Choroidal vessel diameter in central serous chorioretinopathy
Supported by Beijing Municipal excellent talent Foundation and Training plan of high-level-health talent of health system (2009-3-32), Beijng, China.
Abstract.
Purpose: To measure the hyporeflective lumen in the choroid of patients with central serous choroidopathy (CSC) and to compare the diameter with that of a control group.
Methods: The prospective comparative observational clinical study included patients with unilateral CSC and a control group of normal subjects, matched in age, gender and refractive error with the study group. Subfoveal choroidal thickness (SFCT) and the largest diameter of choroidal hyporeflective lumen as surrogates for the choroidal vessels were measured by enhanced depth imaging optical coherence tomography (OCT).
Results: The study group included 15 Chinese patients and the control group 15 control subjects. Mean SFCT was significantly (p = 0.04) larger in the affected eyes (455 ± 73 μm) than in the contralateral unaffected eyes (387 ± 94 μm), in which it was significantly (p = 0.005) larger than in the control group (289 ± 71 μm). In a parallel manner, the mean diameter of the largest hyporeflective lumen was larger, but not significantly larger (p = 0.18) in the affected eyes (305 ± 101 μm) than in the in the contralateral unaffected eyes (251 ± 98 μm), in which it was significantly (p = 0.001) larger than in the control group (140 ± 40 μm). Largest vessel diameter was significantly (p < 0.001; correlation coefficient: 0.73) correlated with the thickness of the total choroid.
Conclusions: In patients with CSC, the affected eyes show larger hyporeflective lumen than the contralateral clinically unaffected eyes and significantly larger than normal control eyes. Assuming these hyporeflective lumens to be choroidal vessels, macular swelling in CSC is markedly associated with vascular engorgement. As also the clinically unaffected eyes showed macular choroidal significant swelling, CSC may have a systemic component with usually asymmetric ocular involvement.
Introduction
Central serous chorioretinopathy (CSC) is a disease affecting predominantly men in the age group 20–50 years and is characterized by an exudative detachment of the retina, often in association with a serous detachment of the underlying retinal pigment epithelium (Laatikainen 1994; Wang et al. 2008; Ahlers et al. 2009; Gueudry et al. 2009). Increased stress and steroid application have been postulated to be associated or causative factors. Various treatment modalities have been suggested for the therapy of CSC, including photodynamic therapy, intravitreal injection of bevacizumab and systemic application of ketoconazole (Golshahi et al. 2010; Ruiz-Moreno et al. 2010; Lee et al. 2011; Pryds & Larsen 2012). Gass was one of the first to discuss an increased permeability of the choriocapillaris resulting in a retinal pigment epithelial detachment and accumulation of fluid into the subfoveal space (Gass 1967). Correspondingly, indocyanine green angiography has revealed a hyperpermeability and vascular congestion of the choroidal vessels in the macula region, in association with a generalized choroidal vascular disturbance (Guyer et al. 1994; Prünte & Flammer 1996; Spaide et al. 1996, 2003; Iida et al. 1999). Assessment of blood flow parameters showed a hyperdynamic circulation within the macular choroid in eyes with CSC (Tittl et al. 2005). In agreement with these findings, spectral domain optical coherence tomography with enhanced depth imaging revealed an increase in the thickness of the macular thickness in eyes with CSC (Spaide et al. 2008; Imamura et al. 2009; Manjunath et al. 2010; Maruko et al. 2010, 2011; Kim et al. 2011). It has been unclear so far, whether the increased thickness of the choroid in eyes with CSC was due to an inter-vascular oedema or due to an engorgement of the vessels in Haller′s and Sattler′s layers of the choroid. We therefore performed this study to assess the vessel dimensions of the choroid in eyes with the CSC as compared with unaffected contralateral eyes and with eyes of a control group of normal subjects.
Methods
The prospective comparative observational clinical study included patients with CSC who presented between March and September 2011 with unilateral loss of central visual acuity, metamorphopsias and characteristics of CSC in fluorescein angiography and spectral domain optical coherence tomography (OCT) such as serous detachment of the central macula and subfoveal leakage of fluorescein starting at the level of the retinal pigment epithelium. The study was approved by the Ethics Committee of the Beijing Tongren Hospital, and informed consent for the examinations was obtained from all patients. The affected eyes of the study group showed the subfoveal leaks in fluorescein angiography corresponding to a hyperfluorescence in indocyanine green angiography. Ophthalmoscopy revealed a circumscribed serous retinal detachment. To avoid the erroneous inclusion of patients with polypoidal choroidal vasculopathy into the study group, we specifically looked for signs typical for this disease, such as branching vascular networks or polypoidal lesions on angiograms and a double layer sign on optical coherent tomograms. These patients were excluded from the study. Other exclusion criteria were any history of corticosteroid intake, previous photodynamic therapy, intravitreal or systemic antivascular endothelial growth factor medications, intake of phosphodiesterase inhibitors such as sildenafil, any disease that might have influenced the choroidal circulation such as pregnancy or endogenous hypercortisolism, uncontrolled diabetes or arterial hypertension, a history of myopia of more than −5 dioptres (spherical equivalent), amblyopia, glaucoma, proliferative retinopathies of any type, epiretinal membranes, a history of any intraocular surgery or any other intraocular disorder such tapetoretinal dystrophy, ocular trauma and macular degenerations including large drusen. If the duration of the symptoms was <6 month, the CSC was classified as acute CSC; if the symptoms lasted for more than 6 months, the stage of the disease was classified as chronic. Among the 15 unaffected fellow eyes in the CSC study group, one eye had a history of acute CSC and two eyes had a history of chronic CSC. Although at the time of the study, these three eyes were free of symptoms of CSC upon fluorescein angiography and OCT examination, they were excluded from the statistical analysis. All patients included into the study experienced the symptoms in the affected study eyes for the first time. A control group included a similar number of patients matched for age, gender and refractive error with the patients of the study group. All eyes were examined using fluorescein angiography and indocyanine green angiography.
All eyes additionally underwent spectral domain OCT (Spectralis®; Heidelberg Engineering, Heidelberg, Germany) with enhanced depth imaging as described by Spaide and colleagues (Spaide et al. 2008). The patients were examined with the OCT device positioned close enough to the eye to produce an inverted image. Seven sections, each comprising 100 averaged scans, were obtained in a 5° × 30° rectangle centred on the macula, and the horizontal section going directly through the centre of the fovea was selected. The OCT images were acquired with the high-speed mode (768 a-scans). The resultant images were viewed and measured with the heidelberg eye explorer software (version 1.5.12.0, Heidelberg Engineering, Heidelberg, Germany). The choroid was measured from the outer portion of the hyper-reflective line corresponding to the retinal pigment epithelium to the inner surface of the sclera (Fig. 1). Within the choroid in the outer layer of Haller and in the intermediate layer of Sattler, we detected hyporeflective lumen in all eyes. Their internal reflective intensity appeared to be similar to the one of the subretinal fluid. The lumens in the choroidal layer were taken as surrogates for choroidal vessels, although that was an assumption. All OCT measurements were performed by two experienced examiners (LHY, WBW) independently of each other. In a first step, all discernible hyporeflective lumens within a zone with a width of 4500 μm centred on the fovea were assessed, and the largest lumen was localized. The region of measurement did not differ between the study group and the control group. In a second step, the diameter of this largest lumen was measured. The diameter of each hyporeflective lumen was assessed perpendicular to Bruch’s membrane always in the region of the widest diameter of the lumen (Fig. 1). The widest measured diameter was taken for the statistical analysis. If the measurements by the two examiners differed by more than 15%, the examiners re-performed the measurement together. If the difference between both measurements was ≤15%, the mean of the two values was used for the statistical analysis. Eyes with a poor image quality due to media opacification or fundus pigmentation were excluded. Eye-tracking function of the OCT device was used during the scan, and 100 scans were averaged by the automatic averaging programme.
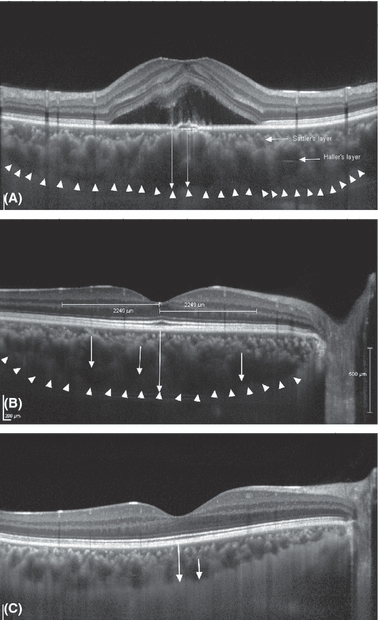
Optical coherence tomographic enhanced depth imaging (horizontal scan) of an eye with central serous chorioretinopathy. (A) Affected eye: The subfoveal choroidal thickness was measured vertically from the outer border of the retinal pigment epithelium to the inner border of the sclera (right long vertical white arrow). The hyporeflective lumens in the choroid layer were measured perpendicular to Bruch’s membrane in the region of its widest diameter (left long vertical white arrow). Horizontal arrows: vessels of Sattler′s layer and Haller′s layer; white arrow heads: choroidal–scleral border. (B) Contralateral unaffected eye of a patient with central serous chorioretinopathy: The subfoveal choroidal thickness was measured vertically from the outer border of the retinal pigment epithelium to the inner border of the sclera (long vertical white arrow). The hyporeflective lumens in the choroid layer were measured perpendicular to Bruch’s membrane in the region of its widest diameter (short vertical white arrows). White arrow heads: choroidal–scleral border. The horizontal lines indicate the region in which the hyporeflective lumen was measured. (C) Normal control eye: The long vertical white arrow indicates the subfoveal choroidal thickness; the short arrow indicates the diameter of hyporeflective lumen.
Statistical analysis was performed using spss for Windows, version 19.0 (IBM-SPSS, Chicago, IL, USA). The measurements were presented as mean ± standard deviation. As all measured parameters showed a Gaussian distribution as tested by the Kolmogorov–Smirnovtest, the study group was compared with control group using a student’s t-test for unpaired samples. For the inter-eye comparison in the study group between the affected eyes and the contralateral unaffected eyes, we used the student’s t-test for paired samples. Proportions were compared using the Chi-squared test. Pearson’s correlation coefficient was calculated to evaluate the correlation between the diameter of the largest hyporeflective lumen and choroidal thickness. A p-value of <0.05 was considered to be statistically significant.
Results
The study group included 15 eyes of 15 Chinese patients (13 men) with unilateral CSC. The mean age was 46.0 ± 8.0 years (median: 48 years; range: 34–61 years), and the mean refractive error was −0.27 ± 0.86 dioptres (median: 0.00 dioptres; range: −2.00 to +0.70 dioptres). The control group consisted of 15 eyes of 15 control subjects with a mean age of 46.5 ± 8.1 years (median: 49 years; range: 33–60 years), and the mean refractive error was −0.51 ± 1.27 dioptres (median: −0.74 dioptres; range: −3.00 to +2.00 dioptres). Due to matching, both groups did not vary significantly in age (p = 0.88), gender (p = 1.00) and refractive error (p = 0.54).
The mean subfoveal choroidal thickness was significantly (p = 0.04) larger in the affected eyes of the study group (455 ± 73 μm) than in the contralateral unaffected eyes (387 ± 94 μm), in which it was significantly (p = 0.005) larger than in the eyes of the control group (289 ± 71 μm). In a parallel manner, the mean diameter of the largest hyporeflective lumen was larger, but not significantly larger (p = 0.18) in the affected eyes of the study group (305 ± 101 μm) than in the in the contralateral unaffected eyes (251 ± 98 μm), in which it was significantly (p = 0.001) larger than in the eyes of the control group (140 ± 40 μm) (Fig. 2). The diameter of the largest lumen was significantly correlated with the thickness of the total choroid (p < 0.001; correlation coefficient r: 0.73) (Fig. 3).
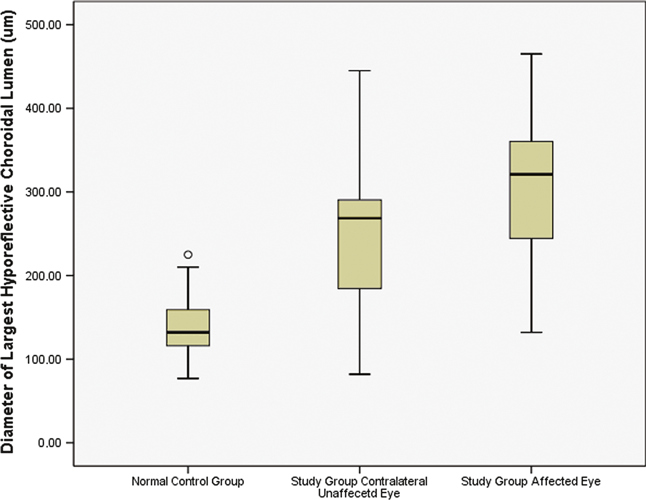
Boxplots showing the largest diameters of hyporeflective lumen in the choroidal layer in affected eyes of patients with central serous chorioretinopathy, the contralateral clinically unaffected eyes of these patients, and a normal control group matched for age, gender and refractive error with the study group. Each boxplot shows the lowest measurement still within 1.5 inter-quartile range of the lower quartile, the lower quartile (bottom of the box), the median (line in the box), the upper quartile (top of the box), the highest measurement still within the 1.5 inter-quartile range of the upper quartile, and an outlier (open circle).
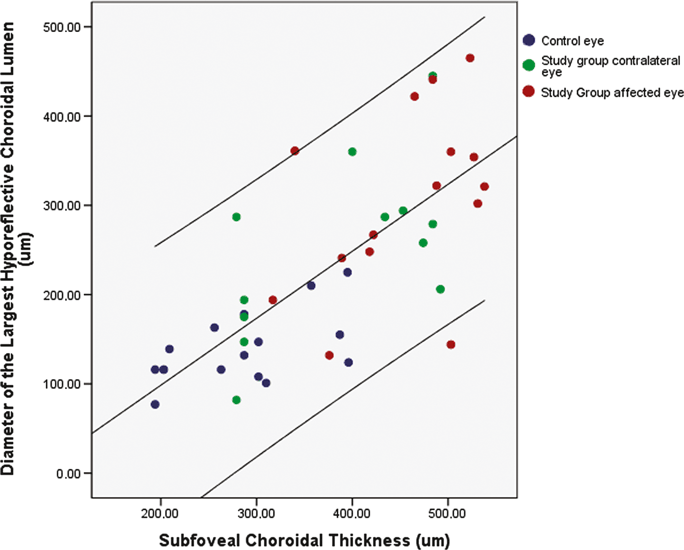
Scattergram showing the correlation between choroidal thickness and the largest diameter of hyporeflective choroidal lumen of eyes affected by central serous chorioretinopathy, contralateral unaffected eyes and normal eyes of a control group (p < 0.001; correlation coefficient r: 0.73; equation of the regression line: Largest Diameter of Choroidal Hyporeflective Lumen (μm) = 0.75 × Choroidal Thickness (μm) – 52 μm); the upper line and lower line indicate the 95% confidence interval.
Discussion
The results of our study demonstrated that in patients with CSC, not only choroidal thickness but also the hyporeflective lumen was larger in the affected eyes and in the asymptomatic contralateral clinically unaffected eyes than in normal eyes of a control group. If one takes the hyporeflective lumen as surrogate for the large choroidal vessels, one may assume that the increased macular choroid thickness in eyes with CSC was mainly due to a dilatation of vessels and that it was not markedly associated with an interstitial oedema. Correspondingly, the thickness of the choroid was correlated with the largest diameter of the hyporeflective lumen. Interestingly, the contralateral clinically unaffected eye of the patients with CSC also showed a significantly increased choroidal thickness as compared to a control group matched for age, gender and refractive error with the study group.
The results of our study agree with previous investigations on an increased choroidal thickness in eyes with CSC as compared to the contralateral eyes of the same patients and as compared to normal control subjects (Spaide et al. 2008; Imamura et al. 2009; Manjunath et al. 2010; Maruko et al. 2010, 2011; Kim et al. 2011). Our results extended the previous observation in that the increased choroidal thickness is mainly due to a dilatation of the choroidal vessels. In a similar manner, studies using indocyanine green angiography reported on congested and dilated choroidal vessels close to the fovea in eyes with CSC (Guyer et al. 1994; Spaide et al. 1996).
Besides the vessel dilatation, an additional role may be played by an interstitial choroidal oedema, because islands of hyperfluorescence were observed on indocyanine angiography in the patients of our study as well as in patients of other studies (Spaide et al. 1996). Hyperpermeability of the choriocapillaris has been considered to be responsible for a focal hyperfluorescence of the choroid. In addition, indocyanine angiographies have suggested a dilatation of the choroidal vessels (Spaide et al. 1996).
The measured diameter of the largest hyporeflective lumen in the choroidal layer was 140 ± 40 μm in the control group (range: 77–225 μm). These figures agree with reported diameters of choroidal vessels as measured by histomorphometry in which the largest vessels had a diameter of up to 300 μm (Jakobiec 1982). Correspondingly, the mean thickness of the subfoveal choroid in the control group of 289 ± 71 μm compared well with the mean thickness of the subfoveal choroid as reported in a pilot study by Margolis and Spaide (287 ± 71 μm) (Margolis & Spaide 2009) and as recently measured in a larger group of healthy Chinese subjects (262 ± 88 μm) (Ding et al. 2011). These comparisons may serve to validate the data obtained in our study.
The reasons have remained unclear why the choroidal vessels showed a dilatation with secondary choroidal thickening in patients with CSC, more marked in the affected eyes than in the contralateral clinically unaffected eyes. As choroidal vessels are innervated by the autonomic system and as ganglion cells have been found in the posterior choroid, the dilatation of the choroidal vessels may have been regulated by innervation (Bergua et al. 1994; May & Lütjen-Drecoll 2005). Additionally, substances such as endothelin-1 or inflammatory cytokines may participate in the pathogenesis of the choroidal swelling in eyes with CSC, because endothelin-1 has been reported to be involved in the autoregulation of the choroidal vasculature (Fuchsjäger-Mayrl et al. 2003; Delgado et al. 2010).
Potential limitations of our study should be mentioned. First, it was a hospital-based study with a relatively small sample size. Despite the relatively small number of patients included into the study, however, the differences between the study and the control group were statistically significant. In addition, the measurements in the contralateral clinically unaffected eyes of the patients with CSC showed an intermediary position between the affected eyes and the normal eyes of the control group. Second, the enhanced depth imaging mode of the OCT technique is an indirect method to image and measure the choroidal vessel. Third, we assumed that the detected lumens in the choroidal layer were the choroidal vessels, with some evidence by comparing histomorphometric measurements of the vessel diameter with our data obtained by the OCT. Fourth, although enhanced depth imaging has provided better visualization of the inner and outer borders of the choroid and in some instances has allowed the differentiation of the layers of Sattler and Haller, this is usually not achieved in all eyes examined in clinical routine. Opacifications of the optic media of the eye, fundus pigmentation, motion artefacts and the lack of adequate depth penetration at a laser wavelength of 840 nm as compared to ‘high-penetration’ OCT prototypes using a laser wavelength of >1000 nm limited the detailed visualization of the choroid. In addition, there may be an effect role of shadowing by the large choroidal vessels as seen with retinal blood vessels on OCT imaging. However, as the large choroidal lumen which one may assume to be large choroidal vessels, showed a mostly circular cross-section and often touched the sclera with their outer wall, it may be unlikely that these structures shadowed other structures beneath them (Fig. 1). Fifth, there is a large range of the choroidal thickness measurements and of the diameter of the largest lumen (2, 3). It reflects the biological variation of the two parameters and also the noise in the examination techniques. Despite the wide range of the measurements, however, the differences between the groups were statistically significant, so that the wide range may finally serve to strengthen the conclusions of the study. Sixth, only one vessel in one OCT scan and for a relatively small study group of 15 patients was measured. In the light of the variation of the choroidal architecture, future studies may be designed to include more patients and the assessment of more vessels per scan and eye.
In conclusion, in patients with CSC, the affected eyes show larger or dilated hyporeflective lumen in the affected eyes than in the contralateral clinically unaffected eyes and significantly larger than in normal control eyes. Assuming these hyporeflective lumens to be choroidal vessels, macular swelling in CSC is markedly associated with vascular engorgement. As also the clinically unaffected eyes showed macular choroidal significant swelling, CSC may have a systemic component with usually asymmetric ocular involvement.




