Diabetic macular ischaemia is associated with narrower retinal arterioles in patients with type 2 diabetes
LG, SDA and KP involved in the design and conduct of the study; SD, TAG, FM, TA and ECA involved in the collection and management of data; LG, SDA, KP, MP, WJJ, WTY, TA and ECA performed the analysis and interpretation of the data; LG and SDA involved in the preparation of manuscript; KP, TAG, MP, WJJ, WTY, FM, TA and ECA involved in review and approval of the manuscript.Drs. Sim and Fruttiger receive funding from Fight For Sight UK, Grant 1987. Drs. Keane, Egan, Sim and Tufail have received a proportion of their funding from the Department of Health's NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology. The views expressed in the publication are those of the author and not necessarily those of the Department of Health.The authors declare that they have no competing interests.
Abstract
Purpose
Diabetic macular ischaemia (DMI) is an important cause of visual loss in patients with diabetes, but its relationship to the larger retinal vessels is unknown. We examined whether retinal vessel calibre is related to DMI.
Methods
Clinic-based case–control study of patients with type 2 diabetes. The presence and severity of DMI was assessed using Early Treatment Diabetic Retinopathy Study (ETDRS) protocols from fundus fluorescein angiographic (FFA) images. Custom software was used to quantify the greatest linear dimension and area of the foveal avascular zone (FAZ). Retinal vessel calibre was measured using a semi-automated software on fundus fluorescein images.
Results
Of 53 patients examined, 18 (34%), 18 (34%) and 17 (32%) had no/mild, moderate and severe DMI, respectively. Persons with moderate or severe DMI had narrower mean retinal arteriolar calibre than persons with no/mild DMI (140.6 μm 95% confidence interval (CI) 134.7, 146.4 versus 150.7 μm, 95% CI 142.5, 158, p = 0.04). The association remained after multivariate adjustment for age, gender, previous panretinal photocoagulation, neovascularization at the disc and elsewhere and diabetic retinopathy severity. Increased FAZ size was also associated with narrower arteriolar calibre. Retinal venular calibre and arteriole to venule ratio (AVR) were not associated with DMI.
Conclusions
Retinal arteriolar narrowing was associated with moderate-to-severe macular ischaemia in eyes with diabetic retinopathy. This suggests that larger vessels other than capillaries may also be associated with DMI.
Introduction
Diabetic macular ischaemia (DMI) is an important cause of visual loss in persons with diabetic retinopathy (Bresnick et al. 1975). The prevalence of DMI is not known precisely but in clinical trials has been reported in approximately 7% of patients with diabetic retinopathy (1995). DMI is diagnosed based on the appearance of the foveal avascular zone (FAZ) on fundus fluorescein angiography (FFA) and is characterized by enlargement and disruption of the margins of the FAZ and abnormalities in the surrounding capillary bed (1995). The presence of DMI is regarded as an adverse prognosis factor and influences clinical decision-making, as persons with DMI are less likely to benefit from interventions such as focal laser and intravitreal therapy (Jonas et al. 2005; Chung et al. 2008).
The pathology of DMI remains poorly understood. The vascular changes that occur in diabetes include thickening of the basement membrane, pericyte loss and endothelial swelling followed by apoptosis which contribute to capillary closure (Nag & Wadhwa 2012). As the FAZ is avascular to begin with, the subsequent loss of surrounding capillaries may result in more severe ischaemia. Neurosensory cellular apoptosis in response to macular ischaemia may also play a role (Catalani et al. 2007). Although the relationship of DMI with capillary closure is well supported by angiographic and histological studies (Kohner & Henkind 1970), the relationship with other larger retinal vessels has not been examined, and there is a need for invivo studies of retinal vascular changes (Bek 2009). Retinal vessels are readily visualized on clinical examination and colour fundus photography, and their calibres are markers of the presence or severity of disease in conditions such as proliferative and non-proliferative diabetic retinopathy (Klein et al. 2012), glaucoma (Mitchell et al. 2005; Amerasinghe et al. 2008) and systemic diseases such as hypertension (Wong & Mitchell 2004), diabetes, (Wong et al. 2002) and stroke (Liew et al. 2012). Knowledge of the relationship of DMI with retinal vessel calibre may thus offer insights into DMI pathophysiology and may provide a non-invasive clinical marker for DMI. In this study, we sought to examine the association of DMI with retinal vessel calibre, as assessed from fundus fluorescein angiograms in persons with type 2 diabetes.
Methods
This study is a substudy of the Diabetic Retinopathy Repair Project, details of which are reported elsewhere (Sim et al. 2013). In brief, clinical and imaging data were collected retrospectively from patients attending a single consultant-led (C.E.) diabetic eye disease retinal clinic at Moorfields Eye Hospital, London, United Kingdom. Data were collected over a 6-month time period (1 December 2010 to 30 June 2011). Approval for data collection and analysis was obtained from the local ethics committee and adhered to the tenets set forth in the Declaration of Helsinki.
All patients referred from the United Kingdom National Screening Committee (UK NSC) – Diabetic Eye Screening Programme (Mackenzie et al. 2011) to the single consultant-led diabetic eye disease clinic at Moorfields Eye Hospital within the time interval described above were eligible to participate if they had a FFA performed at their first hospital visit (the majority of cases) or at a later visit within 6 months of the first visit. Retinopathy/maculopathy grades were obtained from standardized electronic reports in the UK NSC – Diabetic Eye Screening Programme. Patient age at time of attendance, gender and presence of ocular comorbidity were obtained from hospital electronic patient records. For this study, only patients attending the clinic with a diagnosis of Type 2 diabetes mellitus were included. Patients with ocular comorbidities, including retinal arterial or venous occlusion, epiretinal membrane, neovascular age-related macular degeneration, inherited macular disease or macular scarring of any aetiology, were excluded.
In patients with bilateral disease of symmetrical severity, a single eye was selected using permuted block randomization for inclusion in the study. In patients with asymmetrical disease, the eye with the more severe ischaemic maculopathy was selected.
Acquisition and analysis of fundus fluorescein angiograms
Grading methods for macular ischaemia
All angiographic images were acquired with a digital retinal camera system (Topcon TRC 50IX; Topcon Medical Systems Inc., Paramus, NJ, USA). One early- to mid-phase image (at 20–40 s), centred on the macula, was chosen for analysis of DMI as in later phases, fluorescence abnormalities commonly impair visualization of the retinal capillary network. The FFA images were chosen for optimal focus and intensity levels which allowed visualization of macular capillaries. No image manipulation was performed prior to grading. Macular ischaemia was dual-graded by two masked assessors using protocols and standard photographs from ETDRS Report No. 19 in which severity is determined by FAZ size, capillary loss or dilatation and retinal arteriolar abnormalities (1995). According to these criteria, DMI was classified as none, questionable, mild, moderate or severe. In the case of disagreement between graders, open adjudication was used to resolve the final grading decision. For the purposes of this study, questionable DMI grades were excluded while none and mild were combined. Further details are given elsewhere (Sim et al. 2013).
Quantification of the foveal avascular zone and other areas of capillary non-perfusion
Quantitative analysis of all images was performed using a validated image viewer and grading software package (‘GRADOR’; Doheny Image Reading Center, Los Angeles, CA, USA) that facilitates planimetric measurements. Using this software, the areas of the FAZ, and other areas of capillary non-perfusion, were assessed in square millimetres (mm2). After manual delineation of each area, measurements were calculated using a scale factor based on the camera's angle of view. The presence of neovascularization at the disc (NVD), elsewhere (NVE) and panretinal photocoagulation scars (PRP) on FFA were also recorded.
Quantification of retinal vessel calibres
Retinal arteriolar and venular calibres were measured from late-phase FFA images (1–2 min) when the retinal arterioles and venules were clearly outlined with fluorescein dye. FFA images had to have sufficient peripapillary vasculature visible to facilitate grading with a validated semi-automated software which measured the calibres of arterioles and venules coursing through a region 0.5–1 disc diameter from the disc margin (Hubbard et al. 1999; Knudtson et al. 2003; Sun et al. 2008). This software has been reported to have high reproducibility (Sun et al. 2008). All images were masked graded by a single grader at the Centre for Vision Research, Sydney, Australia. These values were then combined using formulas to derive the central retinal artery and vein equivalent for that eye (Knudtson et al. 2003). Arteriole to venule ratio (AVR) was calculated as the ratio of mean arteriolar to venular calibres.
Inclusion criteria
For inclusion in the study, all angiographic image sets had to be of sufficient quality to allow (i) visualization of FAZ capillaries to allow grading of DMI severity and (ii) visualization of arterioles and venules exiting from the disc to allow measurement of arteriolar and venular calibres. We included all 17 eyes with severe DMI, all 18 with moderate DMI and randomly selected 18 eyes with none/mild DMI to serve as controls.
Statistical analysis
Statistical analyses were performed using SAS version 9 (SAS Institute Inc., Cary, NC, USA). Regression models were constructed to examine the association of vessel calibres with DMI severity grades, greatest linear dimension and area of FAZ. Models were adjusted for age, gender, previous PRP, NVE, NVD and UK NSC grading (as a marker of diabetic retinopathy severity). p for trend was estimated modelling vessel calibres as continuous variables. We report p-values and 95% confidence intervals.
Results
Of 53 patients examined, 18 (34%), 18 (34%) and 17 (32%) had none/mild, moderate and severe DMI, respectively. Baseline characteristics are shown in Table 1, where patients with moderate or severe DMI were younger, more likely to be male, have had previous PRP laser, NVD and NVE, compared to patients with no/mild DMI, although these differences were not statistically significant.
| Diabetic macular ischaemia | p-Value | ||
|---|---|---|---|
| None/Mild (n = 18) | Moderate/Severe (n = 35) | ||
| Age (years) | 66.1 (13.8) | 60.1 (12.5) | 0.12 |
| Male | 10 (55.6) | 22 (62.9) | 0.77 |
| Previous panretinal photocoagulation laser | 2 (11.1) | 7 (20.0) | 0.70 |
| Neovascularization at disc | 1 (5.6) | 3 (8.6) | 0.99 |
| Neovascularization elsewhere | 0 (0) | 6 (17.1) | 0.08 |
- Numbers are mean (±standard deviation) or N (%).
Table 2 shows that persons with moderate or severe DMI had narrower mean retinal arteriolar calibre than persons with no DMI. The association of narrower arterioles with moderate/severe DMI remained after multivariate adjustment for age, gender, previous panretinal photocoagulation, neovascularization at the disc and elsewhere and UK NSC diabetic retinopathy grades. Increasing severity of DMI was also associated with narrower arterioles, with multivariate adjusted mean arteriolar calibres of 150.6, 142.1 and 139.1 μm in participants with none/mild, moderate and severe DMI, respectively (p-trend = 0.04). Retinal venular calibre and AVR were not associated with DMI. Excluding patients with PRP (n = 10) from our analyses attenuated the statistical significance of the results but did not change the direction of effect [adjusted arteriolar calibres of 153.3, 146.9 and 143.5 μm in participants with none/mild, moderate and severe DMI, respectively (p-trend 0.09)].
| Diabetic macular ischaemia severity | N | Mean arteriolar calibre, μm (95% CI) | Mean venular calibre, μm (95% CI) | Mean arteriole to venule ratio (AVR) | |||
|---|---|---|---|---|---|---|---|
| Unadjusted | Multivariate adjusteda | Unadjusted | Multivariate adjusteda | Unadjusted | Multivariate adjusteda | ||
| None | 18 | 150.7 (142.5, 158.9) | 150.6 (143.0, 158.1) | 240.6 (227.4, 253.9) | 243.1 (230.3, 256.0) | 0.63 (0.60, 0.66) | 0.62 (0.59, 0.66) |
| Moderate | 18 | 142.4 (134.2, 150.7) | 142.1 (134.9, 149.3) | 238.8 (225.6, 252.1) | 238.8 (226.6, 251.1) | 0.60 (0.57, 0.63) | 0.60 (0.56, 0.63) |
| Severe | 17 | 138.6 (130.1, 147.1) | 139.1 (131.1, 147.1) | 226.8 (213.2, 240.5) | 224.2 (210.6, 237.7) | 0.61 (0.58, 0.65) | 0.62 (0.59, 0.66) |
| p-trend | 0.04 | 0.04 | 0.23 | 0.12 | 0.25 | 0.64 | |
| None | 18 | 150.7 (142.5, 158.9) | 150.4 (142.9, 157.8) | 240.6 (227.3, 254.0) | 242.1 (229.1, 255.1) | 0.63 (0.60, 0.66) | 0.63 (0.59, 0.66) |
| Moderate/Severe | 35 | 140.6 (134.7, 146.4) | 140.7 (135.5, 146.0) | 233.0 (223.4, 242.6) | 232.2 (223.2, 241.3) | 0.61 (0.58, 0.63) | 0.61 (0.59, 0.63) |
| Difference | 10.1 (0.1, 20.2) | 9.6 (0.2, 19.1) | 7.6 (−8.8, 24.0) | 9.9 (−6.6, 26.3) | 0.03 (−0.01, 0.07) | 0.02 (−0.03, 0.06) | |
| p-Value | 0.04 | 0.04 | 0.36 | 0.23 | 0.18 | 0.44 | |
- CI = confidence intervals.
- a Multivariate models adjusted for age, gender, previous panretinal photocoagulation, neovascularization at the disc or elsewhere.
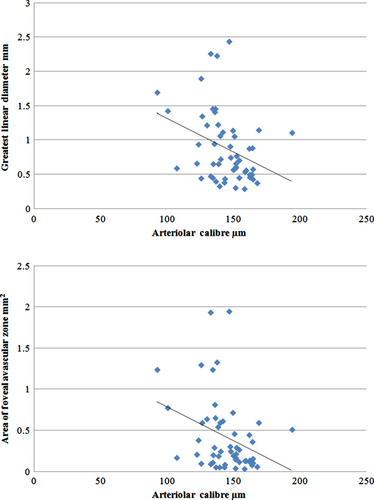
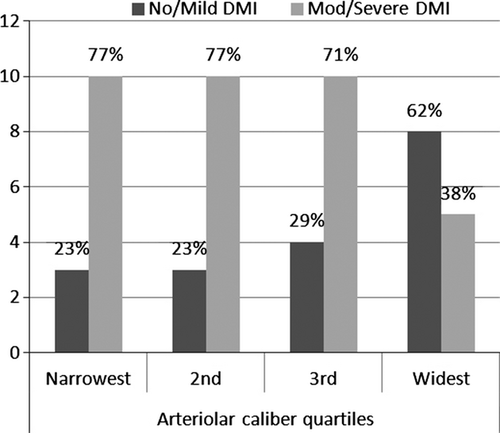
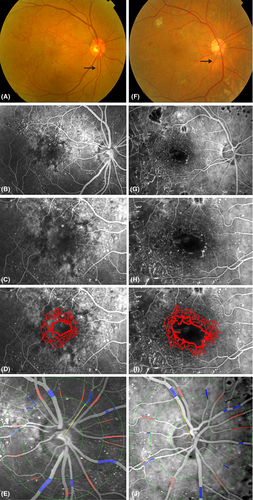
Increased FAZ (greatest dimension and area of ischaemia) was also associated with narrower arteriolar calibre (Table 3). Each standard deviation decrease in arteriolar calibre was associated with increases of 0.21 mm (95% CI 0.05, 0.38) in diameter and 0.19 mm2 (95% CI 0.04, 0.33) in area (Fig. 1). Although associated, the coefficient of correlation between these measures is modest. Figure 2 shows that in our sample, 77% of persons in the narrowest quartiles of arteriolar calibre had DMI, compared with 38% in persons in the largest quartiles. Venular calibre and AVR were not associated with FAZ diameter or area. In Fig. 3, the relationship of larger FAZ (3I) and narrower arterioles (3F) in a person with DMI is highlighted when compared to the FAZ and arterioles in a patient without DMI (3D, A).
| Size of foveal avascular zone | Per SD decrease in arteriolar calibre | Per SD decrease in venular calibre | Per SD decrease in arteriole to venule ratio (AVR) | |||
|---|---|---|---|---|---|---|
| Unadjusted | Multivariate adjusteda | Unadjusted | Multivariate adjusteda | Unadjusted | Multivariate adjusteda | |
| Greatest linear dimension (mm) | 0.17 (0.04, 0.31) | 0.21 (0.05, 0.38) | 0.11 (−0.03, 0.26) | 0.15 (−0.001, 0.31) | 0.08 (−0.06, 0.22) | 0.04 (−0.12, 0.19) |
| p-value | 0.01 | 0.01 | 0.11 | 0.06 | 0.28 | 0.62 |
| Area of ischaemia (mm2) | 0.15 (0.03, 0.27) | 0.19 (0.04, 0.33) | 0.09 (−0.04, 0.21) | 0.12 (−0.02, 0.26) | 0.08 (−0.05, 0.20) | 0.04 (−0.09, 0.18) |
| p-value | 0.02 | 0.01 | 0.17 | 0.08 | 0.22 | 0.53 |
- SD = standard deviation; CI = confidence intervals.
- a Multivariate models adjusted for age, gender, previous panretinal photocoagulation, neovascularization at the disc or elsewhere.
Discussion
Diabetic macular ischaemia as a cause of significant visual loss in persons with diabetes has received relatively little attention due, in part, to difficulties in establishing and quantifying the condition which usually requires a FFA (1995). In this study, we report that narrower retinal arteriolar calibre measured on FFA is associated with DMI. Mean arteriolar calibre was 10.1 μm narrower in persons with moderate/severe DMI compared to persons with none/mild DMI. Narrower arteriolar calibre was also associated with more severe grades of DMI, larger FAZ diameter and area. These associations persisted after adjustment for markers of peripheral ischaemia such as NVD, NVE, prior PRP and UK NSC diabetic retinopathy grading score. Venular calibre and AVR were not associated with DMI. These data suggest that retinal arteriolar calibre may be a quantitative marker of presence and severity of DMI, and highlight that vessels other than capillaries are affected in DMI.
There are few studies with which to compare our results. The major epidemiological studies on diabetic retinopathy did not perform FFA on participants and hence did not report prevalence or associations of DMI. Narrower arteriolar calibre, measured from colour photographs in persons with diabetes, is associated with lower limb amputation (Klein et al. 2007; Roy et al. 2012) and peripheral neuropathy (Ding et al. 2012) and may thus be a marker for some ischaemic diabetic complications. In persons without diabetes, narrower arterioles are also associated with small vessel lacunar stroke (Lindley et al. 2009) and reduced myocardial perfusion (Wang et al. 2008) and are a prognostic marker for coronary heart disease (McGeechan et al. 2009). Persons with retinal arteriolar narrowing have increased markers of endothelial dysfunction (Hemminki et al. 2007). Taken together, these results suggest that retinal arteriolar narrowing may be a marker for ischaemic tissue, both in the retinal and systemic microcirculation.
Arteriolar autoregulation is impaired in persons with type 1 (Justesen et al. 2010) and type 2 (Lott et al. 2012) diabetes. One interpretation of our findings is that impaired autoregulation leads to narrower arterioles which may contribute to a propensity to DMI by reducing available blood flow. However, an alternative interpretation is that arteriolar narrowing may occur in response to reduced metabolic needs in DMI. Our cross-sectional analysis is unable to discriminate between these two hypotheses, and future longitudinal studies are needed to clarify the temporal sequence of events.
The pathophysiology underlying these associations is unclear but may be related to alterations in levels of oxidative stress and inflammation in DMI, which have been found to influence retinal arteriolar calibres (Daien et al. 2013). Retinal vessel vasodilation is known to be impaired in diabetes and prediabetes, and the presence of DMI may be a more severe manifestation of this tendency, leading to narrower arterioles overall in persons with DMI (Lott et al. 2013). It is also important to note that our observations are not due to vasoconstriction from topical dilating drops, as these have been found to not influence vessel calibres (Vandewalle et al. 2013). Further research on DMI could focus on using other non-invasive haemodynamic measurement techniques (Pemp et al. 2013) and measuring vessel calibres at different points in the vascular branching network, which may be differently affected (Torring et al. 2014).
We also examined venular calibre but did not observe any association with DMI. Other studies have reported wider retinal venules are strongly associated with increasing severity of retinopathy (Klein et al. 2006; Kifley et al. 2007), incidence and progression of retinopathy (Klein et al. 2004, 2012) and in particular proliferative diabetic retinopathy. Thus, it is interesting that in contrast to these well-established associations of venular widening with peripheral ischaemia, our study did not detect any associations with central DMI. This may be partly because venular widening is related to vascular endothelial growth factors (VEGF) (Tolentino et al. 1996, 2002) which would not be expected to be raised to the same extent in DMI as in peripheral ischaemia (Malik et al. 2005). Our study therefore suggests that arteriolar and venular calibre may be differentially related to peripheral/generalized and central/localized ischaemia; further work contrasting arteriolar and venular calibre measurement in eyes with DMI and proliferative retinopathy will establish if this hypothesis is correct.
Our findings may have clinical implications. DMI is difficult to diagnose without resorting to invasive FFA which carries a small but definite morbidity. Recent studies with high-speed Fourier domain Optical Coherence Tomography (OCT) report foveal ganglion cell layer loss correlates with DMI diagnosed on FFA (Byeon et al. 2009). Our findings suggest arteriolar narrowing may also be a marker for DMI. Although arterioles are readily visualized on ophthalmoscopy, it is difficult to quantify their calibre clinically. Nonetheless, an overall impression of generally widened or narrowed arterioles may be obtained, as shown in Fig. 3. Figure 2 shows that in our sample, three quarters of persons in the narrowest quartiles of arteriolar calibre had DMI, suggesting that clearly narrowed arterioles may signal to some extent the presence of DMI. Larger, prospective studies are needed to further investigate whether this sign is clinically useful.
Strengths of this study include measurement of DMI and vessel calibres from the same series of FFAs, use of validated methods of grading both variables, and multivariable adjustment for retinopathy severity and peripheral ischaemia (i.e. UK NSC grade, PRP, NVE, NVD). Limitations include firstly, the lack of adjustment for blood pressure as these data are not routinely collected from our eye clinic. It is possible that this may have confounded our results. It is also possible that adjustment for blood pressure may not alter our findings, as hypertension does not appear to be associated with DMI (Shukla et al. 2004). Another limitation is that we could not adjust for other potential confounders such as smoking, HbA1c, diabetes duration, cholesterol and body mass index as these data are not routinely collected in our eye clinics. Future studies exploring the issues raised here should aim to collect and adjust for these potential confounders. Secondly, we measured vessel calibres from FFAs, rather than colour fundus photographs as reported in most epidemiological and clinical work. We do not believe this would result in significant bias as others have shown high correlation and only very slight differences in calibre measurements from FFA and colour photographs (Pakter et al. 2011). Finally, the sample size is relatively small due to the relative rarity of severe and moderate stages of DMI, of which we included all the cases we had with gradable images. Although we did not detect a significant association between venular calibre and DMI at p < 0.05, there was a trend for narrower venular calibre to be associated with increasing severity of DMI, greatest linear dimension and area of ischaemia. It is possible that a larger sample size may have detected a significant association.
In summary, we report that narrower retinal arterioles were associated with the presence and severity of DMI. Narrower arterioles were also associated with larger FAZ diameter and area. These associations were independent of markers of peripheral ischaemia and suggest measurement of retinal arteriolar calibre may be useful to further characterize and understand underlying vascular changes in eyes with DMI.