Imbalance between pro-apoptotic and pro-survival factors in human retinal pericytes in diabetic-like conditions
Part of this work has been presented as an oral/poster communication at the 25th European Association for the Study of Diabetes – Eye Complications Study Group (EASDec), Torino (Italy), 26th–28th June 2015 and at the 51st EASD Annual Meeting, Stockholm (Sweden), 14th–18th September 2015. This research was supported by the EUROCONDOR project, grant agreement number 278040, funded by the European Commission's Seventh Framework Programme (theme FP7-HEALTH-2011.2.4.3-1) and grant SAF2015-65267-R/FEDER (Ministerio de Economía y Competitividad, Spain). This publication reflects the views only of the Authors, and the European Commission cannot be held responsible for any use which may be made of the information contained therein.
Abstract
Purpose
Loss of pericytes is one the key events in the pathogenesis of diabetic retinopathy. We have previously demonstrated that human retinal pericytes (HRP) are more vulnerable to intermittent than stable high glucose concentrations, with an increase in apoptosis. Our aim was to explore the expression of molecules involved in pro-apoptotic and survival pathways in pericytes cultured in stable/intermittent high glucose and/or hypoxia, to clarify the mechanisms of action of these diabetic-like stressing stimuli.
Methods
Human retinal pericytes (HRP) were exposed intermittently at 48-hr intervals to high/physiological glucose for 8 days (intHG) and/or hypoxia over the last 48 hr. Control cells were kept in stable physiological and high glucose. Cell proliferation and apoptosis were assessed. The expression of pro-apoptotic and pro-survival molecules was evaluated by Western blotting. Caspase-8 translocation from the cytoplasm into the nucleus was checked by Western blotting of nuclear versus cytoplasmic fractions and immunofluorescence.
Results
Hypoxia, alone and combined with intHG, increased HRP apoptosis and decreased proliferation. Pro-apoptotic molecules increased in HRP cultured in these conditions, while some survival markers decreased. Conversely, in stable HG, pro-apoptotic molecules were stable or even decreased, and survival factors increased. Translocation of caspase-8 from cytoplasm into nucleus indicates a primary role for this molecule in inducing apoptosis.
Conclusion
Diabetic-like conditions are able to stimulate pericyte apoptosis through activation of pro-apoptotic molecules, leading to an imbalance between pro-apoptotic and survival signalling pathways, with caspase-8 playing a pivotal role. Our identification of such intermediates could help finding new therapeutic approaches for the prevention of diabetic retinopathy.
Introduction
Pericytes modulate vascular permeability, including the blood–brain and blood–retinal barriers, and regulate endothelial cell (EC) proliferation, migration and survival (Armulik et al. 2005). Early loss of retinal capillary pericytes, together with thickening of the basement membrane, is one key event in the pathogenesis of diabetic retinopathy (DR), as it may lead to failure of control on endothelial proliferation and, consequently, to abnormal angiogenesis (Gerhardt & Betsholtz 2003; Armulik et al. 2005).
Although intervention studies have clearly linked severity of the microvascular complications of diabetes with duration and severity of hyperglycaemia (DCCT 1993; UKPDS 1998), other factors may play a role in their pathogenesis. In particular, hypoxia caused by capillary closure and non-perfusion is a recurrent condition in the diabetic retina, leading to increased production of vascular endothelial growth factor (VEGF), the primary cause of proliferative DR (Nyengaard et al. 2004). It is well understood that loss of pericytes is primarily due to the effects of hyperglycaemia (Beltramo & Porta 2013), but, more recently, a contribution of hypoxia to the pericyte loss has also been addressed (Aplin & Nicosia 2016). We have previously demonstrated that human retinal pericytes (HRP) are more vulnerable to intermittent than stable high glucose concentrations, with an increase in their apoptosis (Beltramo et al. 2009a,b). Activation of death receptors and mitochondrial injury caused by stress stimuli has been associated with cell damage and apoptosis in the retina of diabetic subjects with DR (Valverde et al. 2013). Nevertheless, the activation of these signalling pathways in pericytes is poorly understood.
Our purpose was therefore to investigate the effects of hypoxia combined with hyperglycaemia on HRP and to identify the pro-apoptotic and survival markers involved in pericyte damage. Identification of such intermediates could help to unravel new therapeutic approaches for the prevention of DR.
Materials and Methods
Cell cultures
Commercially purchased HRP (Cambrex Bio Science, Rockland, ME, USA) were stabilized in our laboratory, by selecting clones stably transfected to express Bmi-1, a transgene that increases telomerase activity, as previously described (Berrone et al. 2009). Human retinal pericytes (HRP) were characterized by immunofluorescence staining for pericyte markers (α-smooth muscle actin, desmin, platelet-derived growth factor receptor ß and proteoglycan NG-2). They retain wild-type HRP morphology and the capability to undergo senescence in response to stress stimuli; thus, they can be used to study the effects of hyperglycaemia (Berrone et al., 2009).
Human retinal pericytes (HRP) were cultured in DMEM supplemented with 10% fetal bovine serum (Sigma-Aldrich, St Louis, MO, USA), at 5.6 mmol/L D-glucose concentration (physiological condition, NG). High glucose concentrations (HG) were obtained by adding D-glucose to a final concentration of 28 mmol/L. Cells were also cultured in intermittent HG conditions (48 hr HG/48 hr NG twice, intHG). All conditions were maintained for 8 days. Hypoxic conditions (hypo) were obtained by keeping cultures in a 5%CO2/94%N2 /1%O2 gas mixture for the last 48 hr.
Cell survival parameters
Human retinal pericytes (HRP) were counted in Bürker chambers by two independent operators after Trypan blue staining. Proliferation was measured as BrdU incorporation (Cell Proliferation ELISA BrdU kit, Roche Diagnostics, Basel, Switzerland) and apoptosis as DNA fragmentation (Cell Death Detection ELISAPLUS kit, Roche). Results were checked by a fluorescent/chemiluminescent assay, which measures viability, cytotoxicity and apoptosis in the same well (ApoTox-Glo™ Triplex Assay, Promega Corporation, Madison, WI, USA). All procedures were carried out according to manufacturers’ instructions.
Protein extraction
To extract total proteins, HRP were lysed using M-PER Mammalian Protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA) added with 10 μl/ml protease inhibitor cocktail kit (Thermo Fisher). Extracts were kept ice-cold and cleared by centrifugation at 20 000 g for 15 min at 4°C. The supernatant was aliquoted and stored at −80°C.
Other sets of cells were processed for extraction of separate cytoplasmic and nuclear fractions using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher), according to the manufacturer's instructions. The two cellular fractions were separated by the addition of different ice-cold extraction buffers to the cell pellet and stored at −80°C. Protein concentration was determined using the Bradford method.
Western blot analysis
Protein (30 μg) was loaded on sodium dodecyl sulphate–polyacrylamide gel and separated by electrophoresis. Gels were transferred to Immobilon membranes (Merck Millipore, Billerica, MA, USA). Membranes were blocked using 5% non-fat dried milk in 10 mmol/l Tris–HCl and 150 mmol/l NaCl pH 7.5 and incubated overnight with relevant antibodies in 0.05% Tween-20, 10 mmol/l Tris–HCl and 150 mmol/l NaCl pH 7.5.
All primary antibodies were used at a 1:1000 dilution. Immunoreactive bands were visualized using the enhanced chemiluminescence (ECL) Western blotting protocol (Millipore). Antibodies used were as follows: anti-FasL (AB-16982, Merck Millipore); anti-Bim (559685) and anti-BclxL (551269, BD Biosciences PharMingen, San Diego, CA); anti-Bid (AF860, R&D Systems, Minneapolis, MN, USA); anti-histone H3 (D1H2) (4499), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (9101) and anti-p44/42 MAPK (Erk1/2) (9102, Cell Signaling, Beverly, MA, USA); anti-phospho-Akt1/2/3(Ser473) (sc-7985-R), anti-Akt1/2/3 (sc-8312), anti-caspase-8 (sc-7890), anti-PCNA (sc-56) and anti-Bax (sc-493, Santa Cruz Biotechnology, Palo Alto, CA, USA); anti-α-tubulin (T0198) and anti-β-actin (A5316, Sigma-Aldrich).
The relative signal strength was quantified by densitometric analysis (imagej software, NIH, USA), and values normalized against α-tubulin. As regards activated proteins, results were expressed as the ratio of active to pro/inactive forms: active caspase-8/pro-caspase-8, truncated Bid (tBid)/Bid, phosphorylated Akt (pAKT)/Akt, phosphorylated MAPK (pMAPK)/MAPK. β-actin and histone H3 were used as cytoplasmic and nuclear loading controls, respectively, in experiments aimed at verifying active caspase-8 nuclear translocation.
Immunofluorescence staining
Pericytes were fixed in ice-cold methanol for 5 min at −20°C, dried at RT for 15 min and rehydrated in PBS for 15 min. Non-specific binding sites were blocked in PBS + 0.2% BSA (blocking solution) for 1 hr at RT. Cells were then incubated overnight at 4°C with an anticleaved-caspase-8 (Asp391) antibody (1 μg/ml in blocking solution; 9496, Cell Signaling). After washing three times with blocking solution, a FITC-conjugated goat anti-rabbit IgG (Sigma-Aldrich), 1/1000 in blocking solution, was added for 1 hr at RT. 4′,6-diamidino-2-phenylindole (DAPI) was used to blue-stain the cell nuclei. Images were taken under a Leica DM 2000 microscope (Leica, Wetzlar, Germany), equipped with a Leica DFC 320 camera and Leica QWin Plus 2003 digital processing and analysis software.
Statistical analysis
Results are expressed as mean ± SD of five independent experiments, normalized against control (NG). Statistical comparisons among groups were carried out by two-tailed Student's t-test for paired data or Wilcoxon's signed-ranks test, as appropriate. Results were considered significant for p ≤ 0.05.
Results
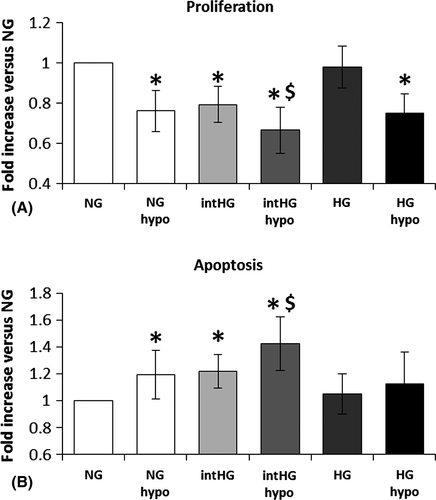
We evaluated HRP proliferation and apoptosis following 8-day exposure to NG, HG and intHG with/without hypoxia, to mimic the diabetic microenvironment in early DR. Our results show that hypoxia is able to decrease HRP proliferation (−24%, p = 0.002 versus NG) and increase their apoptosis (+19%, p = 0.045), similar to intHG, and has a synergic effect with the latter (proliferation: −24%, p = 0.001, and apoptosis: +42%, p =0.003 versus NG; Fig. 1A,B).
We investigated the apoptotic and survival molecules that were activated and/or decreased under these stress experimental conditions. Regarding pro-apoptotic markers, we studied the expression of Fas ligand (FasL), Bid and its truncated active form (tBid), Bim, Bax, p53, p53 upregulated modulator of apoptosis (PUMA), calpain-2, pro/active caspase-8 and caspase-9, while the pro-survival factors examined were phosphorylated Akt (pAkt Ser473)/Akt, phosphorylated mitogen-activated protein kinase (MAPK)/MAPK, BclxL and proliferating cell nuclear antigen (PCNA).
We found that five pro-apoptotic molecules (FasL, active caspase-8, tBid, p53 and Bax) were significantly increased in HRP cultured in intHG conditions, in both normoxia and hypoxia (Fig. 2). In particular, FasL increased dramatically in comparison with NG in all experimental conditions, reaching a 39-fold increase in intHG + hypo (p = 0.013; Fig. 2A). Active/pro-caspase-8 increase in intHG + hypo reached +70% in comparison with NG (p = 0.023) and was significantly higher than NG + hypo and intHG (p = 0.003 versus both; Fig. 2B). t-Bid/Bid registered a threefold increase in intHG alone (p = 0.003) and intHG + hypo (p = 0.001 versus NG; Fig. 2C), while p53 increased fourfold in intHG and fivefold in intHG+ hypo (p = 0.001 both versus NG; Fig. 2D). Finally, Bax underwent a 2.1–2.7-fold increase in intHG (p = 0.036) and intHG + hypo (p = 0.006) versus NG, respectively (Fig. 2E).
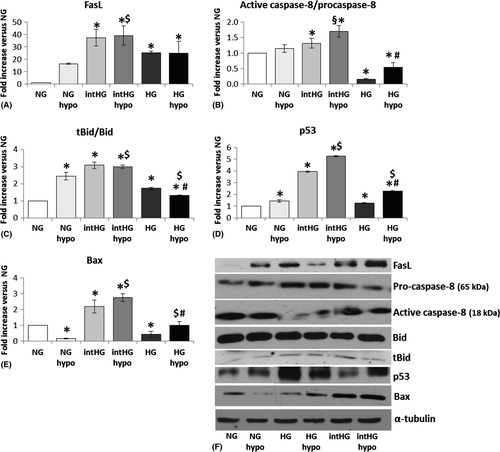
It is interesting to notice that among the pro-apoptotic molecules, only FasL was increased in stable HG/HG+ hypo, to an extent comparable with intHG/intHG + hypo (25-fold increase versus NG, HG: p = 0.001, HG+ hypo: p = 0.015; Fig. 2A). The other mediators were only slightly increased (tBid/Bid, p53; Fig. 2C,D) or even significantly decreased (active/pro-caspase-8, Bax) (Fig. 2B,E). In particular, caspase-8 expression reached −85% in HG (p = 0.001) and −50% in HG+ hypo (p = 0.037 versus NG; Fig. 2B).
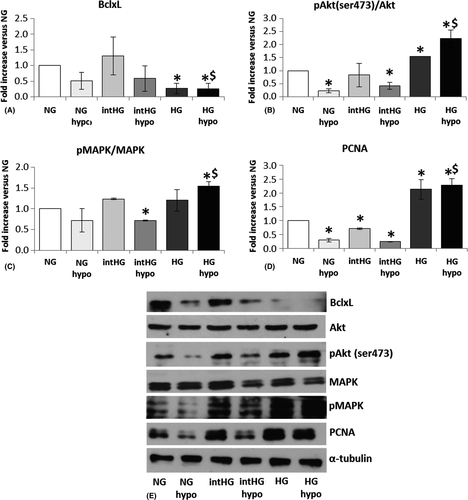
Regarding pro-survival markers, BclxL expression was substantially stable in intHG conditions, while it was the only survival molecule to decrease in stable HG and HG + hypo (−75%, p = 0.016 versus NG and p = 0.017 versus HG; Fig. 3A). We observed a −78% decrease in Akt phosphorylation (Ser473) in NG + hypo (p = 0.003 versus NG) and −49% in intHG + hypo (p = 0.018), together with a concomitant increase in HG and HG + hypo (+55%, p = 0.001 and +122%, p = 0.022 versus NG, respectively; Fig. 3B). Mitogen-activated protein kinase phosphorylation showed a decrease in intHG + hypo (−40%, p = 0.001 versus NG) and an increase in HG + hypo (+54%, p = 0.014 versus NG and 0.017 versus NG + hypo; Fig. 3C). Finally, PCNA expression underwent a marked decrease in NG + hypo (−70%, p = 0.003), intHG (−29%, p = 0.004) and intHG + hypo (−75%, p = 0.001 versus NG), and a major increase in stable HG (+110%, p = 0.03) and HG + hypo (+120%, p = 0.010 versus NG; Fig. 3D).
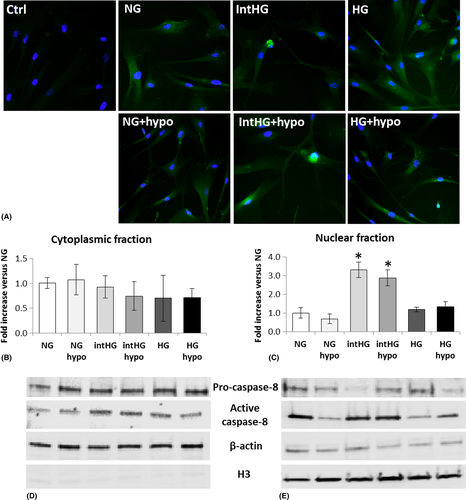
We did not find any change in the expression of the other pro-apoptotic factors analysed (caspase-9, caspase 3, PUMA, calpain-2; data not shown). This was rather surprising regarding caspase-3, which is activated by caspase-8 and considered one of the main executioners of apoptosis. We therefore hypothesized that caspase-8 could act by a direct translocation from the cytoplasm into the nucleus to induce DNA fragmentation. We checked this hypothesis by immunofluorescence staining and protein expression in cytoplasmic versus nuclear enriched fractions. As shown in Fig. 4A, immunolocalization of active caspase-8 into the nucleus was evident in HRP cultured in intHG conditions, but not in HG. This was confirmed by the finding of a threefold increase in the ratio active/pro-caspase-8 in the nuclear fraction of HRP cultured in intHG and intHG + hypo (p = 0.017 and 0.021 versus NG, respectively) (Fig. 4D,E), while no significant change was found into the cytoplasmic fraction (Fig. 4B,C).
Discussion
In this work, we show that hypoxia, alone and combined with intHG, was able to decrease HRP proliferation and increase their apoptosis, while the two stress stimuli together may have a synergic effect. Some pro-apoptotic molecules were significantly increased in HRP cultured in intHG with or without hypoxia, concurrently with decreased expression of survival markers, leading to an imbalance in favour to apoptosis. Conversely, in stable HG conditions, most of the pro-apoptotic molecules studied were unchanged or even decreased, and survival molecules increased. Translocation of caspase-8 from the cytoplasm into the nucleus indicates a pivotal role for this molecule in inducing apoptosis.
Loss of pericytes, together with thickening of the basement membrane, is considered one of the early hallmarks of DR. The primary involvement of hyperglycaemia in these events is well known (Beltramo & Porta 2013), with either direct or mediated actions (Beltramo et al. 2014; Mazzeo et al. 2015), but similar effects of hypoxia have been also recently described (Aplin & Nicosia 2016). Hypoxia, a recurrent event in DR, is due to non-perfusion following closure of capillaries and may cause an increase in VEGF, the major effector of proliferative DR (Nyengaard et al. 2004). In our experimental conditions, hypoxia was able to reduce pericyte proliferation and increase apoptosis, similar to fluctuating glucose concentrations, while the two factors together concurred in exacerbating this phenomenon. The reduction in the number of pericytes in the capillary wall, due to these concurrent events, determines loss of control on EC proliferation, which, together with VEGF overexpression, may lead to abnormal neoangiogenesis (Shweiki et al. 1992; Nyengaard et al. 2004).
Cell damage and apoptosis in the diabetic retina have been linked to the activation of death receptors and mitochondrial injury caused by stress stimuli (Valverde et al. 2013). Nevertheless, there is a lack of knowledge regarding the molecular signalling pathways involved in pericyte loss.
Apoptosis, a genetically determined programmed cell death, occurs physiologically during development and aging to maintain cell homeostasis, but it also works as a defence mechanism against external injuries (Norbury & Hickson 2001). The molecular mechanisms of apoptosis are highly complex, but two main pathways, the extrinsic and the intrinsic, have been described (Elmore 2007; Kiraz et al. 2016). The former involves activation of transmembrane receptors belonging to the superfamily of tumour necrosis factor (TNF) receptors, containing the so-called death domain, which transmits the death signal from the cell surface to the intracellular pathways (Ashkenazi & Dixit 1998). One of the best characterized ligand–receptor complexes containing the death domain is Fas/FasL (Elmore 2007). The binding of FasL to Fas receptor (FasR) results in the activation of a cascade of intracellular factors, which finally leads to the cleavage of pro-caspase-8 to active caspase-8 (Wajant 2002). The intrinsic pathway activation involves a series of non-receptor mediated stimuli (such as viral infections, radiation and also hypoxia), acting directly within the cells (Elmore 2007) and responsible for changes in the inner mitochondrial membrane. This, in turn, results in opening of the mitochondrial permeability transition pore, loss of the mitochondrial transmembrane potential and release of pro-apoptotic proteins (mainly cytochrome c; Saelens et al. 2004). The control and regulation of these apoptotic mitochondrial events are delegated to members of the Bcl-2 family (Cory & Adams 2002), which can be pro- (for instance, Bax, Bid, Bim, PUMA) or anti-apoptotic (such as Bcl-2 and BclxL), with p53 playing a critical role in their regulation (Schuler & Green 2001).
We therefore investigated the expression of some of the major mediators of the referred pathways. We found a dramatic overexpression of FasL in all our experimental conditions, reaching its peak when pericytes were cultured in intHG plus hypoxia. Moreover, active caspase-8 was significantly increased in intHG plus hypoxia, leading to hypothesize a primary involvement of the extrinsic pathway in the apoptosis of HRP. However, our results also demonstrate, in the same stress conditions (intHG with/without hypoxia), an increase in the expression of Bax, which classically belongs to the intrinsic mitochondria-mediated pathway. We can therefore hypothesize that both pathways may act synergically to induce diabetes-related pericyte apoptosis. This is supported by our finding of overexpression of tBid and p53, both acting as crosstalk mediators between the two pathways: FasL/FasR may cause mitochondrial damage through the caspase-8-mediated cleavage of Bid (Li et al. 1998; Esposti 2002), while p53 involvement has been described in both the intrinsic and extrinsic pathways of apoptosis and can also be directly activated by caspase-8 (Kiraz et al. 2016).
Regarding the pro-survival factors, our results show substantial stability of BclxL in intHG conditions with/without hypoxia and decreased phosphorylation of Akt (Ser473) and MAPK, and of PCNA levels. Phosphatidylinositol 3-kinase (PI3K)/Akt pathway is essential for cell survival and growth during development and carcinogenesis. Akt is a kinase inactive in the cytoplasm until it is phosphorylated in Ser473. Activation of Akt promotes cell survival and proliferation, by inhibiting some of the pro-apoptotic Bcl-2 family members (Zhang et al. 2011). Proliferating cell nuclear antigen is involved in DNA replication and repair, as it works as an auxiliary protein of DNA polymerases (Prosperi 1997), and its expression is positively regulated by phosphorylated MAPK (Machalińska et al. 2015).
Taken together, our data suggest an imbalance between pro-apoptotic and pro-survival factors in human retinal pericytes cultured in diabetic-like conditions (intermittent HG and hypoxia) and confirm our previous observations of decreased Bcl-2 to Bax ratio in HRP cultured in fluctuating glucose (Beltramo et al. 2009a).
Interestingly, pericytes cultured in stable HG showed a rather different behaviour. In this condition, we only found an increase in FasL levels comparable to intHG condition, as p53 and tBid increased to a much lesser extent, and active caspase-8 and Bax levels were even decreased. Concurrently, the cell proliferation marker PCNA and the phosphorylated levels of Akt and MAPK, pro-survival signalling intermediates, were significantly augmented, thus indicating a trend towards increased proliferation, rather than a modulation of the anti-apoptotic BclxL. In fact, BclxL expression is usually stimulated in response to the activation of caspase-8, as a compensatory anti-apoptotic mechanism (Elmore 2007). This diverse behaviour of human pericytes when cultured in intermittent HG as opposed to stable HG is in agreement with our previous observations (Beltramo et al. 2009a,b), showing that these cells, differently from other animal-derived pericytes, are much more sensitive to fluctuating glucose conditions mimicking the situation in diabetic patients, while they seem to develop rather a passive resistance to stable hyperglycaemic-like conditions.
The extrinsic and intrinsic apoptotic pathways finally converge into the activation of the executioner caspases, which, in turn, trigger endonucleases, leading to DNA fragmentation, and proteases, degrading nuclear and cytoskeletal proteins (Elmore 2007; Kiraz et al. 2016). Caspase-3 is generally considered the most important among the executioner caspases and is activated by the initiator caspases (caspase-8, caspase-9 or caspase-10). However, we could not observe any significant difference in caspase-3 expression in our experimental settings. This could be explained by the fact that our study model mimics the diabetic microenvironment in early DR, while the activation of pro-apoptotic molecules involved in the opening of the mitochondrial permeability transition pore, which induces the activation of caspase-3, may occur in later stages. Our finding of caspase-8 translocation from the cytoplasm into the nucleus in intHG conditions is in agreement with previous observations showing that active caspase-8 may relocate into the nucleus and act directly as an executioner caspase, cleaving PARP-2, a member of the poly(ADP-ribose) polymerase family involved in DNA repair (Benchoua et al. 2002; Arroba et al. 2005) and thus bypassing caspase-3.
In conclusion, diabetic-like conditions are able to stimulate pericyte apoptosis through the activation of pro-apoptotic molecules, thus leading to an imbalance between pro-apoptotic and survival signalling pathways. In this work, we show for the first time in our knowledge that both extrinsic and intrinsic apoptotic pathways are involved, with caspase-8 playing a primary and independent role in triggering apoptosis. Our identification of the intermediates involved in diabetes-induced pericyte apoptosis could help find new therapeutic approaches for the prevention of DR at early stages.




