Peripapillary microcirculation in Leber hereditary optic neuropathy
Abstract
Purpose
In this prospective observational comparative case series, we aimed to study the peripapillary capillary network with spectral-domain optical coherence tomography angiography (OCT-A) in Leber hereditary optic neuropathy (LHON).
Methods
Twelve eyes of six individuals, of these three males (five eyes) after clinical onset of visual impairment were imaged by OCT-A with scans centred on optic discs. Control group consisted of 6 eyes with no visual impairment.
Results
The three affected individuals lost vision 6 years (at age 22 years), 2 years and 3 months (at age 26 years) and 1 year and 2 months (at age 30 years) prior to OCT-A examination. All five affected eyes had alterations in density of the radial peripapillary microvascular network at the level of retinal nerve fibre layer, including an eye of a patient treated with idebenone that underwent almost full recovery (best corrected visual acuity 0.87). Interestingly, the other eye showed normal ocular findings 14 months after onset. Results of OCT-A examination in this eye were unfortunately inconclusive due to a delineation error. At the level of the ganglion cell layer differences could be also noted, but only in two severely affected individuals. There were no differences between unaffected mutation carriers and control eyes.
Conclusion
Optical coherence tomography angiography scans confirmed that the peripapillary microvascular network is highly abnormal in eyes manifesting visual impairment due to LHON. These findings support the hypothesis that microangiopathy contributes to the development of vision loss in this mitochondrial disorder.
Introduction
Leber hereditary optic neuropathy (LHON, OMIM #535000) is a rare disorder caused by mutations in mitochondrial DNA. It is the one of the most common inherited optic neuropathy with a reported prevalence of 31 000–50 000 (Yu-Wai-Man et al. 2003; Spruijt et al. 2006; Puomila et al. 2007; Gorman et al. 2015). The onset of symptoms occurs mainly in the second and third decade of life. Typically, there is a subacute loss of vision accompanied by optic disc hyperaemia, pseudoedema, peripapillary blood vessel abnormalities such as telangiectasia and tortuosity, and swelling of the retinal nerve fibre layer (RNFL) (Meyerson et al. 2015; Hwang et al. 2017). As the disease progress to chronic atrophic phase, optic disc pallor develops reflecting the death of retinal ganglion cells (RGCs) (Mashima et al. 2003; Corajevic et al. 2018; Himori et al. 2017).
Optical coherence tomography angiography (OCT-A) provides high-resolution images of retinal and peripapillary capillaries by non-invasive visualization of vascular flow via motion contrast (de Carlo et al. 2015). This novel technique has several advantages over fluorescein angiography which has been the gold standard to evaluate retinal circulation in LHON patients (Smith et al. 1973; Nikoskelainen et al. 1977; Chalmers & Schapira 1999). Apart from greater resolution, OCT-A visualizes vascular structures in an arbitrarily selected depth of retinal layers without the need to administer a contrast agent (Spaide et al. 2015; Kiyota et al. 2018). To date, OCT-A imaging has been shown only in six genetically confirmed cases with visual loss due to LHON (De Rojas et al. 2016; Gaier et al. 2016; Ghasemi Falavarjani et al. 2016; Takayama et al. 2017).
The purpose of this study was to evaluate the radial peripapillary capillary network in a series of individuals affected by vision loss due to LHON and asymptomatic mitochondrial DNA (mtDNA) mutation carriers in comparison to control eyes, and thus to improve our understanding of the pathogenetic mechanisms involved in the disease development and to assess the utility of OCT-A in clinical management of individuals with pathogenic mtDNA mutations.
Materials and Methods
Six individuals (12 eyes) from four families, all confirmed carriers of one the three prevalent pathogenic LHON mtDNA mutations (Table 1) and six healthy control eyes of three unrelated individuals were enrolled into the study.
| ID/gender/age | Mutation | Idebenone treatment/ start after the onset | Onset | Time span after onset | RNFL global/temporal quadrant (μm) | Axial length (mm) | Subjective refractive error (DS) | BCVA | Colour vision | Contrast sensitivity | Optic head nerve description | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | ||||
| 1/M/26 years | m.11778G>A | N | 19 years + 8 months | 20 years + 2 months | 6 years | 47/18 | 53/34 | 23.62 | 23.61 | 0 | 0 | CF | 0.016 | D | D | D | 0.00 | Pallor more pronounced temporally | Pallor more pronounced temporally |
| 2/M/22 years | m.11778G>A | Y/22 months | 20 years + 1 month | 20 years + 2 months | 2 years + 3 months | 58/23 | 62/23 | 22.99 | 22.96 | 0 | 0 | 0.05 | 0.05 | D | D | 0.75 | 0.75 | Temporal pallor | Temporal pallor |
| 3/M/30 years | m.14484T>C | Y/1 month | N | 29 years + 3 months | 1 years + 2 months | 93/82 | 63/39 | 26.78 | 26.62 | −6.0 | −6.0 | 1.0 | 0.87 | P | D | 1.65 | 1.20 | Normal | Temporal pallor |
| 4/M/11 years | m.11778G>A | N | N | N | N/A | 100/70 | 101/72 | 22.47 | 22.56 | 0 | 0 | 1.0 | 1.0 | P | P | 1.65 | 1.65 | Normal | Normal |
| 5/M/18 years | m.11778G>A | N | N | N | N/A | 97/72 | 102/72 | 23.67 | 23.70 | 0 | 0 | 1.0 | 1.0 | P | P | 1.50 | 1.50 | Normal | Normal |
| 6/F/41 years | m.11778G>A | N | N | N | N/A | 96/71 | 90/73 | 25.01 | 25.89 | −2.5 | −4.0 | 1.0 | 1.0 | P | P | 1.65 | 1.65 | Normal | Normal |
- BCVA = best corrected visual acuity; CF = counting fingers; D = defect; DS = dioptre sphere; F = female; LE = left eye; M = male; N = none; N/A = not applicable; P = pass; RE = right eye; RNFL = retinal nerve fibre layer; UA = unavailable data; Y = yes.
All subjects underwent ocular examination including measurement of best corrected visual acuity (BCVA) using Early Treatment Diabetic Retinopathy Study charts and extrapolated into decimal values. Visual field was assessed using standard automated perimeter (M-700, Medmont International, Nunawading, Australia). Axial length was measured by IOLMaster 500 (Carl Zeiss Meditec AG, Jena, Germany). The normal range, as established in younger adults, was taken as 23.60 ± 1.15 mm (Tsai et al. 1992). Next, we performed spectral-domain OCT (SD-OCT; Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany) of the macula and peripapillary RNFL thickness measurements by circular scans 3.5–3.6 mm in diameter around the optic disc. The normal range of global RNFL thickness was regarded as 97.41 ± 11.73 μm in children and 97.52 ± 9.83 μm in adults. Temporal quadrant normal values were considered as 90.44 ± 20.49 μm in children and 83.65 ± 14.98 μm in adults (Chung et al. 2016). Colour vision was tested using Lanthony desaturated 15 hue test (more than one major crossing was considered as abnormal) (Shoji et al. 2009) and contrast sensitivity with Pelli–Robson charts (values <1.50 were regarded as abnormal) (Leat et al. 1999).
Optical coherence tomography angiography (Spectralis OCT2, Heidelberg Engineering GmbH, Heidelberg, Germany) was performed between February and April 2017 by one skilled technician (MM). The scans with a 3 × 3 mm field of view were centred on the optic disc. Images from several depths were compared between eyes.
The study followed the Helsinki Declaration and all individuals or their legal representatives signed informed consent.
Results
Basic demographic characteristics and clinical details of the study participants are provided in Table 1. Five individuals (1, 2, 4–6) harboured m.11778G>A in a homoplasmic state, individual 3 was homoplasmic for m.14484T>C (Table 1). Patient 2 is a brother of an unaffected carrier 5 and subject 4 is a son of individual 6.
The clinical course of LHON in individuals 1 and 2 was typical, with bilateral sequential visual loss resulting into RNFL thinning more pronounced in the temporal quadrant, visible optic disc pallor, visual field defects (Fig. 1A–D), decreased contrast sensitivity and colour vision deficiency (Table 1).
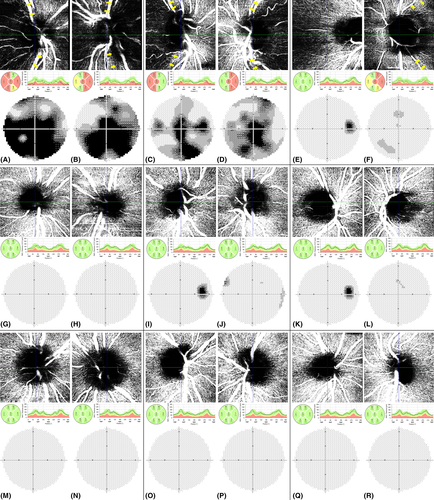
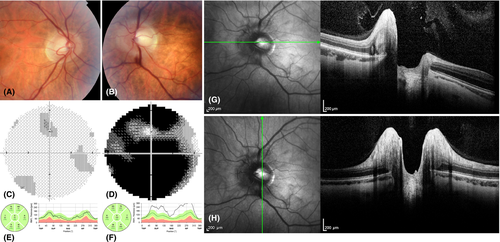
Patient 3 experienced visual loss in the left eye at the age of 29 years and 3 months; 27 days after the onset BCVA was 0.032 with profound visual field loss (Fig. 2D), colour vision defects and contrast sensitivity decrease. Pseudoedema of the left optic disc was documented by SD-OCT (Fig. 2F–H). In the right eye clinical findings were normal with BCVA 1.0 and no decrease in contrast sensitivity or colour vision deficiency. A mild central visual field defect was detected, which was attributed to a learning curve (Fig. 2C). Leber hereditary optic neuropathy (LHON) diagnosis was confirmed by molecular genetic testing and idebenone treatment (900 mg/day) started 27 days after the onset. The left eye recovered significantly and at the point of OCT-A examination, 14 months after disease manifestation, BCVA improved to 0.87 and only few mild central visual field defects could be documented (Fig. 1F). Significant RNFL thinning confirmed atrophy of the left optic disc (Table 1, Fig. 1F). Examination of the right eye showed no pathology (Fig. 1E). Consistent with high myopia the axial length in this patient was bilaterally above the normal limits.
Subjects 4 was 11 years old at the time of OCT-A examination. He tested positive for the presence of m.11778G>A after LHON manifestation in his younger brother in whom OCT-A imaging could not be reliably performed due to age. Neither he nor his parents noticed any decrease of BCVA at any point. Consistent with this his ocular examination was normal (Table 1, Fig. 1G,H). Individuals 5 and 6, also carriers of m.11778G>A, had completely normal ocular findings including RNFL measurements (Table 1, Fig. 1I–L) when compared to control eyes (Fig. 1M-R).
Optical coherence tomography angiography demonstrated peripapillary capillary dropout at the level of RNFL, that is, from the internal limiting membrane (ILM) to RNFL posterior boundary in all eyes with LHON manifestation (i.e. both eyes of individuals 1, 2 and the left eye of individual 3) (Fig. 1A–D,F). Because of problems with automatic peripapillary delineation of RNFL (without the possibility of manual correction) in the right eye of individual 3, we had to decentre the scan slightly temporally. Therefore, comparison with other scans was not reliable (Fig. 1E). No differences were found between the unaffected eyes of carriers and controls (Fig. 1G-R).
At the level of ganglion cell layer differences could be also noted but only in the two severely affected individuals 1 and 2 (Fig. S1). No major abnormalities were observed in microvascularization between the inner and outer plexiform layer (Fig. S1). In the left eye of individual 2 OCT-A imaging suggested possible capillary drop out in the deep vascular plexus, in contrast to the clinically more severely affected fellow eye. We have concluded that this is most likely to be an artefact as the vessel density in all quadrants appeared similar. Overall the vascular network, plotted from total retina (ILM to Bruch membrane) demonstrated differences between the affected eyes and normal controls with a reduction of capillary density corresponding to the disease severity (Fig. S1).
Discussion
Optical coherence tomography angiography is a novel, safe and rapid method that offers dissection of optic disc vascular perfusion and thus may help to differentiate various types of optic nerve disorders. To the best of our knowledge, this is the first study evaluating capillary network by OCT-A in a series of individuals carrying mtDNA mutations causing LHON, including three affected subjects and three unaffected carriers. Previously the microvascular network visualized by OCT-A has been reported in six cases with manifest visual impairment due to LHON (De Rojas et al. 2016; Gaier et al. 2016; Ghasemi Falavarjani et al. 2016; Takayama et al. 2017).
We have documented reduction in radial peripapillary microvascular network in all 5 affected eyes of three individuals. A reduction of the peripapillary microvascular network can also be observed in other chronic optic neuropathies. In the LHON patients reported herein in the chronic phase of the disease capillary dropout was restricted to the RNFL and RGC layer, which maybe an important factor in differential diagnosis in unsolved cases with optic atrophy, as for example in patients with multiple sclerosis alterations of both the superficial and deep vascular plexus have been reported (Feucht et al. 2018).
The limitations of the current study are that quantitative assessment of capillary densities could not be performed because of lack of available software. Also, due to the impossibility of performing the OCT-A repeatedly we cannot comment on artefacts and variability in between different examinations.
There are several theories as to how mitochondrial dysfunction may influence blood flow, including a direct effect on endothelial cell and vascular smooth muscle viability (Chiong et al. 2014; Goveia et al. 2014). Unfortunately, we were not able to image a patient in the acute phase of LHON due to the rarity of the condition. A previous study using OCT-A in a carrier of m.11778G>A with optic disc pseudoedema has however shown microangiopathy in the fellow, subclinical eye suggesting that OCT-A may serve as a useful ancillary diagnostic tool to support early diagnosis, thus maximizing the chance for successful therapeutic intervention (Gaier et al. 2016). Hence we believe that current evidence supports the hypothesis that microangiopathy contributes to the visual loss in LHON.
Apart from OCT-A imaging we also document the disease course in an individual with unilateral LHON 14 months after the disease onset, which is a rare finding, described in the literature only in a few adult cases (Jacobson et al. 1998; Dandekar et al. 2002; Nagai et al. 2005; Sugisaka et al. 2007). Twenty-seven days after the disease manifestation, there was a significant increase of RNFL thickness in superior, inferior and nasal quadrants while in the temporal quadrant it was within the normal range. This is in line with most recent prospective observations (Hwang et al. 2017).
In conclusion, OCT-A results confirm that optic neuropathy in LHON patients is associated with decreased microcirculation primarily in the RNFL and RGC layer. Studies with larger sample sizes are necessary to assess the clinical usefulness of OCT-A in individuals with pathogenic mutations in mtDNA in terms of disease onset prediction, differential diagnosis and recovery. Results of OCT-A imaging in LHON patients in this and previous studies raise the expectation that the method could be used in diagnosing and monitoring function and RGC apoptosis, and thus further aid the unravelling of the pathogenesis of mitochondrial disorders.




