to the underestimation of OSA in a clinical situation (Young et al. 1993).
To date, no population-based study has evaluated the causality of OSA and NAION over time. In this study, we found that the patients with OSA had a greater hazard of developing NAION than those without OSA (HR: 1.66, p = 0.019). Although the incidence of NAION has been shown to be lower in the age 30–39 years compared to other older groups (age 30–39 years: 2.3, age 40–49 years: 5.2, age 50–59 years: 9.44; age ≥60 years: 14.79 per 100 000 Person-Years, respectively) (Lee et al. 2016), our study showed the first time that the patients with OSA aged 30–39 years had a significantly higher risk of developing NAION than those in other age groups (HR: 6.30, p < 0.01), But in the years 40–69, which account for a large portion of our NAION patients, there is no difference in rates and risk of NAION between the OSA group and control group. There are some explanations for these findings. First, OSA group ages < 40 years might have more severe OSA and compromised ocular hemodynamics than the other OSA groups. Li et al. collected 350 patients with suspected OSA who underwent overnight polysomnography (PSG) and found 61% of patients had severe OSA [apnoea/hypopnea index (AHI) ≥ 30] with mean age of 43.7 ± 10.3 years. They concluded OSA patients in East Asia tend to be more severe and younger. Yu et al. reviewed 69 OSA patients and also showed severe OSA group had younger onset age (mean: 38 ± 10 years) than moderate (15 ≤ AHI < 30) and normal-to-mild (AHI < 15) group. Meanwhile, this severe OSA group had significant lower vessel densities around peripapillary area than other groups on optical coherence tomography angiography (OCTA) measurement (Yu et al. 2017). Second, those with optic disc anomalies such as crowded optic disc or disc drusen are associated with younger onset of NAION (Preechawat et al. 2007; Sun & Liao 2017), as opposed to more vascular risk factors for older NAION (Sun & Liao 2017). It is possible that more severe OSA coinciding with these optic disc anomalies contribute to the higher risk of NAION in OSA group aged <40 years than other OSA groups. On the contrary, the risk of NAION was a little higher without statistical significance in OSA group than control group aged 40–49, 50–59 and 60–69 years. As NAION is most common in patients aged >50 years and would naturally increase with age (Cestari et al. 2016), several vascular risk factors contributing to NAION are also associated with OSA; therefore, the effect of OSA on NAION could be diluted in the group aged 40–69 years. Although there is no statistical significance, our study also showed a trend that there is a peak of risk of NAION in patients with OSA aged 70–79 years, and finally, this trend flips completely the other way in those over 80. This could be a statistical bias due to relative small sample size in this group.
There are several limitations to this study. First, as the database used did not include actual medical records, the severity of NAION and OSA and associated data such as visual acuity or apneic duration could not be surveyed. Which eye suffered from NAION or whether there was bilateral involvement was also unknown. Second, the diagnoses in the database were made by different physicians; thus, the standard for diagnosis may be different. Finally, it is possible that patients with arteritic anterior optic neuropathy were also enrolled in this study as it has the same diagnostic code as NAION. Even though arteritic anterior ischaemic optic neuropathy has been reported to have a high incidence, ranging from 20.4 to 32.8 per 100 000 in individuals older than 50 years in Scandinavian countries (Lee et al. 2008), the incidence of arteritic anterior ischaemic optic neuropathy reported in Taiwan is low and there is only one biopsy-proven case report (Cheng et al. 2010), and thus, the effect of this limitation may be minimal.
Conclusions
In this study, there was a strong association between NAION and OSA such that OSA may contribute to the development of NAION. Therefore, OSA should be screened in NAION patients and then treated once the diagnosis is confirmed given that management for OSA may help prevent new NAION events in the fellow eye.




