Earlier immunomodulatory treatment is associated with better visual outcomes in a subset of patients with Vogt-Koyanagi-Harada disease
Abstract
Purpose
To evaluate clinical outcomes of first-line immunomodulatory therapy (IMT) and prednisone alone or late IMT in Vogt-Koyanagi-Harada disease.
Methods
Retrospective cohort study of 152 patients with Vogt-Koyanagi-Harada disease evaluated in a referral uveitis clinic in Chile from 1985 to 2011. Medical records of these patients were reviewed. Demographic data, clinical evaluation, type of treatment, functional outcomes, glucocorticoid (GC) dose and complications were recorded. Multivariate logistic regression was used to identify prognostic factors of poor response to GC.
Results
There were no significant differences between first-line IMT group and prednisone alone/late IMT group in terms of visual acuity (VA) improvement, complications and GC sparing effect. There was a trend for a higher frequency of systemic adverse effects leading to discontinuation of treatment in patients receiving IMT than in those receiving prednisone (14.6% and 6.5%, respectively). The subgroup of patients with poor response to GC who showed functional improvement had a significantly earlier time to IMT initiation than the patients who had no improvement. We identified following prognostic factors of poor response to GC: VA ≤20/200, fundus depigmentation, chronic disease and tinnitus at diagnosis. Patients with a prognostic factor (excluding tinnitus) and VA improvement had an earlier IMT initiation than those who had worse functional outcome.
Conclusion
There were no differences in outcomes between first-line IMT and prednisone alone/late IMT in the entire VKH group. However, in a subset of patients, there was a significant better functional outcome with earlier IMT initiation.
Introduction
Vogt-Koyanagi-Harada syndrome (VKH) is a systemic inflammatory disorder of unknown cause, characterized by the involvement of the eyes, auditory system, meninges and skin (Bordaberry 2010; Rajendram et al. 2005; Read et al. 2001). In the eyes, it causes bilateral granulomatous panuveitis with exudative retinal detachments leading, in some cases, to a significant reduction in visual acuity (Rajendram et al. 2005). Multiple therapeutic regimens have been used to treat VKH, including subtenon, oral and intravenous glucocorticoids (GC), T-cell inhibitors, antimetabolites, alkylating agents and, more recently, biologic therapy (Errera et al. 2011; Byon et al. 2011; Kacmaz et al. 2010; Gangaputra et al. 2009; Baker et al. 2006; Jabs et al. 2000). To date, oral GC remain as the cornerstone of VKH treatment, based on favourable functional outcomes and low side-effects reported in several studies (Moorthy et al. 1995; Cuchacovich et al. 2010; Read et al. 2006; Nazari & Rao 2012). However, despite appropriate treatment with steroid therapy, many patients suffer recurrences and associated complications (Sukavatcharin et al. 2007). Therefore, non-steroidal immunomodulatory therapy (IMT) has become important in the treatment of VKH. However, when and to whom IMT should be initiated is still an open question. In small retrospective series, IMT as first-line treatment for VKH has shown to be effective (Paredes et al. 2006). Unfortunately, these drugs are associated with significant adverse effects in patients with ocular inflammatory disease as well as in other inflammatory processes (Errera et al. 2011; Kempen et al. 2008; Okada 2005). It is also known that a significant proportion of patients could respond to GC as monotherapy (Jabs et al. 2000; Read et al. 2006; Abu El-Asrar et al. 2013; Kruh & Foster 2012; Lai et al. 2009).
Therefore, it would be useful to identify prognostic factors that could predict treatment response and to allow a more personalized therapeutic approach for patients with VKH disease.
This report describes in a retrospective cohort of 152 patients with VKH disease, one of the largest reports published so far, the outcomes and patients’ characteristics at presentation that may predict visual outcomes and treatment response to either (1) prednisone alone group (or late IMT) or (2) IMT as first-line therapy group.
Materials and Methods
This study evaluated a retrospective cohort (Grimes & Schulz 2002) of patients with the diagnosis of VKH obtained from the database of the Uveitis Department of the Salvador's Hospital (Santiago, Chile) from January 1985 to December 2011. The Uveitis Department of the Salvador's Hospital is the Public National Referral Centre for the Country. Most of patients with VKH were referred to us by ophthalmologists from hospitals throughout Chile.
The database, implemented in 1985, gathered prospectively personal and demographics data, family history and complete ophthalmologic examination of patients admitted and followed up in our department in a standardized manner. A detailed history with regard to systemic and diseases was taken from each patient. The ophthalmologic evaluation included best-corrected visual acuity, intraocular pressure, slit-lamp biomicroscopy, ophthalmoscopy under mydriasis and ancillary testing in some cases (fundus fluorescein angiography, indocyanine green angiography, optical coherence tomography and B-scan ultrasonography). Patients were examined further when additional systemic disease was suspected.
Inclusion and exclusion criteria
The study admitted patients with VKH, according to the diagnostic criteria revised by the international nomenclature committee in uveitis (Read et al. 2001; da Silva et al. 2009; Rao et al. 2007). They were considered only if they fulfilled a minimum of 3-month follow-up in our institution, with at least 6 month of the disease evolution. Alternative diagnoses (i.e. syphilis, tuberculosis) were ruled out during our initial evaluation.
Outcomes measurements
Data extracted included visual acuity measured at the initial visit (by Snellen chart), first-line treatment received (GC or IMT), initial dosing, timing of initial dosing and the total treatment length. If more than one drug was used, the reason for the indication was stated. Finally, drug adverse effects and complications associated with VKH were also obtained. All patients where followed by rheumatologist along with ophthalmologist. In this regard, periodic blood tests were performed depending on the clinician's criteria at a given time-point of the follow-up. Therefore, in our cohort, complications related to therapy were stated when any of these tests were altered and not assessed by a specific time-point. GC or IMT intolerance was considered when patient had an adverse effect leading to discontinuation of treatment.
Based on the treatment, patients were initially divided in two groups as previously described by Paredes et al. (2006):
- Group 1: prednisone alone or late IMT
- Group 2: first-line IMT
‘Late’ was defined as IMT given after 6 months of VKH diagnostic, and ‘first-line’ was defined as IMT given within 6 months of diagnosis.
On a second analysis, patients were classified as ‘early first-line IMT’ if they received IMT within 6 weeks of diagnosis, because of that interval of time is used in our clinical practice for defining treatment response. Therefore, probably this time-point could reflect more accurately the first-line treatment concept.
During follow-up, poor response to GC or GC-resistance was defined as follows (Kim & Yu 2007):
- Persistent retinal detachment.
- Absence of vision improvement, defined as an increment of visual acuity less than two Snellen lines.
- Absence of inflammatory improvement, defined as persistent or worsening of inflammation despite steroid treatment, no achieving 2-step decrease in level of inflammation or decrease to grade 0+, as described by the ‘Standardization of Uveitis Nomenclature for reporting clinical data’ consensus (Jabs et al. 2005).
SUN criteria were published in 2005. Prior to that date, the report of ocular inflammation was not standardized worldwide. However, previously to this report, the use of cumulative (+) signs to state the level of uveitis was used in our centre and recorded in every patient chart. Hence, in our study, a SUN criterion to evaluate ‘improvement’ was used in all data set, retrospectively based on the report of inflammation as (+) signs in older data, and after 2005 with the recorded SUN report.
Immunomodulatory therapy respo-nse was evaluated in terms of clinical course of the disease that determines to continue with the same IMT or to change the therapy.
Glucocorticoid sparing effect was defined as a reduction in the prednisone dose to 10 mg per day or less, 5 mg per day or less and 0 mg per day while maintaining inactive uveitis at 6 and 12 months (Pasadhika et al. 2009).
This study was approved by the Ethics Committee of the Salvador's Hospital. The protocol complies with the contents of the Declaration of Helsinki.
Statistics
Descriptive statistics were calculated for the whole cohort and subgroups, including frequency distribution and means or medians as appropriate. Univariate analyses were performed using Student's t-tests for comparing mean age and time differences to IMT initiation between groups, and chi-square tests were used for comparing all the remaining differences of proportions between groups. To determine clinical predictors of poor response to treatment with GC, we used the binary logistic regression test. p values ≤0.05 were considered as a statistically significant difference [SPSS statistics software (version 20.0, Chicago, IL, USA)].
Results
Baseline characteristic of study population
A total of 152 patients were included in the study. Patient characteristics at diagnosis are summarized in Table 1. The mean age at presentation was 35 years. Most patients were female (73%) and fulfilled the criteria for probable VKH diagnosis. The neurological findings were presented most frequently than integumentary findings at diagnosis (36% and 13%, respectively). The mean follow-up period was 57 months. Visual Impairment was common, with 75% of eyes having visual acuity 20/50 or worse and 53% of eyes having visual acuity 20/200 or worse.
| Age (yrs) | 35.8 ± 15a |
| Sex | |
| Male, n pts (%) | 40 (26.3) |
| Female, n pts (%) | 112 (73.7) |
| Follow-up (months) | 57 ± 67.6a |
| Time to diagnosis (days) | 64.2 ± 55.8a |
| VKH diagnosis, n pts (%) | |
| Probable | 84 (55.2) |
| Incomplete | 61 (40.1) |
| Complete | 7 (4.6) |
| Integumentary findings, n pts (%) | 20 (13.1) |
| Alopecia | 6 (3.9) |
| Poliosis | 7 (4.6) |
| Vitiligo | 14 (9.2) |
| Neurological findings, n pts (%) | 56 (36.8) |
| Tinnitus | 53 (34.8) |
| Meningismus | 9 (5.9) |
| Cerebrospinal fluid pleocytosis | 7 (4.6)b |
| Visual acuity n eyes (%) | |
| 20/40 or better | 73 (24) |
| 20/50 to 20/100 | 67 (22) |
| 20/200 or worse | 164 (53.9) |
- yrs = years, pts = patients.
- a Mean ± SD.
- b Lumbar puncture was performed in 11 patients.
Treatment modalities
All 152 patients were initially treated with oral prednisone at a dose of 1 mg/kg per day for at least 4 weeks and then this dose was tapered slowly according the disease activity. 49 patients (32.2%) received first-line IMT while 103 patients (67.7%) received either prednisone alone or late IMT as previously defined (Paredes et al. 2006). There were no statistically significant differences in clinical and demographic features at diagnosis between both groups (Table 2).
| First-line IMT | Late IMT or glucocorticoid alone | |
|---|---|---|
| (98 eyes, 49 pts) | (206 eyes, 103 pts) | |
| Age (yrs) | 35.6a | 35.9a |
| Sex (Female), n pts (%) | 37 (75.5) | 75 (72.8) |
| Follow-up (months) | 58a | 56.4a |
| Visual acuity, n eyes (%) | ||
| 20/40 or better | 20 (20.4) | 53 (25.7) |
| 20/50 to 20/100 | 24 (24.4) | 43 (20.8) |
| 20/200 or worse | 54 (55.1) | 110 (53.4) |
| Visual acuity improvement, n eyes (%) | 67 (68.3) | 125 (60.6) |
| Complications, n pts (%) | ||
| Cataract | 11 (22.4) | 36 (34.9) |
| Glaucoma | 8 (16.3) | 16 (15.5) |
| Ocular hypertension | 5 (10.2) | 23 (22.3) |
| Sunset glow fundus | 17 (34.7) | 51 (49.5) |
- IMT = immunomodulatory therapy, pts = patients, yrs = years.
- a Mean.
During the follow-up, a total of 89 patients were treated with IMT, 76 patients received only 1 IMT and 13 patients received 2 IMT. The immunomodulatory drugs used were as follows: azathioprine 1–2 mg/kg/day (54 patients), cyclophosphamide 1.5–2.5 mg/kg/day (33 patients), methotrexate 20–25 mg/week (nine patients), cyclosporin 3–5 mg/kg/day (five patients) and chlorambucil 0.1 mg/kg/day (one patient).
There were no significant differences in IMT response among our patients. The addition or the switch to another IMT was carried out in six patients who received azathioprine (11.1%), three patients who received cyclophosphamide (9.1%) and one patient who received methotrexate (11.1%). We observed a worse response in cyclosporin group, but this subgroup is too small for analysis (3 of the 5).
Functional outcomes and complications
There were no significant differences between first-line IMT group versus prednisone alone/late IMT group in terms of visual acuity improvement (68.3% and 60.6%, respectively; p > 0.05), complications (cataract, glaucoma, ocular hypertension and sunset glow fundus) and GC sparing effect at 6 and 12 months (Tables 2 and 3).
| First-line IMT | Late IMT or Glucocorticoid alone | p | |
|---|---|---|---|
| Outcomes with 6 months of therapy | (68 eyes, 34 patients) | (122 eyes, 61 patients) | |
| Controlled inflammation and prednisone dose ≤ 10 mg/day | 9 (26.4) | 19 (31.1) | 0.81 |
| Controlled inflammation and prednisone dose ≤ 5 mg/day | 1 (2.9) | 13 (21.3) | 0.01 |
| Controlled inflammation and prednisone dose 0 mg/day | 1 (2.9) | 6 (9.8) | 0.4 |
| Outcomes with 12 months of therapy | (64 eyes, 32 patients) | (110 eyes, 55 patients) | |
| Controlled inflammation and prednisone dose ≤ 10 mg/day | 20 (62.5) | 38 (69) | 0.36 |
| Controlled inflammation and prednisone dose ≤ 5 mg/day | 16 (50) | 28 (50.9) | 0.82 |
| Controlled inflammation and prednisone dose 0 mg/day | 6 (18.7) | 15 (27.2) | 0.44 |
- n patients (%)
- IMT = immunomodulatory therapy.
- a Data from patients/eyes available for each group at indicated time-points.
In the group that received early first-line IMT, despite of the fact that IMT was initiated as early as 6 weeks, we did not find any significant differences between both groups in terms of visual acuity improvement at any time-point (1-month, 3-month and final follow-up) as shown in Table 4.
| VA improvement | |||
|---|---|---|---|
| 1 month | 3 months | Final follow-up | |
| Early first-line IMT | 34 (58.6) | 35 (72.9) | 51 (63.7) |
| Late IMT or glucocorticoids alone | 60 (54.1) | 64 (65.9) | 141 (62.9) |
- n eyes (%).
- VA = visual acuity, IMT = immunomodulatory therapy.
- a Data from eyes available for each group at indicated time-points.
In a further analysis, we evaluated whether patients who received prednisone alone had different outcomes as compared to patients who started late IMT. We observed a significantly higher percentage of VA improvement in the subgroup who received prednisone alone [85 eyes (67.4%)] in comparison with the subgroup who received late IMT [40 eyes (50%)] (p = 0.01).
A significant difference in VA improvement was also observed between first-line IMT group and late IMT subgroup (68.3% versus 50%, p = 0.01).
Adverse events
There was a trend, although not statistically significant, for a higher frequency of systemic adverse effects leading to discontinuation of treatment in patients receiving IMT than in those receiving prednisone (14.6% versus 6.5%; p = 0.18). The most common side-effects leading to discontinuation of treatment in patients using IMT were gastrointestinal upset (5.6%) and bone marrow suppression (5.6%), followed by elevated liver enzymes (2.2%) and haemorrhagic cystitis (1.1%). On the other hand, the most common side-effects in patients treated with GC were metabolic disturbances (hyperglycaemia, hypertension, Cushing's syndrome).
Earlier IMT initiation is associated with better outcomes in VKH patients with poor response to glucocorticoids
As there were no significant differences in functional outcomes and complications between patients treated with first-line IMT versus prednisone alone/late IMT group, we evaluated if in a subgroup of patients, such as those with poor response to GC, the time of IMT initiation could be associated with a different functional outcome. Therefore, we compared the time elapsed to the IMT initiation in patients with poor response to GC regarding to visual improvement.
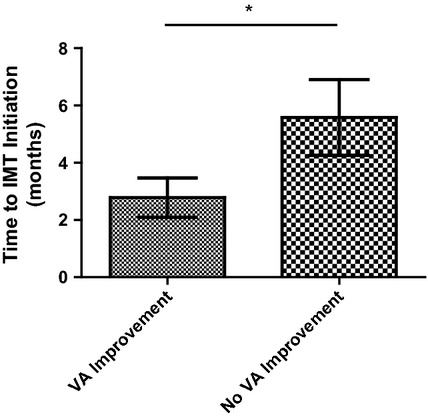
As shown in Fig. 1, those patients with poor response to GC, who experienced a functional improvement, had a significantly earlier time to IMT initiation (2.78 ± 0.68 months) than the patients who had no improvement (5.58 ± 1.32 months) (p = 0.04).
Prognostic factors for poor response to GC
If an earlier IMT will lead to a better functional outcome in GC non-responder patients with VKH, it would be clinically important to identify this subset of patients as early as possible. Therefore, we looked for clinical characteristics that could be associated with poor response to GC in patients with VKH.
The prognostic factors analysed are summarized in Table 5. At diagnostic evaluation, best-corrected visual acuity ≤20/200, fundus depigmentation, tinnitus and chronic disease were associated with poor response to GC treatment.
| OR | p | |
|---|---|---|
| BCVA ≤20/200 | 1.8 | 0.02 |
| Fundus depigmentation | 1.62 | 0.04 |
| Tinnitus | 1.67 | 0.04 |
| Chronic disease | 1.21 | 0.01 |
- BCVA = best-corrected visual acuity.
To further explore whether the group of patients with these prognostic factors would effectively have a better functional outcomes if the IMT was introduced earlier, we compared the time of IMT initiation for patients with or without functional improvement. As shown in Table 6, IMT initiation was significantly earlier among subgroup of patients with fundus depigmentation or chronic disease at diagnosis that had a visual acuity improvement, compared to those patients with no functional improvement. We also observed a difference in patients with BCVA ≤20/200, but that was not statistically significant (p = 0.11). We did not find differences in subgroup with tinnitus.
| Time to IMT initiation (months) | |||
|---|---|---|---|
| VA improvement group | No VA improvement group | p | |
| BCVA ≤ 20/200 | 4.5 | 9.1 | 0.11 |
| Fundus depigmentation | 4.4 | 12.3 | 0.02 |
| Tinnitus | 5.2 | 5.2 | 0.96 |
| Chronic disease | 6.5 | 14.5 | 0.01 |
- IMT = immunomodulatory therapy, VA = visual acuity, BCVA = best-corrected visual acuity.
Discussion
Glucocorticoid treatment for patients with VKH has been considered as the mainstay approach. Early, high-dose and prolonged therapy is the basis to achieve inflammation control and thus to prevent complications (Errera et al. 2011; Lai et al. 2009).
The well-known ocular and systemic complications of GC (Baker et al. 2006; Jabs et al. 2000; Read et al. 2006; Kruh & Foster 2012) have determined that many uveitis specialists lean towards the use of IMT in the management of patients with VKH (Errera et al. 2011; Okada 2005). However, in our study, IMT failed to reduce the incidence of systemic side-effects leading to discontinuation of treatment. Even more IMT had a tendency for a higher number of these adverse events.
Another reason to use IMT as a first-line therapy is to achieve a better functional outcome. Paredes et al. (2006) evaluated first-line IMT in a cohort of 13 patients with VKH. They found a superior functional outcome in comparison with prednisone alone or delayed IMT.
In our study, we evaluated retrospectively both treatment strategies in a National Referral Centre. In a cohort of 152 patients with VKH, we observed no significant differences in visual acuity improvement, ocular complications and steroid sparing effect between both groups. The present VKH cohort is similar in terms of age and gender to the patients included by Paredes et al. However, this apparent contradictory results could be explained because in the studied VKH cohort by Paredes et al. (2006), 9 of 13 included subjects had visual acuity ≤20/200 at diagnosis, which we have identified as a possible prognostic factor associated with poor response to GC (Table 5) and thus configuring a subset of patients which our data suggest will have a better functional outcome with an earlier IMT (Table 6), as was shown by Paredes et al.
Other clinical characteristics at diagnosis associated with poor response to GC were fundus depigmentation, tinnitus and chronic disease (Table 5). Recent published data concur with our results (Abu El-Asrar et al. 2013). In a retrospective analysis of 87 patients (174 eyes) with VKH, Abu El-Asrar et al. showed that visual acuity in patients with acute disease had no significant difference between IMT and no IMT groups. Furthermore, in chronic recurrent disease, the use of IMT showed a statistically significant improvement of visual acuity, reaching a 59% of final VA of 20/20 with the use of IMT, compared to 30% of final VA of 20/20 in the group where IMT was not indicated. According with these data, we observed in our cohort that chronic disease was a risk factor to poor response to GC therapy (Table 5). Moreover, we found that in this specific group, the delay of the initiation of IMT was associated with a worse VA outcome (Table 6). Conversely, these observations were not replicated in initial acute onset disease subgroup of patients, where prednisone or IMT showed no differences in final VA. These findings suggest that IMT indication for patients with VKH must consider the duration of the disease, because its use may improve final VA in chronic disease, but have poor or no impact in acute onset patients.
Therefore, our results suggest that may not be necessary to use IMT in all patients with VKH, as they could have no benefits over the GC therapy alone and also increase the risk of significant side-effects (Errera et al. 2011; Okada 2005). On the other hand, even when the patients with VKH as a whole did not show a further benefit with the IMT as first-line therapy, it is important to point out that the patients with poor response to GC that had an early addition of IMT had a significant better outcome (Fig. 1). These results suggest that first-line IMT could be a better therapeutic approach to this subset of patients with VKH. We observed that the mean time to IMT initiation was 2.7 months in the subgroup with better functional outcomes. Therefore, it would be a 2.7–month window to assess response to GC. However, this time interval has to be considered carefully, because it represents an average and these data are from a retrospective analysis. Prospective clinical trials are needed to determine the best time-point to categorize patients with VKH in terms of GC response and to decide IMT initiation.
In this regard, the present VKH cohort aims to provide information to personalize therapy to improve visual outcomes and does not pretend to compare outcomes within different IMT drugs, as other studies have carried out (Cuchacovich et al. 2010).
Based in our study results and the above literature review, we propose that the assessment of prognostic factors for poor response to GC at diagnosis of patients with VKH could contribute to decide the best choice of treatment for each patient, initiating early IMT when any of these factors are present.
To our knowledge, this study is one of largest cohort for VKH described. However, as a retrospective study, the data arisen from this study may have the biases associated with this design. For example, as we are a referral centre, it is possible that patients that respond well to initial therapy could have not been referred and are under-represented in our results. Moreover, treatment decision could be determined by severity or another non-reported confounding factor. Nevertheless, given the fact that we observed no differences between groups in terms of our primary outcome VA (Table 2), biases associated with severity status are less likely.
Therefore, prospective, randomized controlled clinical trials are needed to determine the efficacy of the suggested personalized strategy of therapy in patients with VKH disease based on clinical evaluation at diagnosis.
In conclusion, we found no differences in outcomes between first-line IMT and prednisone alone or late IMT in the overall VKH group in Chile. However, we observed a significant early IMT initiation in patients with visual improvement in the poor response to GC subgroup. We also described prognostic factors for early identification of that VKH subgroup and we propose a personalized management in VKH disease.