The reduction of temporal optic nerve head microcirculation in autosomal dominant optic atrophy
Abstract
Purpose
To evaluate the optic nerve head (ONH) microcirculation in autosomal dominant optic atrophy (ADOA) patients.
Methods
This study comprised 22 eyes of 12 ADOA patients, diagnosed according to clinical findings including family history and the presence of mutations in the OPA1 gene. Twenty-four normal eyes of 24 age-matched subjects, with either the right or left eye randomly selected for use, served as controls. Circumpapillary retinal nerve fibre layer thickness (cpRNFLT) and mean blur rate (MBR) in the ONH were determined with optical coherence tomography (OCT) and laser speckle flowgraphy (LSFG), respectively. For each ONH quadrant (superior, temporal, inferior and nasal), the MBR and cpRNFLT ratio was also calculated by dividing tissue MBR in that quadrant by tissue MBR in the entire ONH and by dividing cpRNFLT in that quadrant by cpRNFLT in the entire ONH respectively.
Results
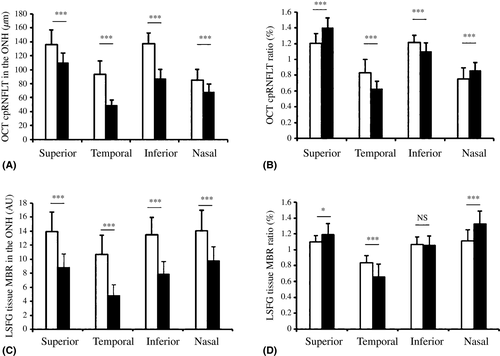
Mean blur rate (MBR) in all quadrants was significantly lower in the ADOA patients than in the controls (p < 0.001 in each). The MBR ratio was significantly lower in the ADOA patients only in the temporal quadrant (p < 0.001). Similarly, cpRNFLT was lower in the ADOA patients in all quadrants (p < 0.001 in each), and the cpRNFLT ratio was lower in the temporal quadrant (p < 0.001).
Conclusion
Reduced blood flow in the temporal optic disc in ADOA patients is associated with reduced temporal cpRNFLT, suggesting that both are caused by damage to the papillomacular bundle. The anatomical characteristics of the papillomacular bundle may make it especially susceptible to mitochondrial dysfunction-induced damage, which occurs in ADOA.
Introduction
Autosomal dominant optic atrophy (ADOA) has a wide variety of characteristics and symptoms and is the most common type of hereditary optic neuropathy (Kjer et al. 1996), with a prevalence estimated to be between 1:12000 and 1:50000 (Eiberg et al. 1994; Kjer et al. 1996; Yu-Wai-Man et al. 2010). The age of onset is in the early decades of life with relatively minor symptoms that are easily overlooked. Visual acuity (VA) ranges from normal to hand movement (Brown et al. 1997), with a majority of patients maintaining functional vision throughout life, although a tendency for visual acuity to decrease with age has been described (Cohn et al. 2008). Typically, the disease progresses slowly, beginning with an insidious onset in childhood. Visual field defects, such as central visual field scotomas, may also be present. The optic nerve head (ONH) can show either temporal or diffuse pallor with optic disc excavation (Kerrison 2001). Colour vision can also be affected, usually resulting in either blue-yellow dyschromatopsia or generalized colour vision deficits (Miyata et al. 2007). In patients with ADOA, the temporal optic disc becomes pale in fundus imaging. Optical coherence tomography (OCT) has enabled the easy observation of retinal morphology. Research using OCT has previously revealed that circumpapillary retinal nerve fibre layer thickness (cpRNFLT) and macular retinal thickness are significantly reduced in patients with ADOA (Ito et al. 2007). However, cpRNFLT and macular retinal thickness are also reduced in patients with glaucoma. Thus, it is necessary to find new techniques to identify ADOA and distinguish it from other diseases.
Recently, advancements in laser speckle flowgraphy (LSFG) have allowed the use of a new measurement parameter for ocular blood flow, mean blur rate (MBR) (Tamaki et al. 1994, 1995; Sugiyama et al. 1996; Araie 2000; Yaoeda et al. 2000; Konishi et al. 2002). Previous reports have shown that LSFG-measured MBR or parameters of MBR could be used to distinguish optic nerve diseases such as glaucoma (Chiba et al. 2011; Yokoyama et al. 2011; Shiga et al. 2013), acute non-arteritic ischaemic optic neuropathy (NAION) and anterior optic neuritis (ON) (Maekubo et al. 2013). Specifically, ONH blood flow was found to decrease in eyes with NAION, while it increased in eyes with anterior ON. Clinically, however, ONH hemodynamics in eyes with ADOA, and the relationship of ONH hemodynamics with cpRNFLT, remain poorly understood.
In this study, we hypothesized that decreased cpRNFLT in the temporal quadrant of the ONH in ADOA patients was associated with decreased ocular blood flow, which might be caused by mitochondrial dysfunction. Therefore, we examined clinical findings, including LSFG-measured ONH microcirculation, in ADOA patients.
Patients and Methods
Patients
This study recruited 12 untreated Japanese members of seven families, all of whom were probands for optic atrophy and had received diagnoses of ADOA. For the case–control comparison, 24 normal eyes of 24 subjects were examined, with the right or left eye randomly selected for inclusion. Subjects were excluded if they had other ophthalmic conditions, such as high myopia (below −8D) or hyperopia (above + 3D). A full history was taken for each patient and an ophthalmologic examination was performed, which included slit-lamp biomicroscopy and fundus photography, in addition to testing for best-corrected VA, intraocular pressure, and the visual field, and LSFG and OCT scanning. All patients were Japanese, with no history of neurological or eye diseases. All subjects were observed as outpatients at the Department of Ophthalmology of Tohoku University in Japan. Informed consent was obtained from all patients for the purpose and procedures of the study. The procedures conformed to the tenets of the Declaration of Helsinki, and the protocol was approved by the Ethics Review Board of Tohoku University.
Blood sampling and mutation analysis
Peripheral venous blood samples (5 ml) were obtained from all subjects, from which genomic DNA was extracted using the NucleoSpin Blood XL (Macherey-Nagel, Duren, Germany), according to the manufacturer's protocol. The samples were then sent to the Casey Eye Institute (Oregon Health and Science University, Portland, Oregon) for analysis. Direct testing for mutations in the OPA1 and OPA3 genes was performed by PCR amplification and next-generation (high-throughput) sequencing of the DNA samples. Conventional Sanger sequencing was also performed on the samples.
Laser speckle flowgraphy
In preparation for LSFG scanning, the pupils of each subject were dilated with 0.4% tropicamide (Mydrin M; Santen Pharmaceutical Co., Ltd., Osaka, Japan). Systolic and diastolic blood pressures and IOP were measured after the patients had rested for 10 min in a sitting position in a dark room. Ocular circulation was then assessed using LSFG (LSFG-NAVI; Softcare, Iizuka, Japan). To evaluate the microcirculation in the optic nerve head, the MBR of optic disc was determined by LSFG-NAVI. The mechanism of LSFG has been described in detail elsewhere (Tamaki et al. 1995; Isono et al. 2003). The MBR ratio was also calculated for each quadrant by dividing tissue MBR in the respective quadrant (i.e. superior, temporal, inferior and nasal) by overall tissue MBR in the optic nerve head. This method was used in our previous research (Aizawa et al. 2014a). We then performed a statistical comparison of the MBR ratio in each quadrant in the two groups.
Optical coherence tomography
The overall cpRNFLT was determined with the 3D OCT-2000-embedded software (ver. 8.11, Topcon Corporation, Tokyo, Japan). After centring a circle scan on the optic nerve head, the software automatically calculated cpRNFLT in each quadrant layer (i.e. superior, temporal, inferior and nasal).
After comparing cpRNFLT in the two groups in each optic nerve head quadrant, we calculated the ratio of cpRNFLT in each quadrant to cpRNFLT in the entire scan area and compared the ratio for each quadrant in the two groups.
Statistical analysis
All data were expressed as mean ± SD. The Mann–Whitney U-test was used for the statistical analysis of changes. Differences were considered significant at p < 0.05. Statistical analysis was performed with the JMP Pro 11 software (SAS Institute Japan, Inc., Tokyo, Japan).
Results
Patients and clinical finding
The clinical characteristics of the normal and ADOA patients are summarized in Table 1. Twenty-two eyes of 12 ADOA patients (mean age: 36.0 ± 15.5) and 24 eyes of 24 normal control subjects as control (mean age: 42.3 ± 12.3) were examined. The visual acuity of the ADOA patients was significantly lower than that of the normal subjects (logMAR: normal, −0.14 ± 0.08, ADOA, 0.51 ± 0.34, p < 0.0001). There were no significant differences in other characteristics, including gender, age, spherical equivalent or intraocular pressure, between the normal and ADOA subjects.
| Normal | ADOA | p Value | |
|---|---|---|---|
| Number of eyes | 24 | 22 | |
| Gender (male:female) | 13:11 | 5:7 | 0.39 |
| Age (years) | 42.3 ± 12.3 | 36.0 ± 15.5 | 0.12 |
| VA (logMAR) | −0.14 ± 0.08 | 0.51 ± 0.34 | <0.0001 |
| Spherical equivalent (diopter) | −3.27 ± 2.27 | −3.93 ± 2.11 | 0.28 |
| IOP (mmHg) | 14.3 ±3.01 | 14.0 ± 3.0 | 0.58 |
| Tissue MBR in quadrant (AU) | |||
| All | 12.66 ± 2.44 | 7.41 ± 1.31 | <0.0001 |
| Superior | 13.91 ± 2.79 | 8.84 ± 1.92 | <0.0001 |
| Temporal | 10.69 ± 2.73 | 4.83 ± 1.51 | <0.0001 |
| Inferior | 13.45 ± 2.52 | 7.87 ± 1.81 | <0.0001 |
| Nasal | 14.06 ± 2.93 | 9.79 ± 1.97 | <0.0001 |
| CpRNFLT in quadrant (micrometer) | |||
| All | 112.83 ± 9.09 | 78.77 ± 8.83 | <0.0001 |
| Superior | 136.00 ± 20.93 | 110.32 ± 13.60 | <0.0001 |
| Temporal | 93.67 ± 18.88 | 49.45 ± 7.18 | <0.0001 |
| Inferior | 137.33 ± 15.17 | 87.04 ± 13.23 | <0.0001 |
| Nasal | 84.79 ± 15.55 | 67.95 ± 11.30 | 0.0004 |
- Differences between the two groups were assessed with Mann–Whitney U test. cpRNFLT = circumpapillary retinal nerve fibre layer thickness; logMAR = logarithm of minimum angle of resolution; MBR = mean blur rate; AU = arbitrary unit; IOP = Intraocular pressure, VA = visual acuity.
Table 2 shows the OPA1 gene mutation status of the seven families included in this study. OPA1 gene mutation was present in six families (c.1374_1377 + 1delTGTAA, c.2942-2_2943delAGTT, c.1178_1179insT, c.2140C>T, c.1374_1377 + 1delTGTAA and c.1305_1305 + 13delGGTAAGGGTTGCAA) and absent in one family. To our knowledge, c.1178_1179insT and c.2140C>T are the first mutations to be reported in patients with ADOA.
| Patient | Pedigree | Gender | Age (years) | VA right/left (logMAR) | Nucleotide change | Amino acid change |
|---|---|---|---|---|---|---|
| 1 | 1 | F | 20 | 1.0/0.82 | c.1374_1377 + 1 delTGTAA | N/A |
| 2 | 1 | F | 24 | 1.0/0.3 | c.1374_1377 + 1 delTGTAA | N/A |
| 3 | 1 | F | 49 | 1.0/1.0 | c.1374_1377 + 1 delTGTAA | N/A |
| 4 | 1 | F | 48 | 0.7/0.52 | c.1374_1377 + 1 delTGTAA | N/A |
| 5 | 2 | M | 25 | 0.4/0.52 | Mutation not found | – |
| 6 | 3 | M | 22 | 0.3/0.3 | c.2942-2_2943delAGTT | N/A |
| 7 | 4 | M | 54 | 0.52/0.3 | c.1178_1179insT | p.Pro315Profs |
| 8 | 5 | M | 30 | 0.3/0.4 | c.2140>T | p.Gln714Ter |
| 9 | 5 | F | 55 | −0.08/0 | c.2140>T | p.Gln714Ter |
| 10 | 6 | F | 46 | 0.15/0.3 | c.1374_1377 + 1 delTGTAA | N/A |
| 11 | 6 | M | 11 | 0.3/0.3 | c.1374_1377 + 1 delTGTAA | N/A |
| 12 | 7 | F | 52 | 1.0/0.7 | c.l305_1305 + 13delGGTAAGG GTTGCAA | N/A |
- Patient 1–4, 8–9, 10–11 are each same pedigrees.
The MBR in each optic disc
In each optic nerve head quadrant (superior, temporal, inferior and nasal), tissue MBR was measured in the two study groups. In every quadrant, tissue MBR was significantly lower in the ADOA patients than in the normal subjects (Table 1 and Fig. 1).
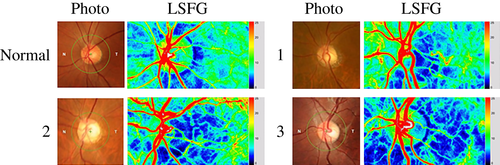
Reduced temporal optic disc circulation and relatively preserved nasal circulation were characteristic of the ADOA patients in this study. Figure 2 shows a representative example of three patients comprising three generations of a single family, all with such characteristic changes in the temporal disc (Fig. 2).
The MBR ratio in the temporal quadrant was lower in the ADOA eyes than in the normal eyes (p < 0.001). In the ADOA patients, we found that the MBR ratio was relatively high in the superior and nasal quadrants.
The cpRNFLT in each optic disc quadrant
To compare RNFL thinning and the decrease in microcirculation in the ADOA eyes and the normal eyes, cpRNFLT was assessed in each quadrant, in the same manner as MBR. The results were similar for MBR and cpRNFLT: in each quadrant, cpRNFLT was significantly lower in the ADOA patients than in the normal subjects (Table 1 and Fig. 1).
As in our analysis of MBR, we calculated the ratio of cpRNFLT in each quadrant and the overall optic nerve head. The cpRNFLT ratio in the temporal quadrant was lower in the ADOA eyes than in the normal eyes (p < 0.001). The RNFLT ratio was also relatively high in the superior and nasal quadrants, and relatively low in the inferior quadrant.
Discussion
We set out to evaluate clinical findings, including ONH microcirculation in ADOA patients. We found that in the ADOA patients, MBR in all quadrants was significantly lower than that in the controls, that the MBR ratio was significantly lower in the temporal quadrant, and that the MBR ratio was significantly higher in the superior and nasal quadrants. These results show that tissue MBR is significantly lower in the temporal area, but not in the superior, inferior or nasal areas. Similarly, cpRNFLT was lower in the ADOA patients in all quadrants, and the cpRNFLT ratio was lower in the temporal and inferior quadrants and higher in the superior and nasal quadrants. Therefore the characteristic pale appearance in fundus imaging of the temporal optic disc in eyes with ADOA might be caused by the fact that cpRNFLT and blood flow are reduced in the temporal ONH while they are relatively preserved in the nasal ONH. Thus, a key finding of our study was that blood flow in the nasal ONH was relatively preserved, while temporal ONH blood flow decreased. The exact reason for this is still unclear, but one possible reason is the anatomy of the papillomacular bundle, which is located in the area leading to the temporal ONH and is composed of RGC axons with relatively small cross-sectional areas. These small cross-sectional areas contain relatively few mitochondria, meaning that the papillomacular bundle has a lower mitochondrial reserve and a greater physiological susceptibility to damage than the larger, magnocellular RGCs. This increased susceptibility is compounded by the poor energy efficiency of the papillomacular bundle, which creates a high demand for mitochondrial energy. The optic nerve is an extension of the central nervous system and has several unique structural features (Carelli et al. 2004). Posterior to the lamina cribrosa, the axons of the optic nerve become myelinated. Myelination allows for efficient saltatory conduction to occur, reducing the demand for mitochondrial energy production. By contrast, the prelaminar RGC axons, including the papillomacular retinal nerve fibre, are unmyelinated and require a high amount of energy to restore electrical potential and for axoplasmic transport. Therefore, in the prelaminar axons, there are few mitochondria serving a high energy demand. Due to these anatomical characteristics, genetic dysfunction of the mitochondria causes damage to the axons of the macular ganglion cells and results in the apoptosis of the RGCs.
Mapping the thickness of the macula and measuring retinal blood flow have been shown to be effective means of diagnosing ADOA. Confirming a previous report (Ito et al. 2007), we found that ADOA patients had lower cpRNFLT than controls. Furthermore, Rönnbäck et al. (2013) observed that the thickness of the ganglion cell layer had a higher predictive value for ADOA than RNFL thickness and that ganglion cell layer thickness in the inferonasal macula was markedly reduced in ADOA patients. These findings support our hypothesis that ADOA predominantly affects the papillomacular bundle. Retinal blood flow in ADOA has thus been the subject of a number of investigations, including that by Gränse et al. (2003). In a follow-up report, Rönnbäck et al. (2014) argued that central retinal vessel narrowing is a consequence of inner retinal hypoplasia or atrophy. Taken together, these studies promise to facilitate the diagnosis of ADOA and shed new light on its characteristics. Thus, changes in blood flow and cpRNFLT are characteristic of ADOA and may be caused by an increased susceptibility to damage of the papillomacular bundle.
OPA1 is a mitochondrial dynamin-related GTPase (Ishihara et al. 2013). A decreased level of the OPA1 protein may cause abnormalities in the mitochondria, resulting in insufficient energy support. Additionally, the papillomacular retinal nerve fibre require high levels of energy supply for axoplasmic transport (Alexander et al. 2000). As shown in the current study, this might lead to a diffuse loss of the axons on the temporal side of the ONH and a reduction in ONH microcirculation. Furthermore, we consider that our finding of a reduction of tissue MBR in the ONH is especially important for future research, because tissue MBR, measured by LSFG, has been found to be suitable for interindividual comparisons, regardless of individual pigmentation (Aizawa et al. 2014b). In addition, we compared the ratio of MBR measurements and RNFL thickness in each quadrant of the optic disc in the ADOA patients and normal subjects, as this can provide information on the allocation of total MBR in each quadrant. We found that both the MBR ratio and the RNFL thickness ratio were significantly and persistently reduced in the temporal quadrant, a finding that reinforced the results of our comparison of the raw MBR and RNFL thickness values. This may reflect the pathogenesis of ADOA and suggests that a reduced MBR ratio might have importance as a reliable marker of reduced blood flow, which may occur as an adaptation to reduced metabolism in ADOA eyes.
ONH microcirculation is reduced in a number of diseases, most notably glaucoma (Chiba et al. 2011; Yokoyama et al. 2011). Indeed, the clinical phenotype of ADOA is similar to glaucoma, making it difficult to differentiate these two diseases in some cases. Thus, we hypothesized that measuring MBR might be a valuable addition to current clinical ADOA diagnostic methods, which are based on cpRNFLT. However, once again, more studies will be needed to confirm this. In ADOA, dysfunction of the mitochondria caused by mutations in the OPA1 gene is thought to be associated with the apoptosis of the RGCs, which leads to optic neuropathy. Similarly, genetic polymorphism of the OPA1 gene has been suspected to be a risk factor for glaucoma (Turkoski 2012), because OPA1 is expressed in RGCs and the optic nerve. In fact, Guo et al. (2012) recently performed a meta-analysis of recent genetic linkage studies of OPA1 polymorphisms in patients with glaucoma and found a specific association with NTG risk in Caucasian individuals only, not in Asian individuals. The specificity of this association suggests that NTG may be a hereditary optic neuropathy with a pathophysiology characterized by mitochondrial dysfunction. Further investigation is needed to validate this pathomechanism.
Our results supported previous findings that OCT could be used as a highly sensitive method of diagnosing ADOA (Rönnbäck et al. 2013). However, in contrast to previous research, our results may have been limited by our inability to perform comparisons with healthy family member, due to cultural constraints imposed by the Japanese setting of the study. Other limitations of this study included a small sample size and a cross-sectional design as a case series enrolling ADOA patients and control subjects. In most cases, we included both eyes of each ADOA patient in our analysis. This was both because MBR varied between the eyes of each patient and because ADOA is a rare, hereditary type of optic neuropathy, making the recruitment of patients very difficult. For these reasons, studies of ADOA (such as those by Ito et al. 2007; Park & Hwang 2015) have conventionally used both eyes. Furthermore, our previous research showed that MBR could decrease in eyes with myopic optic discs (Aizawa et al. 2014c). Therefore, to prevent high myopia influencing the results, we excluded eyes with a spherical equivalent < −8.00 dioptres from both groups. Thus, we believe that the results of this clinical analysis of carefully selected subjects support the view that measurement of MBR and cpRNFLT would be helpful in diagnosing ADOA.
In conclusion, we found that MBR in all quadrants was significantly lower in ADOA patients than in controls and that the MBR ratio was significantly lower in ADOA patients only in the temporal quadrant. Similarly, cpRNFLT was lower in the ADOA patients in all quadrants, and the cpRNFLT ratio was lower only in the temporal quadrant. Anatomical characteristics of the optic nerve cause the papillomacular bundle to be susceptible to damage caused by mitochondrial dysfunction, which occurs in ADOA. Our results thus suggest that damage to the papillomacular bundle causes reduced blood flow in the temporal ONH and cpRNFLT in ADOA patients.