Initial experience with CytoSorb therapy in patients receiving left ventricular assist devices
Konstantin Zhigalov, Jef Van den Eynde, Alina Zubarevich, Thomas Chrosch, Lukas Goerdt, and Arian Arjomandi Rad contributed equally to the manuscript.
Abstract
Background
The use of left ventricular assist devices (LVAD) in patients with advance heart failure is still associated with an important risk of immune dysregulation and infections. The aim of this study was to determine whether extracorporeal blood purification using the CytoSorb device benefits patients after LVAD implantation in terms of complications and overall survival.
Materials and Methods
Between August 2010 and January 2020, 207 consecutive patients underwent LVAD implantation, of whom 72 underwent CytoSorb therapy and 135 did not. Overall survival, major adverse events, and laboratory parameters were compared between 112 propensity score-matched patients (CytoSorb: 72 patients; non-CytoSorb: 40 patients).
Results
WBC (p = .033), CRP (p = .001), and IL-6 (p < .001), significantly increased with LVAD implantation, while CytoSorb did not influence this response. In-hospital mortality and overall survival during follow-up were similar with CytoSorb. However, patients treated with CytoSorb were more likely to develop respiratory failure (54.2% vs. 30.0%, p = .024), need mechanical ventilation for longer than 6 days post-implant (50.0% vs. 27.5%, p = .035), and require tracheostomy during hospitalization (31.9% vs. 12.5%, p = .040). No other significant differences were observed with regard to major adverse events during follow-up.
Conclusions
Overall, our results showed that CytoSorb might not convey a significant morbidity or mortality benefit for patients undergoing LVAD implantation.
Abbreviations
-
- ALT
-
- alanine transaminase
-
- BMI
-
- body mass index
-
- BSA
-
- body surface area
-
- BUN
-
- blood urea nitrogen
-
- CRP
-
- C-reactive protein
-
- ECLS
-
- extracorporeal life support
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- HR
-
- hazard ratio
-
- IL-6
-
- interleukin 6
-
- INTERMACS
-
- Interagency Registry for Mechanically Assisted Circulatory Support
-
- IQR
-
- interquartile range
-
- LDH
-
- lactate dehydrogenase
-
- LVAD
-
- left ventricular assist device
-
- NYHA
-
- New York Heart Association
-
- PCT
-
- procalcitonin
-
- PRBC
-
- packed red blood cells
-
- ST-RVAD
-
- short-term right ventricular assist device
-
- WBC
-
- white blood cell
1 BACKGROUND
In patients with end-stage heart failure, implantable left ventricular assistive devices (LVAD) have emerged as a treatment option to bridge critically ill patients to heart transplants, known as bridge-to-transplant therapy, or to temporarily support patients until their cardiac functions recovers, known as bridge-to-recovery therapy.1 However, due to the presence of hardware, driveline exposure, surgical stress, and the inflammatory state associated with heart failure, patients with LVAD are at an increased risk of immune dysregulation and infectious complications.2, 3 In particular, elevation in plasma interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-α (TNFα) levels between pre- and post-surgery has been associated with worse post-implantation morbidity and mortality, due in large part to the development of multiorgan failure.4 Therefore, in order to attenuate the elevated pro-inflammatory cytokine levels and the associated adverse clinical outcomes, it has been postulated that extracorporeal blood purification could improve the overall survival rate and morbidity of patients undergoing LVAD implantation.
CytoSorb is a recently licensed extracorporeal hemoadsorption blood purification device that contains biocompatible polystyrene divinylbenzene beads which are capable of adsorbing medium molecular weight molecules—such as pro- and anti-inflammatory cytokines, bilirubin, myoglobin, exotoxins, and certain drugs—and through a combination of hydrophobic interactions and size exclusion, removing them from circulation.5
Literature suggests that CytoSorb use is associated with improved clinical outcomes in cardiac surgery when the cartridges have been inserted into the cardiopulmonary bypass (CPB) circuit for the removal of inflammatory cytokines in the blood. Promising results have been demonstrated in heart transplantation, surgical management of acute infective endocarditis, and in patients with severe post-CPB systemic inflammation response syndrome.5-11 However, there remains a lack of literature surrounding the use of CytoSorb therapy in the implantation of LVAD and its efficacy in the realm of survival and morbidity.
With this in mind, the aim of this study was to determine whether CytoSorb therapy benefits patients after LVAD implantation in terms of complications and overall survival. A retrospective, nonrandomized, single institution study comparing patients who received implantation of a LVAD with or without concomitant postoperative CytoSorb therapy was performed and presented.
2 MATERIALS AND METHODS
2.1 Study population and design
This study conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the local Ethical Committee (20-9283-BO). We retrospectively reviewed the data of 207 consecutive patients who underwent LVAD implantation at our institution between August 2010 and January 2020. All patients who received either HeartMate III™ (St. Jude Medical—Abbott, MN, USA) or a HeartWare™ (Medtronic, MN, USA) LVAD devices were eligible. Intraoperatively, the Cytosorb filter was used at the discretion of the operators depending on the following clinical criteria at the day of surgery: fever, severely elevated inflammatory markers, and/or hemodynamic instability requiring inotropic support. Cytosorb filter was included via side arm directly into the CPB circuit and the filtration was maintained over the whole CPB-time.
Data were obtained from a prospectively collected institutional LVAD database that includes detailed information on patients’ demographics, baseline clinical characteristics, and their laboratory and hemodynamic parameters, as well as intraoperative variables and postoperative outcomes. Patients were followed up based on information available in their electronic medical records, as well as through telephone interviews. Minimal period of follow-up for all patients was until discharge or death.
The study population was divided into two groups: Group 1—patients who received CytoSorb therapy (n = 72) and Group 2—patients who did not receive CytoSorb therapy (n = 135). Baseline patient characteristics and intraoperative data were collected and compared between groups. Laboratory parameters including white blood cell (WBC) count, C-reactive protein (CRP), creatinine, blood urea nitrogen (BUN), total bilirubin, alanine transaminase (ALT), lactate dehydrogenase (LDH), procalcitonin (PCT), and interleukin 6 (IL-6) were also collected, both preoperative and postoperative (within 7 days following surgery).
2.2 Outcome measures
The primary endpoint was overall survival after LVAD implantation. Secondary endpoints were major adverse events in-hospital and during follow-up. Changes in laboratory parameters were also investigated.
2.3 Statistical analysis
Continuous variables were checked for normality using the Shapiro-Wilk test and the difference between groups was tested with the t-test or Mann-Whitney U-test accordingly. Categorical variables are expressed as frequency and proportion, and differences were assessed with the chi-square test. To account for baseline differences between groups, genetic propensity score matching was performed using the R package “MatchIt” (v 3.0.2.). Matching factors included all unbalanced variables (p ≤ .1), that is, coronary artery disease, hyperlipidemia, intra-aortic balloon pump, operative time, CPB time, HeatWare LVAD model, tricuspid valve surgery, ventricular septum defect closure, preoperative creatinine, and preoperative blood urea nitrogen. Two-way ANOVA was used to determine the effect of LVAD implantation and CytoSorb therapy on the levels of laboratory parameters. Kaplan–Meier curves were used to study the effect of CytoSorb therapy on survival during follow-up, with the log-rank test used for statistical significance in this estimate. To study the occurrence of major adverse events during follow-up while taking into account the different follow-up of the two cohorts, incidence rates per person-year were calculated and compared. A p-value of < .05 was considered statistically significant. All analyses were performed using R Statistical Software (version 4.0.2. 2020-06-22, Foundation for Statistical Computing, Vienna, Austria).
3 RESULTS
3.1 Baseline characteristics and intraoperative data
A total of 207 patient with end-stage heart failure underwent implantation with a LVAD at our institution between August 2010 and January 2020. Baseline characteristics and intraoperative data are given in Tables 1 and 2, respectively. Before propensity score matching, patients in the CytoSorb group had a lower proportion hyperlipidemia (36.1% vs. 55.6%, p = .008) and were less often on intra-aortic balloon pump (5.6% vs. 17.0%, p = .019). They also had shorter operative times [174 min (149–238) vs. 214 min (185–256), p = .001] and CPB time [72 min (62–99) vs. 85 min (73–108), p < .001]. Patients who received CytoSorb also more commonly received Heartmate III (22.2% vs. 3.7%, p < .001) and had concomitant tricuspid valve surgery (6.9% vs. 0.7%, p = .011). Propensity matching resulted in a selection of 72 patients in the CytoSorb group and 40 patients in the non-CytoSorb group; baseline and intraoperative variables were comparable between the two groups.
| Characteristics | Non-matched (n = 207) | Matched (n = 112) | ||||
|---|---|---|---|---|---|---|
| CytoSorb (n = 72) | Non-CytoSorb (n = 135) | p-value | CytoSorb (n = 72) | Non-CytoSorb (n = 40) | p-value | |
| Demographic data | ||||||
| Age, years | 58 (48–65) | 60 (53–65) | .217 | 56.3 ± 12.3 | 57.9 ± 9.5 | .448 |
| Female, n (%) | 11 (15.3) | 26 (19.3) | .476 | 11 (15.3) | 9 (22.5) | .485 |
| BMI, kg/m2 | 25 (22–29) | 26 (23–29) | .171 | 24.6 (22.2–28.4) | 25.6 (23.5–27.9) | .625 |
| BSA, m² | 1.96 (1.81–2.19) | 1.98 (1.83–2.13) | .809 | 1.99 ± 0.25 | 1.95 ± 0.23 | .385 |
| Comorbidities, n (%) | ||||||
| Arterial hypertension | 45 (62.5) | 96 (71.1) | .205 | 45 (62.5) | 28 (70.0) | .554 |
| Coronary artery disease | 36 (50) | 86 (63.7) | .056 | 36 (50.0) | 22 (55.0) | .756 |
| Hyperlipidemia | 26 (36.1) | 75 (55.6) | .008 | 26 (36.1) | 17 (42.5) | .643 |
| Smoking history | 39 (54.2) | 82 (60.7) | .361 | 39 (54.2) | 26 (65.0) | .361 |
| Atrial fibrillation | 28 (38.9) | 58 (43.0) | .571 | 28 (38.9) | 20 (50.0) | .348 |
| Diabetes | 24 (33.3) | 47 (34.8) | .831 | 24 (33.3) | 10 (25.0) | .481 |
| Disease of peripheral arteries | 12 (16.7) | 17 (12.6) | .421 | 12 (16.7) | 4 (10.0) | .494 |
| Chronic obstructive pulmonary disease | 13 (18.1) | 32 (23.7) | .348 | 13 (18.1) | 7 (17.5) | 1.000 |
| Stroke | 6 (8.3) | 7 (5.2) | .374 | 6 (8.33) | 2 (5.00) | .709 |
| Infection | 19 (26.4) | 30 (22.2) | .502 | 19 (26.4) | 12 (30.0) | .850 |
| Myocardial infarction | 32 (44.4) | 60 (44.4) | 1.000 | 32 (44.4) | 18 (45.0) | 1.000 |
| Percutaneous cardiac intervention | 31 (43.1) | 47 (34.8) | .244 | 31 (43.1) | 12 (30.0) | .247 |
| Cardiac resynchronization therapy | 28 (38.9) | 45 (33.3) | .426 | 28 (38.9) | 14 (35.0) | .839 |
| Implantable cardioverter defibrillator | 38 (52.8) | 86 (63.7) | .127 | 38 (52.8) | 26 (65.0) | .292 |
| Pulmonary hypertension | 34 (47.2) | 71 (52.6) | .462 | 34 (47.2) | 19 (47.5) | 1.000 |
| Preoperative pericardial effusion | 10 (13.9) | 11 (8.1) | .193 | 39 (54.2) | 26 (65.0) | .207 |
| Primary diagnosis, n (%) | ||||||
| Ischemic cardiomyopathy | 33 (45.8) | 68 (50.4) | .534 | 33 (45.8) | 17 (42.5) | .887 |
| Dilated cardiomyopathy | 36 (50.0) | 63 (46.6) | .647 | 36 (50.0) | 21 (52.5) | .955 |
| Toxic cardiomyopathy | 2 (2.8) | 2 (1.5) | .519 | 2 (2.78) | 1 (2.50) | 1.000 |
| Other indication | 1 (1.40) | 2 (1.5) | .958 | 1 (1.39) | 1 (2.50) | 1.000 |
| Cardiorespiratory conditions | ||||||
| Mechanical ventilation, n (%) | 17 (23.6) | 27 (20.0) | .545 | 17 (23.6) | 7 (17.5) | .607 |
| Intra-aortic balloon pump, n (%) | 4 (5.6) | 23 (17.0) | .019 | 4 (5.56) | 4 (10.0) | .453 |
| ECLS, n (%) | 14 (19.4) | 28 (20.7) | .825 | 14 (19.4) | 9 (22.5) | .889 |
| Ejection fraction, % | 15 (14–20) | 15 (10–20) | .766 | 16.5 (15.0–21.2) | 15.0 (10.0–22.0) | .316 |
| INTERMACS profile, n (%) | ||||||
| 1–2 | 40 (55.6) | 67 (49.6) | .416 | 40 (55.6) | 18 (45.0) | .382 |
| 3–5 | 32 (44.4) | 68 (50.4) | .416 | 32 (44.4) | 22 (55.0) | .382 |
| Device strategy at the time of implantation, n (%) | ||||||
| Destination therapy | 54 (75.0) | 98 (72.6) | .709 | 54 (75.0) | 28 (70.0) | .726 |
| Bridge to candidacy | 6 (8.3) | 6 (4.4) | .254 | 6 (8.33) | 3 (7.50) | 1.000 |
| Bridge to transplant | 12 (16.7) | 31 (23.0) | .288 | 12 (16.7) | 9 (22.5) | .613 |
- Abbreviations: BMI, body mass index; BSA, body surface area; ECLS, extra corporeal life support; TAPSE, tricuspid annular plane systolic excursion.
| Characteristics | Non-matched (n = 207) | Matched (n = 112) | ||||
|---|---|---|---|---|---|---|
| CytoSorb (n = 72) | Non-CytoSorb (n = 135) | p-value | CytoSorb (n = 72) | Non-CytoSorb (n = 40) | p-value | |
| Durations, min | ||||||
| Operation | 174 (149–238) | 214 (185–256) | .001 | 174 (149–234) | 188 (163–236) | .446 |
| Cardiopulmonary bypass | 72 (62–99) | 85 (73–108) | <.001 | 72.5 (61.8–99.5) | 73.0 (65.8–98.5) | .729 |
| LVAD model, n (%) | ||||||
| Heartmate III | 16 (22.2) | 5 (3.7) | <.001 | 16 (22.2) | 4 (10.0) | .174 |
| HeartWare | 56 (77.8) | 130 (96.3) | <.001 | 56 (77.8) | 36 (90.0) | .174 |
| Isolated procedure, n (%) | 59 (81.9) | 120 (88.9) | .164 | 59 (81.9) | 36 (90.0) | .388 |
| Concomitant procedures (also in various combinations), n (%) | 13 (18.1) | 15 (11.1) | .164 | 13 (18.1) | 4 (10.0) | .388 |
| Tricuspid valve surgery | 5 (6.9) | 1 (0.7) | .011 | 5 (6.94) | 1 (2.50) | .418 |
| Atrium septum defect closure | 1 (1.4) | 1 (0.7) | .650 | 1 (1.39) | 0 (0.0) | 1.000 |
| Aortic valve replacement | 4 (5.6) | 6 (4.4) | .723 | 4 (5.56) | 1 (2.50) | .653 |
| Left ventricular aneurysm | 1 (1.4) | 1 (0.7) | .650 | 1 (1.39) | 0 (0.0) | 1.000 |
| Coronary artery bypass graft | 0 (0.0) | 2 (1.5) | .299 | 0 (0.0) | 0 (0.0) | 1.000 |
| Ventricular septum defect closure | 2 (2.8) | 0 (0.0) | .052 | 2 (2.78) | 0 (0.0) | .537 |
- Abbreviation: LVAD, left ventricular assist device.
3.2 Laboratory parameters
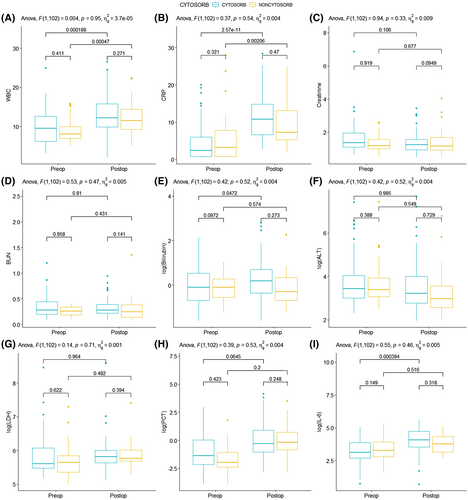
Pre- and postoperative laboratory values are presented in Table 3. A graphical presentation with pairwise comparisons is given in Figure 1. All laboratory parameters were similar between both groups (Figure 1). Likewise, two-way ANOVA showed that CytoSorb therapy had no influence of laboratory parameters. LVAD implantation was associated with a significant increase in WBC (p = .033) and CRP (p = .001), and IL6 (p < .001), whereas all other parameters remained stable. No significant interaction terms were found between CytoSorb and timepoint (pre- vs. postoperative), indicating that CytoSorb therapy did not modify the response of the studied laboratory parameters to LVAD implantation.
| Characteristics | CytoSorb (n = 72) | Non-CytoSorb (n = 40) | ANOVA model | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | p-value CytoSorb | p-value timepoint | p-value interaction | |
| WBC, ×109/L | 10.9 ± 5.08 | 13.0 ± 5.10 | 10.7 ± 4.16 | 13.0 ± 4.74 | .947 | .033 | .951 |
| CRP, mg/L | 6.97 ± 5.53 | 11.6 ± 5.74 | 8.76 ± 8.84 | 11.5 ± 7.92 | .707 | .001 | .545 |
| Creatinine, mg/dl | 1.47 ± 0.45 | 1.46 ± 0.66 | 1.13 ± 0.50 | 1.44 ± 0.94 | .377 | .634 | .333 |
| BUN, mg/dl | 0.34 ± 0.16 | 0.35 ± 0.32 | 0.26 ± 0.12 | 0.34 ± 0.32 | .538 | .522 | .470 |
| Total bilirubin, mmol/L | 1.63 ± 1.72 | 2.50 ± 3.73 | 1.18 ± 0.75 | 1.63 ± 2.30 | .302 | .200 | .776 |
| ALT, U/L | 161.8 ± 345.4 | 126.4 ± 349.3 | 243.1 ± 586.9 | 93.8 ± 223.0 | .933 | .409 | .516 |
| LDH, U/L | 427.1 ± 298.5 | 465.5 ± 700.0 | 482.4 ± 432.2 | 416.8 ± 356.5 | .924 | .889 | .706 |
| PCT | 1.44 ± 3.55 | 4.66 ± 11.5 | 1.03 ± 2.04 | 1.59 ± 2.31 | .292 | .135 | .534 |
| IL-6 | 67.7 ± 48.85 | 82.9 ± 64.7 | 39.6 ± 37.1 | 35.0 ± 34.7 | .513 | <.001 | .459 |
- Abbreviations: ALT, alanine transaminase; BUN, blood urea nitrogen; CRP, C-reactive protein; IL-6, interleukin 6; LDH, Lactate dehydrogenase; PCT, procalcitonin; WBC, white blood cell count.
3.3 Overall survival
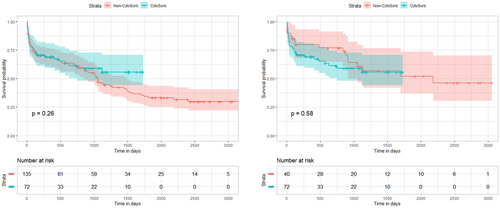
In-hospital mortality occurred in 19 (26.4%) patients who received CytoSorb, in contrast to 6 (15.0%) patients in the matched cohort of patients who did not receive CytoSorb (p = .250) (Table 4). Over a median follow-up of 1.18 years (interquartile range (IQR) 0.30–3.04 years) in the CytoSorb group and 2.67 years (IQR 0.37–5.33, p = .021) in the non-CytoSorb group, there was no difference in overall survival between both groups (log rank: p = .580) (Figure 1). Causes of death were similar between both groups (Table 4).
| Characteristics | Non-matched (n = 207) | Matched (n = 112) | ||||
|---|---|---|---|---|---|---|
| CytoSorb (n = 72) | Non-CytoSorb (n = 135) | p-value | CytoSorb (n = 72) | Non-CytoSorb (n = 40) | p-value | |
| Mortality, n (%) | ||||||
| In-hospital mortality | 19 (26.4%) | 30 (22.2%) | .617 | 19 (26.4%) | 6 (15.0%) | .250 |
| Survival, % | ||||||
| Follow-up period, years | 1.18 (0.30–3.04) | 2.29 (0.22–4.13) | .091 | 1.18 (0.30–3.04) | 2.67 (0.37–5.33) | .021 |
| 6 months | 70.8% (61.0%–82.1%) | 70.8% (63.4%–78.9%) | 70.8% (61.0%–82.1%) | 79.7% (68.0%–93.3%) | ||
| 1 year | 69.1% (59.2%–80.8%) | 67.6% (60.1%–76.8%) | 69.1% (59.2%–80.8%) | 79.7% (68.0%–93.3%) | ||
| 2 year | 61.3% (50.4%–74.5%) | 61.2% (53.3%–70.2%) | 61.3% (50.4%–74.5%) | 76.9% (64.8%–91.4%) | ||
| 3 year | 59.1% (48.0%–70.7%) | 46.5% (38.4%–56.4%) | 59.1% (48.0%–70.7%) | 60.8% (46.5%–79.6%) | ||
| Causes of death, n (%) | ||||||
| Cardiopulmonary failure | 9 (12.5) | 15 (11.1) | .766 | 9 (12.5) | 4 (10.0) | .768 |
| Infection | 6 (8.3) | 23 (17.0) | .086 | 6 (8.3) | 5 (12.5) | .518 |
| Cerebrovascular accident | 7 (9.7) | 19 (14.1) | .368 | 7 (9.7) | 3 (7.50) | 1.000 |
| Multiorgan failure | 13 (18.1) | 36 (26.7) | .165 | 13 (18.1) | 7 (17.5) | 1.000 |
| Bleeding | 1 (1.4) | 4 (3.0) | .482 | 1 (1.4) | 1 (2.50) | 1.000 |
| Unknown | 2 (2.8) | 15 (11.1) | .038 | 2 (2.8) | 2 (5.0) | .616 |
3.4 Major adverse events
In-hospital major adverse events are given in Table 5. In the matched analyses, the incidence of respiratory failure (54.2% vs. 30.0%, p = .024), the need for mechanical ventilation for longer than 6 days post implant (50.0% vs. 27.5%, p = .035), and tracheostomy (31.9% vs. 12.5%, p = .040) were all higher in patients who received CytoSorb therapy (Table 6). No other significant differences were observed with regard to major adverse events (Figure 2).
| Characteristics | Non-matched (n = 207) | Matched (n = 112) | ||||
|---|---|---|---|---|---|---|
| CytoSorb (n = 72) | Non-CytoSorb (n = 135) | p-value | CytoSorb (n = 72) | Non-CytoSorb (n = 40) | p-value | |
| Major bleeding, n (%) | ||||||
| Need for Revision | 18 (25.0) | 27 (20.0) | .406 | 18 (25.0) | 6 (15.0) | .319 |
| Major infection, n (%) | 28 (38.9) | 39 (28.9) | .143 | 28 (38.9) | 8 (20.0) | .066 |
| Localized non-device infection | 11 (15.3) | 11 (8.1) | .113 | 11 (15.3) | 2 (5.0) | .131 |
| Driveline infection | 3 (4.2) | 2 (1.5) | .231 | 3 (4.17) | 0 (0.0) | .551 |
| Pneumonia | 13 (18.1) | 21 (15.6) | .644 | 13 (18.1) | 5 (12.5) | .618 |
| Sepsis | 14 (19.4) | 21 (15.6) | .477 | 14 (19.4) | 4 (10.0) | .300 |
| Respiratory failure, n (%) | 39 (54.2) | 48 (35.6) | .010 | 39 (54.2) | 12 (30.0) | .024 |
| Ventilation over six days post implant | 36 (50.0) | 47 (34.8) | .034 | 36 (50.0) | 11 (27.5) | .035 |
| Reintubation | 17 (23.6) | 22 (16.3) | .200 | 17 (23.6) | 4 (10.0) | .130 |
| Tracheostomy | 23 (31.9) | 29 (21.5) | .098 | 23 (31.9) | 5 (12.5) | .040 |
| VV-ECLS | 1 (1.4) | 10 (7.4) | .066 | 1 (1.39) | 2 (5.00) | .290 |
| Right heart failure, n (%) | ||||||
| Mild right heart failure | 15 (20.8) | 15 (11.1) | .058 | 15 (20.8) | 8 (20.0) | 1.000 |
| Moderate right heart failure | 17 (23.6) | 24 (17.8) | .316 | 17 (23.6) | 4 (10.0) | .130 |
| Severe right heart failure | 10 (13.9) | 24 (17.8) | .472 | 10 (13.9) | 5 (12.5) | 1.000 |
| ST-RVAD | 3 (4.2) | 8 (5.9) | .591 | 3 (4.17) | 2 (5.00) | 1.000 |
| Hepatic dysfunction, n (%) | 6 (8.3) | 14 (10.4) | .637 | 6 (8.33) | 2 (5.00) | .709 |
| Acute renal dysfunction, n (%) | 30 (41.7) | 62 (45.9) | .557 | 30 (41.7) | 15 (37.5) | .818 |
| Neurological dysfunction, n (%) | 5 (6.9) | 12 (8.9) | .627 | 5 (6.94) | 4 (10.0) | .719 |
| Ischemic stroke | 4 (5.6) | 8 (5.9) | .914 | 4 (5.56) | 3 (7.50) | .699 |
| Intracranial hemorrhage | 2 (2.8) | 5 (3.7) | .726 | 2 (2.78) | 1 (2.50) | 1.000 |
| Psychiatric episode, n (%) | 0 (0.0) | 6 (4.4) | .069 | 0 (0.0) | 4 (10.0) | .015 |
| Hemolysis, n (%) | 1 (1.4) | 1 (0.7) | .650 | 1 (1.39) | 0 (0.0) | 1.000 |
| LVAD thrombosis, n (%) | 4 (5.6) | 3 (2.2) | .206 | 4 (5.56) | 1 (2.50) | .653 |
| Thromboemboly, n (%) | 2 (2.8) | 0 (0.0) | .052 | 2 (2.78) | 0 (0.0) | .537 |
- Abbreviations: LVAD, left ventricular assist device; PRBC, packed red blood cells; ST-RVAD, short-term right ventricular assist device; VV-ECLS, veno-venous extracorporeal life support.
| Characteristics | Non-matched (n = 207) | Matched (n = 112) | |||||
|---|---|---|---|---|---|---|---|
| CytoSorb (n = 72) | Non-CytoSorb (n = 135) | p-value | CytoSorb (n = 72) | Non-CytoSorb (n = 40) | p-value | ||
| FU length in person-years | 122.92 | 363.92 | 122.92 | 126.17 | |||
| FU outcome | |||||||
| Death | Cases, n (%) | 28 (38.9) | 81 (60.0) | 28 (38.9) | 17 (42.5) | ||
| Rate/1000 person-years (95% CI) | 0.23 (0.14–0.31) | 0.22 (0.17–0.27) | .916 | 0.23 (0.14–0.31) | 0.13 (0.07–0.20) | .085 | |
| Ongoing LVAD support | Cases, n (%) | 42 (58.3) | 42 (31.3) | 42 (58.3) | 20 (50.0) | ||
| Rate/1000 person-years (95% CI) | 0.34 (0.24–0.45) | 0.12 (0.08–0.15) | <.001 | 0.34 (0.24–0.45) | 0.16 (0.09–0.23) | .004 | |
| LVAD exchanged | Cases, n (%) | 3 (4.2) | 3 (2.2) | 3 (4.2) | 2 (5.0) | ||
| Rate/1000 person-years (95% CI) | 0.02 (−0.003 to 0.05) | 0.008 (−0.001 to 0.02) | .227 | 0.24 (−0.003 to 0.05) | 0.02 (−0.006 to 0.04) | .635 | |
| Weaned of LVAD | Cases, n (%) | 0 (0.0) | 5 (3.7) | 0 (0.0) | 2 (5.0) | ||
| Rate/1000 person-years (95% CI) | 0 (0–0) | 0.02 (0.002–0.03) | .025 | 0 (0–0) | 0.02 (−0.006 to 0.04) | .157 | |
| Heart transplant | Cases, n (%) | 3 (4.2) | 5 (3.7) | 3 (4.2) | 1 (2.5) | ||
| Rate/1000 person-years (95% CI) | 0.02 (−0.003 to 0.05) | 0.01 (0.002–0.03) | .488 | 0.02 (−0.003 to 0.05) | 0.008 (−0.008 to 0.02) | .308 | |
| FU adverse events | |||||||
| Ischemic stroke | Cases, n (%) | 6 (8.3) | 17 (12.6) | 6 (8.3) | 5 (12.5) | ||
| Rate/1000 person-years (95% CI) | 0.05 (0.01–0.08) | 0.05 (0.02–0.07) | .927 | 0.05 (0.01–0.09) | 0.04 (0.05–0.07) | .730 | |
| Intracranial hemorrhage | Cases, n (%) | 8 (11.1) | 22 (16.3) | 8 (11.1) | 5 (12.5) | ||
| Rate/1000 person-years (95% CI) | 0.07 (0.02–0.11) | 0.06 (0.04–0.09) | .860 | 0.07 (0.02–0.10) | 0.04 (0.01–0.07) | .381 | |
| Thoracic bleeding | Cases, n (%) | 6 (8.3) | 15 (11.1) | 6 (8.3) | 3 (7.5) | ||
| Rate/1000 person-years (95% CI) | 0.05 (0.01–0.09) | 0.04 (0.02–0.06) | .737 | 0.05 (0.01–0.09) | 0.02 (−0.003 to 0.05) | .301 | |
| Gastro-intestinal bleeding | Cases, n (%) | 12 (16.7) | 22 (16.3) | 12 (16.7) | 8 (20.0) | ||
| Rate/1000 person-years (95% CI) | 0.10 (0.04–0.15) | 0.06 (0.04–0.09) | .230 | 0.1 (0.04–0.15) | 0.06 (0.02–0.11) | .342 | |
| LVAD thrombosis | Cases, n (%) | 14 (19.4) | 20 (14.8) | 14 (19.4) | 9 (22.5) | ||
| Rate/1000 person-years (95% CI) | 0.11 (0.05–0.17) | 0.05 (0.03–0.08) | .073 | 0.11 (0.05–0.17) | 0.07 (0.02–0.12) | .271 | |
| Driveline infection | Cases, n (%) | 16 (22.2) | 46 (34.1) | 16 (22.2) | 17 (42.5) | ||
| Rate/1000 person-years (95% CI) | 0.13 (0.07–0.19) | 0.13 (0.09–0.16) | .92 | 0.13 (0.07–0.19) | 0.13 (0.07–0.20) | .921 | |
| Device malfunction | Cases, n (%) | 2 (2.8) | 7 (5.2) | 2 (2.8) | 3 (7.5) | ||
| Rate/1000 person-years (95% CI) | 0.02 (−0.07 to 0.04) | 0.0.2 (0.005–0.03) | .828 | 0.02 (−0.01 to 0.04) | 0.02 (−0.003 to 0.05) | .675 | |
| Right heart failure | Cases, n (%) | 3 (4.2) | 18 (13.3) | 3 (4.2) | 3 (7.5) | ||
| Rate/1000 person-years (95% CI) | 0.02 (−0.04 to 0.05) | 0.05 (0.03–0.07) | .171 | 0.02 (−0.003 to 0.05) | 0.02 (−0.003 to 0.05) | .975 | |
| Number of readmission during FU period | Cases, n (%) | 1 (0–3) | 1 (0–4) | 1 (0–3) | 2 (0–6.25) | ||
| Rate/1000 person-years (95% CI) | 0.09 (−0.01 to 0.02) | 0.003 (−0.003 to 0.008) | .530 | 0.01 (−0.01 to 0.02) | 0.02 (−0.01 to 0.04) | .577 | |
- Abbreviation: LVAD, left ventricular assist device.
4 DISCUSSION
4.1 Main findings
In this study, we analyzed the outcomes of 112 propensity score-matched patients with advanced heart failure who underwent LVAD implantation between August 2010 and January 2020. We found that WBC, CRP, and IL-6 significantly increased with LVAD implantation and that CytoSorb did not influence this response. In-hospital mortality and overall survival during follow-up were similar with CytoSorb. However, patients treated with CytoSorb were more likely to develop respiratory failure, need mechanical ventilation for longer than 6 days post implant, and require tracheostomy during hospitalization. During follow-up, no significant differences were observed with regard to major adverse events. Overall, our results showed that CytoSorb might not convey a significant morbidity or mortality benefit for patients undergoing LVAD implantation.
4.2 Comparison with the literature
The use of LVADs in patients with end-stage heart failure who are not eligible for heart transplantation has been increasing over the last decades.12 While this increase has been accompanied by an improvement in LVAD outcomes and a reduction in LVAD-related complications, this technology still carries some potentially major risks. Extensive research in the field has underlined the increased inflammatory profile which patients with heart failure present with.13, 14 The release and the levels of pro-inflammatory cytokines in heart failure have been found to be directly proportional to cardiac performance and deterioration, allowing to follow its progression.9, 10, 15-17 The pre-existing inflammatory profile of LVAD recipients, coupled with the inflammation derived from LVAD implantation and from CPB, expose the patients to a plethora of inflammatory outcomes, thus offering some theoretical context to the use of CytoSorb in this cohort of patients.3, 18-20 Indeed, in line with the published literature, our results illustrate an increase in inflammatory markers, namely CRP (p = .001) and IL-6 (p < .001), coupled with an increase in WBC (p = .033) following LVAD implantation.
The outcomes of CytoSorb application in cardiac surgery have been reported in different trials, leading to inconsistencies in results and failing to demonstrate its efficacy in improving patient outcomes.11, 14, 15 Our results have been consistent with the latest published trials in cardiac surgery, illustrating that CytoSorb did not modify the increase in postoperative inflammatory markers following LVAD implantation. In their pilot randomized control trial, Poli et al assessed differences in cytokines levels in 30 elective cardiac surgery patients.11 Levels of several cytokines including IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, TNF-α, and IFN-γ were measured both intraoperatively and postoperatively, with no significant decrease reported in the CytoSorb group.11 Similarly to our findings, reported levels of in-hospital mortality were also unvaried by CytoSorb use. These results have also been supported by a more recent randomized trial by Taleska Stupica et al comparing the use of CytoSorb versus glucocorticoids during CPB surgery.14 Indeed, when compared to the control group, the CytoSorb group did not present with any significant changes in 30-day mortality and inflammatory markers including TNF-α, IL-1β, IL-6, and IL-8. Similar findings were also reported by a previous trial published by Bernardi et al who in 32 patients undergoing CPB found no significant differences in perioperative values of IL-6, IL-18, IL-1β, TNF-α, and HMGβ1.15 The only exception in the study were postoperative levels of IL-10, which were increased in the control group 24- and 48-h post-bypass but not in the CytoSorb group, although no elaboration on its possible implications was offered.15 It is worth to note that although our results align with the previously mentioned three trials, our study was only able to include WBC, CRP, IL-6, and PCT levels, thus potentially not providing a full inflammatory picture of our patients.
Nevertheless, the outcomes of severely ill patients undergoing cardiac surgery with concomitant CytoSorb seem to suggest the existence of a potential advantage. In patients with infective endocarditis, Träger et al in a case series13 and Haidari et al in a retrospective study of 58 patients17 found levels of IL-6 to be reduced postoperatively with CytoSorb. Sepsis-related death was also found to be significantly lower with the use of CytoSorb, although overall 30-day mortality was unvaried.17
With regard to overall survival and mortality, data in the literature on the use of CytoSorb in LVAD patients scarce and results inconsistent. Our results show no significant difference in mortality and overall survival with the use of CytoSorb in LVAD implantation. Taleska Stupica et al also reported 1-year mortality of their patients receiving CytoSorb not be significantly different when compared to the control group.14 Nevertheless, unlike the previously mentioned randomized control trials, we found that patients treated with CytoSorb were more likely to develop respiratory failure, need mechanical ventilation for longer than 6 days post-implant, and require tracheostomy during hospitalization. This contrasts with reports of the use of CytoSorb in non-cardiac surgical setting, where it has been suggested to potentially help improve lung function through pulmonary metabolism modulation.18, 19 Furthermore, potential advantages of CytoSorb use with continuous renal replacement therapy in COVID-19 patients has been reported, with improved lung function (paO2/FiO2 ratio) 30-day post-treatment.20 However, the validity and accuracy of these small-scale studies results is low and makes it difficult to generalize them.
4.3 Study limitations
This study was a retrospective analysis from a single medical center over a span of 10 years. Results might therefore have been affected by the decision criteria used to select patients for LVAD implantation, which were likely influenced by the growing experience of the center with these procedures and their management. Additionally, the indications for Cytosorb implementation, duration of treatment, and patients’ selection were at the discretion of the operators and were unfortunately not available in the database. Furthermore, given the long timeframe of this study, changes in surgical, anesthesiology, and nursing staff might have had an influence on our results. Our study also did not take into consideration the wide range of ILs, and pro- and anti-inflammatory markers which form part of inflammatory processes.
5 CONCLUSION
In summary, for the first time, this study reports on the outcomes of CytoSorb in patients receiving LVAD therapy. Our results showed that CytoSorb might not convey a significant morbidity or mortality benefit for patients undergoing LVAD implantation. There persists the need for larger scale randomized control trials to evaluate the potential role of CytoSorb in improving outcomes of LVAD recipients.
ACKNOWLEDGMENTS
Open access funding enabled and organized by ProjektDEAL.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTIONS
Concept/design, data collection, data interpretation, drafting the article, critical revision of the article, and approval of the article: Konstantin Zhigalov. Concept/design, data interpretation, drafting the article, critical revision of the article, and approval of the article: Jef Van den Eynde. Data interpretation, drafting the article, critical revision of the article, and approval of the article: Thomas Chrosch, Lukas Goerdt, Arian Arjomandi Rad, Alina Zubarevich, and Robert Vardanyan. Data interpretation, critical revision of the article, and approval of the article: Michel Pompeu Barros Oliveira Sá, Peter Luedike, Nikolaus Pizanis, Achim Koch, Bastian Schmack, and Markus Kamler. Concept/design, Data interpretation, critical revision of the article, and approval of the article: Arjang Ruhparwar and Alexander Weymann.




