Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of on-demand therapy every 4 or 8 weeks
Abstract
Purpose
Intravitreal anti-vascular endothelial growth factor (VEGF) injections are an effective treatment for neovascular age-related macular degeneration (nARMD). Bevacizumab appears to be a cost-effective off-label anti-VEGF alternative to ranibizumab, but an optimal injection schedule has not yet been determined. In this study, we investigate whether on-demand bevacizumab treatment every 8 weeks is non-inferior to on-demand bevacizumab every 4 weeks in treating nARMD.
Methods
A total of 120 nARMD patients were randomly assigned to an on-demand regimen of intravitreal bevacizumab (IVB) every 4 (n = 60) or 8 weeks (n = 60). The primary outcome was visual acuity (VA) change after 1 year of treatment.
Results
Visual acuity (VA) improved between baseline and 1 year in both treatment groups. The mean change in the VA score at 1 year was not significantly different between bevacizumab administration on-demand every 4 weeks [5.6 ± 10.2 Early Treatment Diabetic Retinopathy Study (ETDRS) letter] or 8 weeks (4.6 ± 12.0 ETDRS letters). A reduction in the central retinal thickness was observed in both groups. At 1 year, the mean decrease in central foveal thickness ranged from 61 ± 90 μm in the 4-week group to 91 ± 83 μm in the 8-week group (p = 0.07). The mean number of IVB treatments during the study period was 8.7 ± 2.3 in the 4-week group and 5.9 ± 1.0 in the 8-week group.
Conclusion
At 1 year, bevacizumab administration on-demand every 8 weeks was non-inferior to administration every 4 weeks. The results strongly suggest that bevacizumab acts longer than 4 weeks in ARMD, reducing the burden of injections for patients.
Introduction
For years, neovascular age-related macular degeneration (nARMD) was regarded as a poorly manageable or untreatable disease, but the treatment of nARMD has evolved over the last decade (Bressler 2004; Friedman et al. 2004). Since 2005, therapy options have become available that slow or stop progression, and even improve visual acuity (VA), in nARMD patients. These drugs target vascular endothelial growth factor (VEGF) and are typically known as anti-VEGF drugs (Brown et al. 2006, 2009; Rosenfeld et al. 2006; Regillo et al. 2008; Schmidt-Erfurth et al. 2011).
Bevacizumab is currently one of the most frequently used anti-VEGF drugs. The popularity of intravitreal bevacizumab (IVB) is primarily due to its cost-effectiveness compared to the alternatives (Brown & Regillo 2007; Brown et al. 2007; Frennesson 2007; Raftery et al. 2007), and the efficacy of off-label IVB compared to its on-label counterpart (CATT Research Group et al. 2011; IVAN Study Investigators et al. 2012) has been well-studied in recent years.
Most studies have focused on a frequency of 4 weeks for therapy, either continuous or on-demand. This regimen is the result of extensive registration research performed for intravitreal ranibizumab (IVR), known by the brand name Lucentis® (Genentech Inc., South San Francisco, CA, USA), an anti-VEGF drug approved by the Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products for all forms of nARMD. Considering the off-label ocular use of IVB and apparent similarities with IVR, a 4-week therapy regimen was also adopted for IVB.
However, several papers have shown differences in the pharmacokinetics of IVR and IVB. The small differences in half-life observed in both animal and human experiments may allow for a longer therapeutic window for IVB than the standard 4 weeks (Bakri et al. 2007a,2007b; Krohne et al. 2008, 2012). Several smaller prospective studies have explored options other than 4 weeks with mixed results (Arias et al. 2008; Sonmez et al. 2011). More recently, attention has shifted to treat and extend (T&E) studies (Jørstad et al. 2017). For example, the LUCAS trial (Berg et al. 2015, 2016) showed equivalent effects of IVB and IVR on VA at 1 year on a T&E schedule, with treatments gradually extended in intervals of 2 weeks to up to 12-week intervals. These promising results in a group of 441 patients showed the viability of a T&E strategy and, thus, a longer therapeutic window.
Ophthalmologists face the daunting task of continuing anti-VEGF treatment in a growing nARMD population. The 4-week therapeutic regimen is often abandoned in daily practice, leading to T&E or longer therapy intervals. The reasons for this change vary, including the clinician's personal experience, continually growing treatment populations, patient preferences and national health system restrictions. However, evidence in favour of a decrease in therapy frequency is still scarce. The efficacy of on-demand IVB was evaluated in the CATT and IVAN trials (CATT Research Group et al. 2011; IVAN Study Investigators et al. 2012). The 4-week on-demand comparison in the IVAN trial showed equivalent results, but in the CATT trial, the results were inconclusive, with a 2.1 letter difference at 52 weeks. Although on-demand treatment or T&E strategies may often be used in clinical practice, guidelines and the results of larger studies do not provide all the answers clinicians hope. Additional information is needed to make sound clinical decisions, especially in IVB use (Real & Luna 2016).
In 2013, we found no significant differences in the change in VA or central foveal thickness (CFT) after 1 year of continuous treatment of patients with nARMD using IVB a continuous 4-, 6- or 8-week therapy strategy (Lushchyk et al. 2013). Here, we report the second phase of that study comparing on-demand IVB every 4 weeks to on-demand IVB every 8 weeks.
Materials and Methods
Study design
A total of 120 patients were enroled from March 2010 to December 2011 in the prospective 1-year, open-label, randomized, controlled non-inferiority trial of two on-demand bevacizumab injection schedules for the treatment of nARMD at Rotterdam Eye Hospital. The eligibility criteria were the same as in the previous study of continuous IVB therapy (Lushchyk et al. 2013): age ≥65 years, VA 20/200 to 20/20 (Snellen equivalent) assessed using the Early Treatment Diabetic Retinopathy Study (ETDRS) VA charts, and previously untreated active choroidal neovascularization due to ARMD. The presence of active leakage was required to establish active choroidal neovascularization. Active leakage was defined as leakage observed using fluorescein angiography (FA) and indocyanine green (ICG) angiography, and the observation of fluid below the retina or the retinal pigment epithelium using spectral-domain optical coherence tomography (OCT).
Patients with other significant ocular disorders affecting VA, a known allergy to FA or ICG dye, compromised immunity or ocular surgery planned during the 1-year follow-up period were excluded from the study. Patients who used coumarin derivatives at the time of inclusion or experienced a clinically significant cerebrovascular accident or myocardial infarction in the 6 months prior to planned inclusion were also ineligible for the study. Although no conclusive information was available at the start of the study, these patients were considered to be at risk for vascular adverse events during 1 year of treatment with an anti-VEGF agent.
The Institutional Review Board and Medical Ethics Committee of Erasmus University of Rotterdam approved this study. All patients provided written informed consent for study participation. Adherence to the study protocol and the tenants of the Declaration of Helsinki were confirmed by a solicited external audit. The study was registered as NTR1174 at http://www.trialregister.nl.
Treatment
Patients were randomly assigned to one of two study groups. Treatment consisted of 1.25 mg IVB in a 0.05 ml solution, but one group was administered IVB on-demand every 4 weeks and the other every 8 weeks. Patients were not given a loading dose of three consecutive injections of IVB; all patients received one IVB treatment at inclusion and retreatment was assessed at subsequent study visits. Patients in the 4-week group were seen for assessment every 4 weeks, and patients in the 8-week group were seen for assessment every 8 weeks. An extra assessment-only visit occurred at 3 and 9 months. The criteria for retreatment were VA loss (≥5 ETDRS letters), increased CFT (≥50 μm), new fluid observed on OCT and/or new haemorrhages visible on fundoscopy. New fluid on OCT was defined as fluid that had been reported for less than three consecutive IVB injections. When the measured VA was not changed (≤5 ETDRS letters) and new fluid on OCT persisted without a change in the CFT (≤50 μm) after three consecutive IVB treatments, therapy was not administered.
Each study visit consisted of best-corrected VA (BCVA), spectral-domain OCT and fundoscopy. A standardized protocol was used to measure VA with ETDRS logMAR charts one (right eye), two (left eye) and R (refraction). Spectral-domain OCT (RTvue – Optovue inc, Fremont, CA, USA) scans were performed using macular thickness maps. Fluorescein angiography (FA) and ICG angiography were performed at baseline and the final follow-up visit.
Outcome measurements
The change in VA between baseline and 1 year was the primary outcome of this study. Secondary outcomes included change in fluid and foveal thickness on spectral-domain OCT, and the proportion of patients with a change in VA of 15 letters or more. Adverse events were ascertained at study visits by study staff questioning the patients, with special emphasis placed on cardiovascular events.
Statistical analysis
The change in VA after 1 year was the primary outcome and assumed to be dependent on the maximum number of bevacizumab injections administered. In this study, the statistical analysis was based on a comparison of the maximal possible number of injections in this study with the 4-week treatment group in the previous study on continuous IVB therapy. Multiple group evaluations were made using this approach and the standard deviation estimated to be 15 letters with a power of 0.80. This resulted in a non-inferiority limit of seven ETDRS letters for a group size of 45 or greater. A minimum of 45 patients per treatment group were needed to find significant differences of seven ETDRS letters or more. Group sizes were set at 60, and inclusion was halted after 120 patients. Dropout was estimated at 25%, which corresponds to the published 23% dropout rate in the anchor trial (Brown et al. 2006).
Efficacy analyses were performed based on an intention-to-treat principle. Study visits missed by patients due to dropout were considered missing data for the purpose of analysis. In this study, we calculated the results using only available data without carrying forward selected data, and available sample sizes are indicated at each study point.
Study groups were compared using chi-squared tests for categorical variables and analysis of variance for continuous variables. All statistical analyses were performed using spss software (IBM Corp., version 24.0., Armonk, NY, USA).
Results
Patients and treatment
Patient characteristics were not significantly different between the two groups at baseline (Table 1). Of the 120 patients who entered the study, VA scores and OCT measurements were available for all follow-up visits for 108 patients. The mean number of IVB treatments during the 1-year study period was 8.7 ± 2.3 in the 4-week group and 5.9 ± 1.0 in the 8-week group.
| Bevacizumab | |||
|---|---|---|---|
| On-demand 4 weeks | On-demand 8 weeks | ||
| Characteristic | (n = 60) | (n = 60) | p values |
| Age, number (%) | |||
| 65–74 years | 22 (36.7) | 18 (30.0) | |
| 75–84 years | 27 (45.0) | 28 (46.7) | |
| ≥85 years | 11 (18.3) | 14 (23.3) | |
| Mean age, years | 77.6 ± 6.8a | 79.1 ± 7.2a | |
| Sex, number (%) | |||
| Male | 24 (40.0) | 22 (36.7) | |
| Female | 36 (60.0) | 38 (63.3) | |
| Race, number (%) | |||
| White | 60 (100) | 60 (100) | |
| Other | 0 (0.00) | 0 (0) | |
| Visual acuity score, number of letters | 60 ± 11a | 63 ± 12a | 0.31 |
| Mean visual acuity in logMAR | 0.47 ± 0.26a | 0.49 ± 0.46a | |
| Total thickness at fovea, μmb | 358 ± 93a | 387 ± 110a | 0.13 |
| Lesion type, number (%) | |||
| Occult | 35 (58.3) | 29 (48.3) | |
| Classic | 1 (1.6) | 0 (0) | |
| Predominantly xlassic | 3 (5) | 2 (3.33) | |
| Minimally classic | 15 (25) | 13 (20.3) | |
| Retinal angiomatous proliferation | 6 (10) | 16 (26.7) | |
| Mean lesion size, fluorescein angiography | 10.24 ± 6.69 | 10.49 ± 7.69 | |
| Mean lesion size, indocyanine green | 9.49 ± 7.27 | 9.52 ± 8.47 | |
- a Plus-minus values are presented as means ± standard deviation (SD).
- b Total thickness at the fovea includes the retina, subretinal fluid, choroidal neovascularisation and pigment epithelial elevation.
Mean change in VA
Visual acuity (VA) improved between baseline and 1 year in both groups. The mean change in VA score at 1 year did not differ significantly for IVB administered on-demand every 4 weeks (5.6 ± 10.2) versus every 8 weeks (4.58 ± 11.97). The highest mean BCVA gain was observed during the first 3 months in both therapy groups (Fig. 1). Adjusting for potential confounders, including baseline VA, baseline lesion type, baseline lesion size and age, did not result in significant differences between the two groups.
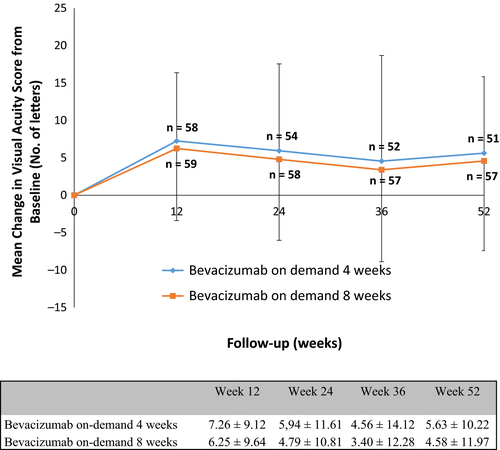
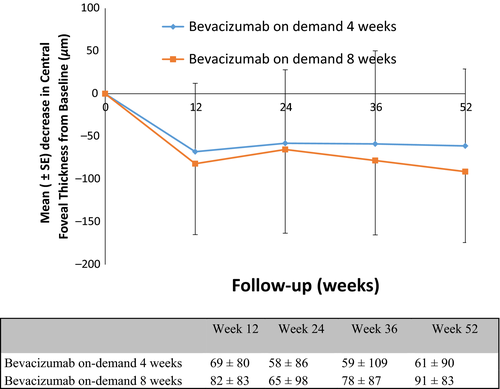
Secondary outcomes
A reduction in CFT on OCT was observed in both groups. At 1 year, the mean decrease in CFT was 61 ± 90 μm in the 4-week group and 91 ± 83 μm in the 8-week group. At 1 year, 3.9% (two of 51) of patients treated every 4 weeks, and 5.3% (three of 57) of patients treated every 8 weeks reported a decrease in VA of 15 letters or more (Fig. 2). An increase in VA of 15 letters or more was measured in 17.6% (nine of 51) of patients in the 4-week group and 21.0% (12 of 57) in the 8-week group (Table 2).
| Outcome | Bevacizumab | p values | |
|---|---|---|---|
| On-demand 4 weeks | On-demand 8 weeks | ||
| (n = 51) | (n = 57) | ||
| Visual acuity score, mean no. letters ± SD | 67.4 ± 13.2 | 67.3 ± 15.4 | 0.98 |
| Change from baseline visual acuity score | |||
| Increase of ≥5 letters, no. (%) | 9 (17.6) | 12 (21.0) | |
| Increase of 5–14 letters, no. (%) | 16 (31.4) | 16 (28.0) | |
| Change of ≤4 letters, no. (%) | 19 (37.3) | 23 (40.4) | |
| Decrease of 5–14 letters, no. (%) | 5 (9.8) | 3 (5.3) | |
| Decrease of ≥15 letters, no. (%) | 2 (3.9) | 3 (5.3) | |
| Mean number of letters ± SD | 5.6 ± 10.2 | 4.6 ± 12.0 | 0.63 |
| Total thickness at fovea, μm | |||
| Mean ± SD | 302 ± 81 | 288 ± 66 | 0.32 |
| Mean change from baseline ± SD | −61 ± 90 | −91 ± 83 | 0.07 |
- SD, standard deviation.
Adverse events and dropouts
The dropout rate and reasons are summarized in Table 3. A total of 12 patients were lost to follow-up, nine in the 4-week group and three in the 8-week group. The main reason for dropout was study protocol compliance (n = 7), followed by death (n = 3). The circumstances of these deaths are indicated in Table 4. No cases of endophthalmitis, pseudo-endophthalmitis or allergic reactions to IVB were reported.
| Bevacizumab | ||
|---|---|---|
| On-demand 4 weeks | On-demand 8 weeks | |
| (n = 60) | (n = 60) | |
| Total SAEs, no. | 13 | 5 |
| Arteriotrombotic event, no. (%) | 1 (1.7) | 0 (0) |
| Cerebrovascular accident, no. (%) | 0 (0) | 0 (0) |
| Transient ischaemic attack, no. (%) | 0 (0) | 0 (0) |
| Retinal pigment epithelium tear, no. (%) | 1 (1.7) | 1 (1.7) |
| Endophthalmitis, no. (%) | 0 (0) | 0 (0) |
| Pseudo-endophthalmitis, no. (%) | 0 (0) | 0 (0) |
| Other ocular event, no. (%) | 1 (1.7) | 1 (1.7) |
| Death, no. (%) | 4 (6.7) | 0 (0) |
| Death from vascular causes, no. (%) | 1 (1.7) | 0 (0) |
| Non oculair event, no (%) | 6 (10.0) | 3 (5.0) |
| Bevacizumab | ||
|---|---|---|
| On-demand 4 weeks | On-demand 8 weeks | |
| (n = 60) | (n = 60) | |
| Total lost to follow-up, no. (%) | 9 (15.0) | 3 (5.0) |
| Exit reason | ||
| Serious adverse event, no. (%) | 1 (1.7) | 0 (0.0) |
| Death, no. (%) | 4 (6.7) | 0 (0.0) |
| Non-compliance, no. (%) | 4 (6.7) | 3 (5.0) |
The mean change in VA between baseline and dropout in the 4-week group (n = 9) was a gain of 5.7 ± 21.6 letters. One patient in this group suffered a loss of 32 ETDRS letters (reason for dropout was a retinal pigment epithelium tear) and another patient lost six letters (reason for dropout was non-compliance). The mean change in VA between baseline and dropout in the 8-week group (n = 3) was a gain of 0.7 ± 14.0 letters. One patient suffered VA loss of 13 ETDRS letters (reason for dropout was non-compliance). No significant difference (p = 0.17) was found between the change in VA at dropout and the change in VA between baseline and completion for patients who completed the 1-year study protocol.
In the 4-week group, a decrease in the CFT of 70 ± 61 μm was measured at dropout compared to the baseline measurement. None of the patients had an increased CFT at dropout compared to baseline. In the 8-week group, an increase in the CFT of 34 ± 48 μm was observed at dropout compared to baseline. No significant difference (p = 0.08) was found between the change in CFT at dropout and the change in CFT between baseline and completion for patients who completed the 1-year study protocol.
The time between the final examination and last injection was 1.6 ± 1.3 months in the 4-week group and 1.6 ± 1.1 months in the 8-week group (p = 0.47). No significant differences were found between outcome (VA, p = 0.27; OCT changes, p = 0.07) and our previously published fixed regimen study (Lushchyk et al. 2013).
Discussion
The large clinical trials performed for the registration of IVR were critical for the acceptance of anti-VEGF therapy as a means of treating nARMD. Treatment with bevacizumab as a cheaper, off-label anti-VEGF substitute for IVR was initially a bold choice for which large comparative trials were performed only recently (CATT Research Group et al. 2011; IVAN Study Investigators et al. 2012; Solomon et al. 2016). The standard dosing frequency for IVB in these trials and clinical practice is 4 weeks, a logical extension of the results seen with ranibizumab. The CATT and IVAN trials did compare 4-week on-demand and continuous IVB therapy. The results of these comparisons were inconclusive, and it stands to reason that a continuous therapy schedule could possibly obtain better results than on-demand therapy.
The optimal administration frequency of IVB for human eyes probably differs from IVR due to differences in molecular architecture and size, and the observed pharmacokinetic differences in the vitreous humour and serum. Currently, no large prospective studies have been published that compare on-demand therapy for bevacizumab over longer therapeutic windows than the standard 4-week trials.
In our previous paper, we set out to compare continuous IVB therapy every 6 and 8 weeks to the standard 4-week therapy. We concluded that an 8-week therapeutic window was a distinct possibility for IVB treatment in nARMD patients. Here, we expand on our previous work to compare on-demand treatment every 8 weeks to more routine on-demand treatment every 4 weeks. Both the previous and current study suggest that 8-week IVB administration is adequate for most patients. In agreement with this finding, T&E trials (Berg et al. 2015, 2016; Jørstad et al. 2017) have also shown that longer treatment cycles for IVB and IVR can yield equivalent results with 1- or 2-year T&E schedules.
At the start of this study in 2010, before much of the literature cited in this manuscript was reported, there was some hesitance to treat nARMD patients on-demand every 8 weeks. As part of the investigation into an optimal bevacizumab treatment algorithm for nARMD, the on-demand study began after finishing a non-inferiority comparison of continuous IVB treatment every 4, 6 or 8 weeks (Lushchyk et al. 2013). Although those results showed no significant differences between 4- and 8-week continuous treatment, the treatment of patients on an 8-week on-demand schedule, effectively resulting in injections less than every 8 weeks, could lead to a suboptimal gain in VA or a preventable loss in VA. Therefore, the treatment algorithm was tailored to be strict on VA and OCT changes that would demand retreatment, and the retreatment of patients in the study may differ from normal clinical on-demand practice. This explains why the number of injections in the 4-week group over the 1-year follow-up was 8.7 of a potential 13 injections, and 5.9 of a potential 7 injections in the 8-week group. No solid study arguments are yet available to extend treatment or follow-up visits beyond 8 weeks in the majority of patients, although clinical practice and T&E studies have shown that it is possible in selected patients (Berg et al. 2015).
The primary outcome of this study was change in VA. There was no significant difference in the change in VA at 1 year between on-demand treatment every 4 weeks and on-demand treatment every 8 weeks. Furthermore, there were no significant differences in the change in CFT and the 15 letter change rate between the two groups. Notably, the final follow-up visit in this study was performed at 52 weeks, and for both groups, the last possible therapy visit was at 48 weeks. However, the time between the final injection in the 8- and 4-week regimens and the final examination at 1 year was 1.6 ± 1.3 and 1.6 ± 1.1 weeks, respectively. In retrospect, this could have influenced the outcome of the study, and this point should be addressed in future studies by choosing different census times for different treatment regimens. There was no difference in outcome compared to the previously published VA outcome using fixed 4- and 8-week injections (p > 0.05).
In this study, the mean change in VA was +5.6 letters in the 4-week group and +4.6 letters in the 8-week group. In the CATT trial of 4-week therapy, +5.9 letters were measured in the on-demand bevacizumab group and +8.0 letters in the continuous IVB group. Furthermore, the LUCAS trial reported a +7.9 letter gain at 1 year with a T&E schedule. Differences between our trial and other published trials may be attributed to the composition of lesion size and lesion type, but mainly the real-life VA inclusion criteria (20/200 to 20/20 Snellen), whereby a significant gain in VA is not always possible.
This study demonstrated that 1 year after presentation, on-demand therapy every 8 weeks is non-inferior to on-demand therapy every 4 weeks within a non-inferiority limit of seven ETDRS letters. Depending on the reaction to IVB, treatment could be limited to a single injection or involve continuous treatment every 8 weeks for as long as needed. From a cost and labour standpoint, this would effectively reduce the current ophthalmic workload by as much as 50% (Vottonen & Kankaanpää 2016).
Clinicians need to address some questions about the on-demand treatment of patients. First, the absence of fluid on OCT and VA gain after IVB is an excellent way to monitor the response to IVB but does not automatically mean that these parameters are the best predictors of future disease activity or indicators of the optimal treatment interval. Suboptimal or undertreatment is a potential risk of on-demand therapy. However, no well-designed on-demand study is currently available to address this issue. Second, treating patients without clear signs of active nARMD, as is the case in a fixed interval regimen or the widely used T&E regimen, could seem counterintuitive to some clinicians and raises questions regarding the balance between preserving vision versus potentially severe adverse events, such as endophthalmitis, and ultimately atrophic changes (Grunwald et al. 2017).
Longer stable intravitreal dosages of bevacizumab theoretically lead to better results, but more research is still needed, although a recent study (Kvannli & Krohne 2017) showed that switching patients from on-demand to T&E can improve VA.
Clinicians have to find a balance between the undertreatment and overtreatment of nARMD patients based on a limited number of studies that give guidance in the everyday use of IVB.
A final issue is the management of patients and the clinicians' own expectations after the first few injections that often result in an initial gain of visual function and anatomical improvement. Disappointment over reactivation of the disease, late visual loss, and the chronicity of nARMD is not rare, although dropouts due to non-compliance were similar in the 4- and 8-week groups over the course of the study. Thus, the search for an optimal treatment algorithm that would fit all patients and all eyes from a clinician's, patient's, and financial perspective is not over and requires new and longer comparative studies.




