The effect of canaloplasty with suprachoroidal drainage combined with cataract surgery – 1-year results
Abstract
Purpose
The purpose of this study was to investigate the safety and efficacy of phacocanaloplasty with suprachoroidal drainage (PCscD) and to compare its intraocular pressure (IOP)-lowering and drug-sparing effect to canaloplasty with suprachoroidal drainage (CscD).
Methods
The study retrospective interventional study included patients with open-angle glaucoma or secondary forms of glaucoma who underwent either CscD or PCscD between the year 2011 and 2014 in Knappschaft Eye Clinic Sulzbach. Primary end-points were IOP reduction and the number of IOP-lowering medication after 12 months. Secondary end-points were intraoperative and postoperative complications.
Results
A total of 328 eyes were included, 193 were treated with CscD and 135 underwent PCscD. Canaloplasty with scD achieved an IOP reduction of 37.0% (from 20.9 ± 3.6 mmHg to 13.2 ± 2.6 mmHg) after 1 year, whereas PCscD showed a significant higher reduction of 47.4% (from 23.2 ± 5.1 mmHg to 12.2 ± 1.7 mmHg). Reduction in IOP-lowering medication was higher after PCscD (from 3.6 ± 0.6 to 0.2 ± 0.5) than after CscD (from 3.5 ± 0.8 to 0.7 ± 1.0). Twelve months after surgery 55.5% in the CscD group and 80.2% in the PCscD group were free of IOP-lowering medication. In both groups, no severe or sight-threatening complications occurred.
Conclusion
Combining cataract surgery and CscD achieves a higher IOP reduction, and patients postoperatively need less IOP-lowering medication than after CscD alone.
Introduction
Due to present demographic development in Western society, a growing number of patients suffer from concomitant glaucoma and cataract. Concerning the advices in published literature for the surgical treatment of these patients, a shift can be noticed moving away from separate procedures towards combined surgery (Casson & Salmon 2001; Friedman et al. 2002; Verges et al. 2005; Rosdahl & Chen 2012). There are various reasons for considering combined surgery: the risk of cataract development increases after an invasive glaucoma surgery (Hylton et al. 2003; Patel & Danesh-Meyer 2013). Cataract surgery alone achieves only a small IOP reduction, insufficient as a treatment option for most glaucoma patients (Shingleton et al. 2006; Shrivastava & Singh 2010). Furthermore, a lot of patients prefer undergoing a combined operation instead of two separate procedures because of the personal effort and inconveniences associated with hospitalization.
Trabeculectomy with all its complications and patient discomfort used to be the gold standard for many years (Rulli et al. 2013; Thederan et al. 2014; Kim et al. 2015). This has changed after Matlach et al. (2015) have demonstrated that IOP reduction from canaloplasty is comparable to that of trabeculectomy (Januschowski et al. 2016). The IOP reduction was shown to be even higher by combining it with phacoemulsification (Bull et al. 2011; Lewis et al. 2011; Tetz et al. 2015). Moreover, canaloplasty has attractive and minimally invasive exit strategies in cases of complicated surgery and in cases of late failure (Seuthe et al. 2015).
After being secreted into the posterior chamber by the ciliary processes, aqueous humour passes through the pupil and is drained in two different pathways: the conventional (trabecular) outflow pathway and the unconventional (uveoscleral) outflow pathway. In the case of the uveoscleral pathway, aqueous humour flows from the anterior chamber by diffusion through the interstitial spaces of the ciliary muscle into the suprachoroidal space (Nilsson 1997). The uveoscleral outflow is guided by the pressure gradient between the anterior chamber and the suprachoroidal space (Emi et al. 1989). Toris et al. (1999) found out that uveoscleral outflow accounts for 54% of the total elimination pathway in young people (20–30 years) and 46% in people over 60. Tamm (2013) reported it to account for up to 57% depending on the measuring method. We could recently show that this uveoscleral pathway can be accessed with canaloplasty as well further improving IOP-lowering potential of this procedure by creating a suprachoroidal drainage (scD). For this purpose, the second deeper scleral flap is not prepared lamellar like in conventional canaloplasty but consists of the whole remaining sclera, thus creating an access to suprachoroidal space. Hence, the outflow of aqueous humour from the unroofed Schlemm's canal does not drain into an intrascleral filtering space but directly under the choroid (Seuthe et al. 2016; Szurman et al. 2016).
The question whether canaloplasty with suprachoroidal drainage (CscD) as an already optimized variation of canaloplasty can further profit from a combination with phacoemulsification is highly relevant in patients who have a comorbidity with cataract or for cases that require drastic IOP reduction.
The aim of this study was to investigate the safety and efficacy of PCscD and to compare its IOP-lowering and topical drug sparing effect to the CscD.
Patients and Methods
This study included patients who were treated with CscD or with PCscD in the Knappschaft Eye Clinic Sulzbach from 2011 to 2014. Patients suffered from open-angle or secondary forms of glaucoma (pseudoexfoliative, pigmentary dispersion or uveitic glaucoma) and showed progression of visual field loss under maximal topical treatment, allergies against topical medication or repeated IOP measurements above target IOP despite of maximal topical treatment. Patients with visually significant cataract underwent PCscD and the others CscD. Surgery was performed by four different surgeons who were all experienced in conducting the surgery technique over years.
Patients who had cyclodestructive interventions or other forms of previous glaucoma surgery as well as cases of juvenile, traumatic or neovascular glaucoma were excluded from our study. Furthermore, those cases that did not have available data after 3 months were excluded. Control visits were performed in the Knappschaft Eye Clinic Sulzbach 3 and 12 months after surgery. Goldmann tonometry was used to assess the IOP. Patients who needed additional glaucoma surgery after one of the two interventions were labelled as unsuccessful.
The study adhered to the tenets of the Declaration of Helsinki and additionally was approved by the local ethics committee (Ethikkommission der Ärztekammer des Saarlandes).
Surgical technique
The surgery technique of CscD has been described recently (Seuthe et al. 2016; Szurman et al. 2016). Under local anaesthesia by peribulbar block, in special cases also under general anaesthesia, a limbal conjunctival incision is performed, and after liberating the sclera from conjunctiva and tenon, a quadrangular superficial scleral flap of 4 × 4.5 mm is created.
In case of PCscD at this point, a clear cornea incision is performed, followed by standard phacoemulsification and implantation of posterior chamber intraocular lens. Then, in both cases, a second deeper scleral flap uncovering the choroid measuring 3.5 × 4 mm is dissected underneath the first flap. Schlemm's canal is easily located and can be unroofed and then probed by a flexible microcatheter (iTRACK™ 250 microcatheter; Ellex Medical Lasers Ltd., Adelaide, SA, Australia). Figure 1 gives an impression of the intraoperative setting at this point. After the 360-degree catheterization, a 10-0 prolene suture (Prolene; Johnson and Johnson Medical GmbH, Ethicon, Norderstedt, Germany) is firmly tightened in the area where Schlemm's canal has been unroofed after 360° viscodilation with hyaluronic acid (Healon® OVD; Abbott Medical Optics Inc., Santa Ana, CA, USA) is performed. The superficial sclera flap is sewed watertight with absorbable suture 8-0 polyglactin (Vicryl; Johnson and Johnson Medical GmbH, Ethicon) to prevent the development of a filtering bleb.
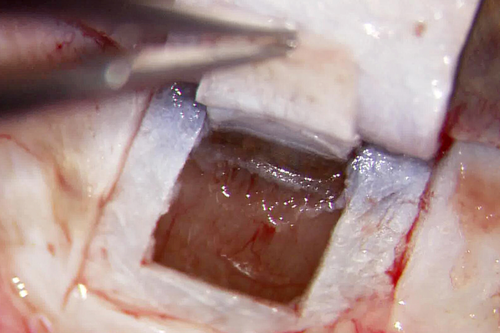
Postoperatively treatment regime consists of topical antibiotics (moxifloxacin) and non-steroidal anti-inflammatory eyedrops. All IOP-lowering medication is stopped on the first day after surgery.
Statistical analysis
The primary outcome measures of this study were the decrease in IOP after 12 months as well as the reduction in IOP-lowering medication. Secondary end-points were the occurrence of intra- and postoperative complications and the necessity of secondary surgical interventions.
Continuous variables concerning IOP were described as box plots showing 5% and 95% quantiles (whiskers), 25% and 75% quartiles (box) and the median. An asterisk marked statistical significance. For paired measurements, Student's t-test was applied, and for repeated measurements analysis of variance, test was taken to calculate statistical significance. The chi-square test was used to test proportions. Fisher's exact test was used to compare complications and secondary surgical interventions between both groups. Values for p < 0.05 were esteemed to reflect significant differences.
Results
Demographics
A total of 328 eyes of 328 patients could be included into this retrospective interventional study. A total of 193 eyes were treated with CscD, and 135 eyes were treated with PCscD.
Of the 193 patients who underwent CscD 85.8% had open-angle glaucoma (OAG), 11.9% pseudoexfoliative glaucoma, 1.0% pigmentary glaucoma, 0.5% uveitic glaucoma and 1.0% other forms of secondary glaucoma.
In the group of combined PCscD, the majority (68.1%) of the patients suffered from primary OAG, 25.2% had pseudoexfoliative glaucoma, 3.0% uveitic glaucoma, 2.2% pigmentary glaucoma and 1.5% other forms of secondary glaucoma.
Table 1 gives an overview on the patient characteristics.
| Canaloplasty + scD | Phacocanaloplasty + ScD | |
|---|---|---|
| Eyes | 193 | 135 |
| Mean age | 67.5 ± 11.9 years | 69.5 ± 7.3 years |
| Primary open-angle glaucoma | 165 (85.5%) | 92 (68.1%) |
| Pseudoexfoliative glaucoma | 23 (11.9%) | 34 (25.2%) |
| Pigmentary dispersion glaucoma | 2 (1.0%) | 3 (2.2%) |
| Uveitic glaucoma | 1 (0.5%) | 4 (3.0%) |
| Other secondary glaucoma | 2 (1.0%) | 2 (1.5%) |
| Pseudophakic | 100 (51.8%) | 0 |
- scD = suprachoroidal drainage.
Intraocular pressure
In the group of CscD, the mean baseline IOP was 20.9 ± 3.6 mmHg. After 3 months, IOP had significantly (p < 0.0001) decreased by 35.7% to 13.5 ± 2.9 mmHg. After 12 months, the mean IOP had significantly (p < 0.0001) decreased by .37.0% to 13.2 ± 2.6 mmHg.
The mean IOP of patients who received PCscD was 23.2 ± 5.1 mmHg before surgery. After 3 months, the IOP was significantly (p < 0.0001) reduced by 46.4% to a mean of 12.4 ± 2.4 mmHg. This decrease in IOP was even more prominent after 12 months with 47.4% to 12.2 ± 1.7 (p < 0.0001).
Hence, we can observe a higher reduction in the group of PCscD than in the group of CscD. This difference is statistically significant (p < 0.0001). An overview on these results is given in Table 2 as well as in Figs 2 and 3.
| Canaloplasty + scD | Phacocanaloplasty + scD | p-Value | |||||
|---|---|---|---|---|---|---|---|
| n | Mean IOP (mmHg) | Reduction | n | Mean IOP (mmHg) | Reduction | ||
| Baseline | 193 | 20.9 ± 3.6 | 135 | 23.2 ± 5.1 | <0.0001 | ||
| 3 months | 193 | 13.5 ± 2.9 | 35.7% | 135 | 12.4 ± 2.4 | 46.4% | <0.0001 |
| 12 months | 174 | 13.2 ± 2.6 | 37.0% | 111 | 12.2 ± 1.7 | 47.4% | <0.0001 |
- scD = suprachoroidal drainage; IOP = intraocular pressure.
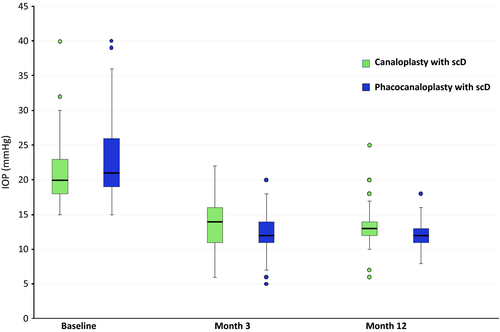
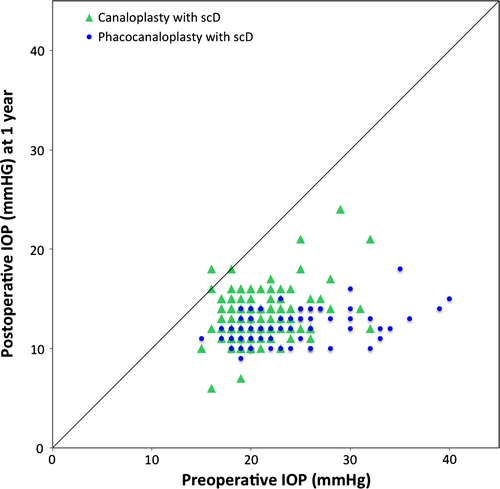
Reduction in pharmacological treatment
In the group of CscD, mean baseline medication consisted of 3.5 ± 0.8 different IOP-lowering agents. Twelve months after surgery patients used 0.7 ± 1.0 eyedrops in the mean and 55.5% of the patients were free of any IOP-lowering medication.
In contrast to that, patients who underwent PCscD used 3.6 ± 0.6 different IOP-lowering agents at baseline visit and showed a reduction to 0.2 ± 0.6 after 12 months. 80.2% of them did not use any IOP-lowering eyedrops after 1 year.
Hence, the percentage of those patients who were free of IOP-lowering medication after 1 year was significantly higher (80.2%) in the PCscD group than in the group of CscD (55.5%) (p = 0.0001).
An overview on these results is given in Table 3.
| Canaloplasty + scD | Phacocanaloplasty + scD | p-Value | |||||
|---|---|---|---|---|---|---|---|
| n | Mean number of medication | Reduction | n | Mean number of medication | Reduction | ||
| Baseline | 193 | 3.5 ± 0.8 | 135 | 3.6 ± 0.6 | 0.0274 | ||
| 3 months | 193 | 0.5 ± 1.0 | 85.6% | 135 | 0.2 ± 0.6 | 94.2% | <0.0001 |
| 12 months | 174 | 0.7 ± 1.0 | 79.1% | 111 | 0.2 ± 0.5 | 93.3% | <0.0001 |
| Free of medication 12 months | 55.5% | 80.2% | 0.0001 | ||||
- scD = suprachoroidal drainage; IOP = intraocular pressure.
Complications
In the group of CscD, 34.2% of the patients showed postoperative hyphema, whereas only 10.4% of the patients who received PCscD had such an anterior chamber haemorrhage. This difference is statistically significant (p < 0.0001). Almost all cases showed spontaneous absorption within the first days. Only a small percentage, 2.1% in CscD group and 2.2% in PCscD group, needed anterior chamber lavage to remove the haemorrhage.
In cases where the targeted IOP was not reached by canaloplasty or phacocanaloplasty and consequently this primary surgery was unsuccessful, additional glaucoma surgery was necessary. In most cases, a minimal invasive 360-degree suture trabeculotomy was performed, as described in an earlier study (Seuthe et al. 2015). In those cases where the IOP afterwards still was not acceptable, a deep sclerectomy had to be performed.
Following CscD 360-degree suture, trabeculotomy was required in 34 cases (17.6%). Nine of these patients (6.7%) had postoperatively such intolerable high IOP despite maximal IOP-lowering medication that this procedure had to be carried out before 3-month follow-up.
After PCscD, 20 patients (15.6%) needed a 360-degree suture trabeculotomy, 10 of them (7.4%) before 3-month follow-up, as IOP was incontrollable high despite maximal pressure-lowering medication.
The necessity of a deep sclerectomy occurred in five cases of the CscD group, hereby two times in the first 3 months. None of the patients in the PCscD group needed such an intervention.
Transient hypotony, defined as an IOP under 5 mmHg, was observed in 10 cases (7.4%) of CscD. Only one patient (0.7%) suffered from transient hypotony in the group of PCscD. In all cases, normotonia was reached without intervention in the first 4 weeks.
There was no occurrence of choroidal detachment or bleeding, filtering bleb formation, haemorrhagic descemet detachment or endophthalmitis in both groups. An overview on the complications is given in Table 4.
| Complication | Canaloplasty + scD n = 193 | Phacocanaloplasty + scD n = 135 | p-Value* |
|---|---|---|---|
| Hyphema | 66 (34.2%) | 14 (10.4%) | <0.0001 |
| Anterior chamber lavage | 4 (2.1%) | 3 (2.2%) | 1.0 |
| Postoperative conservatively uncontrollable IOP | 9 (6.7%) | 10 (7.4%) | 0.63 |
| Necessity of 360-degree trabeculotomy | 34 (17.6%) | 20 (15.6%) | 0.55 |
| Necessity of deep sclerectomy | 5 (2.6%) | 0 | 0.08 |
| Transient hypotony | 10 (7.4%) | 1 (0.7%) | 0.03 |
- scD = suprachoroidal drainage; IOP = intraocular pressure, *Fisher's exact test.
Discussion
This is the first study comparing PCscD to single CscD over a follow-up period of 12 months in a large patient cohort. Our data show that by combining phacoemulsification and CscD, a statistically significantly higher IOP reduction of 47.4% after 12 months, as well as a significant reduction in IOP-lowering medication (0.2 eyedrops after 12 months and 80.2% of patients not needing topical therapy) can be achieved. Our data are even better than existing studies when comparing conventional canaloplasty to conventional phacocanaloplasty.
A couple of studies showed that cataract surgery itself can achieve an IOP reduction in glaucoma patients, even if this effect turned out to be rather small (Shingleton et al. 2006; Shrivastava & Singh 2010). But if cataract surgery is combined with canaloplasty in one procedure, a better IOP reduction can be observed than after both procedures performed separately (Shingleton et al. 2008; Bull et al. 2011; Lewis et al. 2011; Tetz et al. 2015). Lewis et al. observed an IOP reduction of 34% (from 23.5 ± 4.5 mmHg to 15.5 ± 3.5 mmHg) after canaloplasty alone (n = 89) in contrast to 42.1% after phacocanaloplasty (from 23.5 ± 5.2 mmHg to 13.6 ± 3.6 mmHg) (n = 27) 36 months after surgery. Bull et al. had quite similar results: canaloplasty alone achieved a significant IOP reduction from baseline 23.0 ± 4.3 mmHg to 15.1 ± 3.1 mmHg, whereas in the phacocanaloplasty group, IOP decreased significantly from 24.3 ± 6.0 mmHg to 13.8 ± 3.2 mmHg. In their 3-year-lasting study, Tetz et al. could also show a higher reduction in IOP-lowering medication by phacocanaloplasty (from baseline 1.5 ± 1.0 decreasing to 0.3 ± 0.5) compared to canaloplasty alone (baseline 1.8 ± 0.8 to 1.1 ± 0.8).
We attribute the fact that our results show a stronger IOP reduction to the extra effect of the scD. This is supported by other studies (Seuthe et al. 2016; Szurman et al. 2016). We showed in this earlier study that in comparison with conventional canaloplasty, the modified technique of CscD yields a stronger IOP reduction (37.1% versus 32.9%) and achieves a higher rate of medication-free patients after 1 year (56.9% versus 45.4.%).
A limitation of this study is definitely its retrospective nature and the short follow-up period. In return, its strong points are the large number of patients and the quite homogenous patient characteristics. It will be worthwhile to conduct a prospective and randomized study on the aforementioned surgical procedures over a longer follow-up period.
Concerning intra- and postoperative complications, we have observed no serious adverse events in both groups. Most frequent complication was the occurrence of anterior chamber haemorrhage leading to postoperative hyphema. Some authors consider that this type of hyphema predicts a better IOP development (Grieshaber et al. 2010; Koch et al. 2011). All the occurred complications in both groups are rather harmless compared to those reported after trabeculectomy or phacotrabeculectomy (Rulli et al. 2013; Kim et al. 2015). This is supported by a number of other studies, which came to the conclusion that the complication rate of canaloplasty is rather low (Thederan et al. 2014; Matlach et al. 2015; Januschowski et al. 2016).
In cases where canaloplasty did not achieve a sufficient and sustained IOP, a 360-degree suture trabeculotomy can be performed as an effective and micro-invasive way to gain an additional IOP-reducing effect after canaloplasty by removing the canaloplasty suture via the anterior chamber (Seuthe et al. 2015).
In conclusion, we could show that by combining cataract surgery and CscD, an even better IOP reduction can be achieved than by CscD alone. Additionally, PCscD delivers a higher drug-sparing effect. Both surgical procedures proved to be safe and effective and comprise the advantage of suprachoroidal access: an easier localization of Schlemm's canal.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.




