Performance of juvenile dusky grouper (Epinephelus marginatus Lowe, 1834) fed different practical diets in an indoor water-recirculation system
Abstract
Juvenile dusky groupers (Epinephelus marginatus Lowe, 1834; 143 g initial body weight) were reared over a 100-day experimental period in a water-recirculated tank system (28°C; 34 g L−1) and fed four different diets, namely a fresh fish diet (whole chub mackerel, Scomber japonicus), a semi-moist diet composed of a 60:40 mixture of a fishmeal-based formulated dry diet and fresh fish, and two fish meal-based dry pelleted diets, with or without supplemental taurine (5.0 g kg-1). Fish grew well on all diets with the exception of fish fed the diet without supplemental taurine (fish exhibiting a significantly lower body weight), with the highest growth response observed with fish fed the semi-moist diet, followed by the artificial diet with taurine and fresh fish respectively (although these differences were not statistically different: p < .05). The estimated essential individual amino acid requirement of juvenile dusky grouper as determined using whole-body amino acid composition was in the range previously reported for grouper species, with the possible exception of isoleucine and tryptophan (lower) and phenylalanine (higher). The present results serve as baseline for further nutritional studies on the dusky grouper Epinephelus marginatus.
1 INTRODUCTION
The dusky grouper (Epinephelus marginatus Lowe, 1834) is a carnivore fish species with demersal feeding habits inhabiting coastal areas on both sides of the Atlantic: from the Mediterranean to South Africa and Madagascar and from the North Brazil until Southern Argentina (Pierre et al., 2008; Rico & Acha, 2003). The species may reach 60 kg living up to 50 years (Harmelin & Harmelin-Vivien, 1999). To date, the culture of the dusky grouper is still in its infancy, as is information concerning its dietary nutrient requirements. As such, studies conducted to date have been limited to test the efficacy of fresh diets such as fresh fish, mussels and other non-specific commercial fish diets (López & Castello-Orvay, 2003; Ramos et al., 2012; Sanches et al., 2007, 2014) and to determine optimum feeding frequencies (Sousa et al., 2019).
Facing this lack of information, the present study was aimed at evaluating the performance of juvenile dusky grouper reared in an indoor water-recirculation tank-based culture system and fed four different practical fish diets: a fresh fish- based diet, a semi-moist (fresh fish: dry diet mixture) and two experimental dry diet formulations with and without supplemental taurine. In addition, grouper whole-body amino acid composition was used as reference for estimation of the individual essential amino acid requirements for the species.
2 MATERIAL AND METHODS
2.1 Experimental fish
Dusky grouper (Epinephelus marginatus) fingerlings were obtained from a local commercial grouper hatchery (Redemar Alevinos, Ilhabela, Brazil) and stocked in a 14,000 L nursery rearing tank receiving seawater (34 g L−1) in a flow-through system housed at the experimental aquaculture facilities of the University of Sao Paulo in Ubatuba. Grouper fingerings were fed on a commercial nursery feed (INVE O.range nursery feed XL 1.2 mm: declared composition: moisture (max) 5.0%, crude protein (min) 55.0%, lipid (min) 13.0%, ash (max) 13.5%; INVE Aquaculture NV, Belgium) until reaching the desired experimental weight for the feeding trial and then randomly allotted to the experimental recirculating-water tank system, and the fish acclimated during 2 weeks. After acclimation period, groupers were individually weighed (mean initial body weight 143.7 ± 2.13 g) and stocked into 16 experimental tanks (4 replicates per treatment), 15 fish/tank, with a density corresponding to 2.15 ± 0.03 kg/m3, and fed the four experimental diets. The experiment was performed in accordance with the Animal Ethics Committee of the Oceanographic Institute of the University of São Paulo (Protocol 001Pesq2018).
2.2 Experimental system
The trial was performed within the Aquaculture Laboratory (LAM) of the University of São Paulo (Oceanographic Institute) in Ubatuba, Southeastern Brazil. An appropriate design of a water-recirculated tank system was employed, composed of 16 × 500-L cylindrical self-cleaning tanks, filled with marine water (34 g L−1 salinity, previously filtered by cartridge 5 µm and UV for disinfection) and with tank lids so as to allow natural light and feed delivery. The tank system was connected to a treatment module with biofilter (plastic media), mechanical filtration and a protein skimmer. Water exchange was maintained at 5% daily so as to allow for evaporative losses and for cleaning, and water flow within each tank maintained at 4.5 L/min, with a backup air-stone place in each tank to maintain optimum oxygen supply.
Water quality parameters (including temperature, salinity and dissolved oxygen) were monitored daily at 9:00 AM for the duration of the experiment using a YSI 85 m (Yellow Springs, Ohio, USA) and were as follows; [mean (SD)]: 28.3 (1.48)°C; 34.3 (0.89) g L−1; 6.17 (0.42) mg L−1 DO and 84.7 (4.21) % DO saturation, respectively. Weekly measurement of total ammonia, nitrite and pH was 0.13 (0.07) mg L−1; 0.54 (0.21) mg L−1; and 7.88 (0.16), respectively (determined using an Alfakit, Florianópolis, Brazil).
2.3 Experimental diets and husbandry
The choice for feed ingredients to compose test diets was related to availability in the marketplace and current use in fish aquafeeds in Brazil. For diet preparation, feed ingredients were previously milled to <550 µm through a hammer mill (MCS 450 Moinhos Vieiras, Tatuí, Brazil). Four experimental diets were tested (Table 2): AquaMar (AQT)—cold-pressed reference practical diet, AquaMar—Taurine (AQ)—composed of AQT without Taurine supplementation, semi-moist (SM)—composed of AQT +fresh fish (60:40, w:w, respectively) and fresh fish (FF)—mainly composed of whole chub mackerel, Scomber japonicus, obtained from local fisheries. Diet preparation (AQT and AQ) ingredients were first mixed in a food mixer (ES-600, Hobart) for 10 min, and then liquid ingredients added (including boiled distilled water) to the ingredient mix to form a semi-moist dough prior to pelleting. The resulting dough was cold pressed (ca. 45°C) and the pellets (10 mm length, 10 mm diameter and sinking pellet) were dried in a forced-air dryer overnight (35–45°C for 18 h). The semi-moist diet was prepared by grinding the fresh fish and then blending with the AQT diet dry ingredient mix, and then blending in the Hobart food mixer for 10 min. The resulting dough was cold pressed (ca. 45°C) and passed through a 10 mm matrix. The fresh fish diet was composed of chopped fish of 10 mm dimension. Dry and semi-moist pellets and fresh fish were stored at −15°C until used. Formulation and proximate composition of the test diets, including the amino acid composition of diets AQT and FF, are shown in Table 1.
| Ingredient % | AquaMard (AQT) | AquaMar-T not Taurine supplementedd, e (AQ) | Semi-moiste, f (SM) | Fresh fishg (FF) |
|---|---|---|---|---|
| Fish mealh | 50.0 | 50.0 | 30.0 | – |
| Poultry by-product meali | 10.0 | 10.0 | 6.00 | – |
| Squid meali | 10.0 | 10.0 | 6.00 | – |
| Blood meali | 3.00 | 3.00 | 1.80 | – |
| Wheat flouri | 10.5 | 11.0 | 6.30 | – |
| Wheat gluteni | 5.00 | 5.00 | 3.00 | – |
| Fish oilj | 5.00 | 5.00 | 3.00 | – |
| Fish hydrolysatek | 4.00 | 4.00 | 2.40 | – |
| Soy lecithin oili | 1.00 | 1.00 | 0.60 | – |
| Mineral and vitamin premixl | 1.00 | 1.00 | 0.60 | – |
| Taurinem | 0.50 | – | 0.30 | – |
| Fresh fishg | – | – | 40.0 | 100.0 |
| Proximate composition | ||||
| Moisture | 5.72 (0.23) | 5.41 (0.18) | 36.3 (0.28) | 72.5 (0.19) |
| Crude protein | 51.1 (0.69) | 50.7 (0.92) | 35.9 (0.62) | 20.0 (0.17) |
| Crude lipid | 12.7 (1.15) | 12.9 (1.04) | 10.8 (0.38) | 4.11 (0.21) |
| Ash | 20.3 (0.87) | 19.9 (0.74) | 11.5 (0.06) | 1.72 (0.05) |
| Crude fibre + NFEa | 10.2 | 11.1 | 5.50 | 1.67 |
| Gross energy (MJ/kg)b | 18.7 | 18.8 | 13.6 | 6.60 |
| Essential amino acids | ||||
| Arginine | 2.92 | NA | NA | 4.28 |
| Histidine | 1.04 | NA | NA | 3.11 |
| Isoleucine | 1.41 | NA | NA | 3.19 |
| Leucine | 3.68 | NA | NA | 5.32 |
| Lysine | 2.73 | NA | NA | 5.84 |
| Methionine | 1.13 | NA | NA | 2.12 |
| Phenylalanine | 1.70 | NA | NA | 2.74 |
| Threonine | 1.79 | NA | NA | 3.33 |
| Tryptophan | 0.28 | NA | NA | 0.49 |
| Valine | 1.89 | NA | NA | 3.62 |
| Taurinec | 1.04 | NA | NA | 0.57 |
| Non-essential amino acids | ||||
| Cystine | 0.85 | NA | NA | 1.56 |
| Tyrosine | 1.23 | NA | NA | 2.37 |
| Serine | 1.89 | NA | NA | 2.80 |
| Glycine | 4.62 | NA | NA | 3.89 |
| Aspartic acid | 3.02 | NA | NA | 7.07 |
| Glutamic acid | 6.32 | NA | NA | 9.50 |
| Proline | 3.21 | NA | NA | 2.64 |
| Alanine | 3.11 | NA | NA | 4.32 |
| Sum of amino acids | 43.4 | NA | NA | 68.7 |
Note
- Proximate composition data expressed as mean (SD), n = 3.
- Abbreviation: NA, Not analysed.
- a Crude fibre + Nitrogen-free extract calculated by difference: 100-moisture–crude protein–ash–crude lipid.
- b Gross energy was calculated using energy equivalents 17.2, 39.2 and 23.4 kJ/g for protein, lipid and crude fibre +NFE, respectively (Cho et al., 1982).
- c Classified as conditionally essential amino acid.
- d Experimental in-house prepared dry cold-pelleted diet.
- e Amino acid composition estimated by values determined in AquaMar and fresh fish diets.
- f Semi-moist diet; fresh fish (predominantly chub mackerel Scomber janponicus): diet AquaMar (40:60 w/w).
- g Minced fresh fish, chub Mackerel (Scomber japonicus).
- h Residue meal from fish processing, Nicoluzzi industria de rações Ltda, Penha, SC, Brazil.
- i Sourced by Guabi Nutrição animal, Campinas, SP, Brazil.
- j Sourced by Polinutri Alimentos S.A., Osasco, SP, Brazil.
- k 100% fish oil (mainly anchovy, sardines, salmon, and tuna), Omegasur S.A., Mar del Plata, Argentina.
- l Aquativ, Diana, Even, France.
- m AquaMar, DSM, São Paulo, SP, Brazil. The following was supplied (mg/kg final feed): ascorbic acid, 250.0; DL-calcium pantothenate 125.0; choline chloride, 750.0; inositol, 400.0; folic acid 6.0; biotin, 0.6; niacin, 100; vitamin A (6,000.0 UI/kg); vitamin D3 (2,000.0 UI/kg),; vitamin E, 350.0; vitamin K3, 5.0; vitamin B1, 35.0; vitamin B2, 25.0; vitaminB6, 35.0;vitamin B12, 0.1; Se, 0.5; Cr, 0.5; Iron, 100.0; Cu, 10.0; Zinc, 150.0; Mg, 25.0; Iodine, 3.0; Cobalt, 0.5.
- n Sweetmix, Sorocaba, SP, Brazil.
Fish was fed daily by hand, once a day (10 AM), until apparent satiation. Previous studies at the laboratory with dusky grouper had shown no significant difference in grouper performance fed once, twice or continuously over a 24 h period. Feeding was conducted randomly on a daily basis, with the first round of feeding followed by a second round of feeding to confirm apparent satiety. Juvenile dusky grouper rapidly accepted all experimental diets, including dry pellets, semi-moist pellets and chopped fresh fish.
2.4 Chemical analysis
Grouper whole-body composition was initially determined in 10 fish followed by sampling 5 fish per tank at the end of the experimental feeding trial. Fish fed diets AQT and FF had amino acid determined in whole body. Ingredients, diets and fish were analysed according to AOAC (2000): moisture determined gravimetrically; samples dried at 105°C until constant weight (24 h for ingredients and diets, 48–72 h for wet diet and whole fish); ash determined gravimetrically after burning samples at 550°C for 6 h (Method 942.05; AOAC, 2000); crude protein by Kjeldahl (N × 6.25) using copper sulphate as catalyser in acid hydrolysis (FOSS Kjeltec™ 8200); and lipid determined with petroleum ether as solvent (FOSS Soxtec™, ST255). Amino acid content in diets (AQT and FF) and fish analysed by HPLC with precolumn phenylisothiocyanate derivatization (Hagen et al., 1989; White et al., 1986) and with tryptophan analysed separately after alkaline hydrolysis using lithium oxide (Lucas & Sotelo, 1980).
2.5 Calculation and statistics
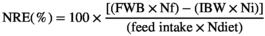
Dietary requirement of essential amino acids (EAA) in dusky grouper was estimated by two methods as follows:
Method 1: The amino acid profile in whole body (fish fed AQT) was used for calculation of A/E ratios as suggested by Arai (1981), A/E = [(individual EAA/total EAA) * 1000], EAA was then estimated considering 2.8% lysine requirement in dry diets for grouper spp. (NRC, 2011) by the formula EAA = (lysine requirement * A/E of each essential amino acid)/(A/E for lysine), as suggested by Kaushik (1998).
Method 2: The requirement is calculated as EAA = [(%CPdiet * Q * Z)/10000], where %CPdiet is crude protein dietary requirement (presently used at 50%), Q = ΣEAA + cystine + tyrosine (%), corresponding to 35% of CP required by the species; and Z is the whole-body content of the amino acid (presently from fish fed AQT diet) expressed as % of Q (Tacon, 1989).
All replicate data were submitted to normality and homoscedasticity check, applied one-way ANOVA to compare diet performance. Differences between means were analysed by post hoc Tukey HSD test and considered significant at p < .05 (ZAR, 1984), using IBM SPSS Statistics (IBM Corp. released 2011).
3 RESULTS
The appetite of juvenile grouper fed the different experimental diets varied considerably over the 100-day experimental period. It was observed that in all treatments, individual fish feed intake alternated between successive high and reduced consumption patterns; none of the dietary treatments showing a continuous and steady feed intake over the feeding trial. In general, a decreased feed intake was observed over a two- to three-day period, following one day of higher consumption. Diet SM exhibited the lowest number of days with reduced feed intake in comparison with the other diets tested. The intervals of reduced feed intake checked in all dietary treatments suggest a discontinuous feeding behaviour under the present experimental conditions.
The performance of grouper fed the different experimental diets over the 100-day experimental period is shown in Table 2. Although survival was 100 w% for all treatments, fish body weight and growth were significantly affected by dietary treatment (p < .05). Final fish body weight and weight gain were significantly higher in fish fed diets AquaMar (AQT) and semi-moist (SM) diet, whereas fish fed the diet not supplemented with taurine (AQ) displayed the lowest growth and weight gain compared with diets AQT and SM, respectively (p < .05). Although feed intake was significantly high in fish fed the high moisture feeds (SM and fresh fish (FF) on a fresh as-fed basis, fish fed FF had the lowest feed intake and overall FCR on a dry matter basis, indicating feed may have been limiting for fish fed the FF diet (Table 2). Likewise, the total energy intake was lower for FF, being significantly lower AQT and SM, with SM being higher than all others. Considering the average cost of feed and fresh fish in Ubatuba (coastal São Paulo State, Brazil) diet, AQT displayed the lowest overall cost per kg grouper produced (US$ 1.9/kg production) compared with other treatments which ranged from US$ 2.01 to 2.64/kg production (Table 2).
| AquaMar (AQT) | AquaMar-T (AQ) | Semi-moist (SM) | Fresh fish (FF) | |
|---|---|---|---|---|
| Initial body weight (g) | 143.2 (1.14) | 142.9 (1.38) | 144.8 (3.30) | 143.9 (2.37) |
| Final body weight (g) | 313.5a (16.5) | 242.6b (22.8) | 322.0a (50.4) | 274.0ab (18.5) |
| Weight gainn (%) | 118.9a (12.0) | 69.7b (15.5) | 121.9a (30.9) | 90.5ab (15.7) |
| SGR1 (%/day) | 0.78a (0.05) | 0.52b (0.08) | 0.79a (0.14) | 0.64ab (0.08) |
| Total feed intake (kg as-is/m2) | 9.25a (0.39) | 7.73a (0.48) | 17.1b (1.88) | 19.0b (1.55) |
| Total feed intake (kg dm/m2) | 8.74a (0.37) | 7.30a (0.45) | 10.7b (1.19) | 5.14c (0.42) |
| Total energy intake (MJ/m³) | 172.9a (7.31) | 146.0ab (9.09) | 232.0c (25.6) | 125.5b (10.3) |
| Daily individual feed intake (g, as-is) | 2.46a (0.11) | 2.05a (0.13) | 4.55b (0.50) | 5.07b (0.41) |
| Survival (%) | 100.0 | 100.0 | 100.0 | 100.0 |
| Production2 (kg/m3) | 11.7a (0.62) | 9.10b (0.86) | 12.1a (1.90) | 10.3ab (0.70) |
| FCR3 | 1.46a (0.21) | 2.12ab (0.32) | 2.69b (0.65) | 3.94c (0.39) |
| FCRDM4 | 1.37ab (0.20) | 2.00c (0.30) | 1.69bc (0.41) | 1.06a (0.10) |
| Feed cost per kg production5 (USD/kg) | 1.90 (0.28) | 2.43 (0.37) | 2.64 (0.64) | 2.01 (0.19) |
Note
- Values are mean (SD) of 4 replicates per treatment. Different superscript letters within the same line indicate significant difference (p < .05).
- 1 Weight gain (%) = (final weight – initial weight)/initial weight * 100.
- 2 SGR—specific growth rate (% day−1) = (ln final weight—ln initial weight)/days * 100.
- 3 Production (kg/m3) = (total final weight per tank) * 1000/tank volume.
- 4 FCR: feed conversion ratio = total feed consumed (g) / [final fish biomass (g)–Initial fish biomass (g)].
- 5 FCRDM: feed conversion ratio = total feed consumed (g, dry mater basis) / [final fish biomass (g) – Initial fish biomass (g)].
- 6 Feed cost per kg production (USD/kg) = Diet price * FCR, prices were considered as: AQT and AQ local industry average prices (1.30 and 1.15 USD/kg respectively), price of fresh fish are based on local fish market (0.51 USD/kg), and the cost of SM (0.98 USD/kg) was calculated from the previous values by the proportion of diet preparation.
Whole-body composition of initially stocked fish showed a higher moisture, crude protein and ash content compared with fish fed the experimental test diets (Table 3). In contrast, whole-body lipid content increased significantly in bigger individuals with the highest values observed in fish fed diet FF (p < .05); these fish also displaying significantly reduced crude protein, ash and moisture contents (p < .05; Table 3). Grouper fed SM showed also displayed a higher crude protein content compared with other test diets (p < .05).
| Initial fish | AquaMar (AQT) | AquaMar-T (AQ) | Semi-moist (SM) | Fresh fish (FF) | |
|---|---|---|---|---|---|
| Moisture | 71.2a (0.79) | 68.8b (0.57) | 67.8c (0.45) | 68.7bc (0.39) | 66.0d (0.92) |
| Crude protein | 59.1a (0.13) | 56.4b (1.57) | 55.2b (0.68) | 59.3a (2.03) | 51.8c (1.33) |
| Crude lipid | 21.3a (0.90) | 24.1ab (1.63) | 24.4b (2.09) | 25.5b (1.98) | 30.6c (1.33) |
| Ash | 18.5a (0.60) | 16.4b (0.61) | 17.0ab (1.04) | 16.5b (1.95) | 14.0c (1.06) |
| Essential amino acids | |||||
| Arginine | 3.55 | 3.63 | – | – | 3.48 |
| Histidine | 1.03 | 1.01 | – | – | 0.98 |
| Isoleucine | 2.11 | 1.95 | – | – | 1.88 |
| Leucine | 3.62 | 3.42 | – | – | 3.32 |
| Lysine | 3.30 | 3.70 | – | – | 3.53 |
| Methionine | 1.53 | 1.48 | – | – | 1.44 |
| Phenylalanine | 2.07 | 2.03 | – | – | 1.95 |
| Threonine | 2.33 | 2.37 | – | – | 2.30 |
| Tryptophan | 0.43 | 0.41 | – | – | 0.44 |
| Valine | 2.31 | 2.17 | – | – | 2.09 |
| Taurine1 | 0.93 | 1.05 | – | – | 0.45 |
| Non-essential amino acids | |||||
| Cystine | 1.21 | 1.84 | – | – | 1.72 |
| Tyrosine | 1.53 | 1.42 | – | – | 1.36 |
| Serine | 2.24 | 2.29 | – | – | 2.24 |
| Glycine | 5.45 | 5.94 | – | – | 5.81 |
| Aspartic acid | 4.56 | 4.67 | – | – | 4.70 |
| Glutamic acid | 7.22 | 6.97 | – | – | 6.78 |
| Proline | 3.23 | 3.32 | – | – | 3.19 |
| Alanine | 3.98 | 4.12 | – | – | 3.96 |
| True protein (∑ of amino acids) | 52.6 | 53.7 | – | – | 51.6 |
Note
- Proximate composition data expressed as mean (SD), n = 4. Different superscript letters within the same line indicate significant difference (p < .05) based on one-way ANOVA followed by Tukey multiple comparison test. –, not determined.
- 1 Classified as conditionally essential amino acid (NRC, 2011).
Amino acid carcass levels in grouper whole body suggest a higher value in initial fish compared with individuals fed the AQT and FF diets. When comparing treatments AQT and FF, individual amino acids were 3 to 5% higher in AQT, exception made only to tryptophan and aspartic acid. Similarly, estimated true protein levels (sum of total amino acids) suggest higher levels in individuals fed the AQT diet compared with FF.
When expressed as per cent of total recovered amino acids, some differences in whole-body amino acid content were observed between treatments, and in particular, fish fed the AQT and FF diets (Table 4). Although it cannot be compared statistically, in raw values, fish fed diet FF displayed a higher content of leucine, threonine, serine and aspartic acid compared with AQT, and the opposite was true for taurine (which was 120% higher in AQT fish). Moreover, the amino acid carcass composition of the dusky grouper suggests that it differs from other marine fish species. For example, Plata pompano (Trachinotus marginatus) showed higher carcass levels of histidine, leucine, lysine, methionine, tryptophan, taurine, tyrosine, serine and proline, whereas for the other marine fish species, essential amino acids plus cystine and alanine values were lower than those observed for dusky grouper. Whole-body amino acids levels were also higher in Atlantic salmon compared with dusky grouper with exception of methionine, cystine, glycine, proline and alanine. Moreover, if compared to the general finfish average presented, whole-body amino acids in dusky grouper showed reduced carcass amino acid levels, with the possible exception of arginine, serine, cystine, proline and alanine (Table 4).
| Dusky grouper (E. marginatus, AquaMar)a | Dusky grouper (E. arginatus, Fresh fish)a | Plata pompano (Trachinotus marginatus)b | Atlantic Salmon (Salmo salar)c | Cobia (Rachycentron canadum)e | Finfishf | |
|---|---|---|---|---|---|---|
| Essential amino acids Arginine | 6.75 | 6.74 | 6.63 | 6.61 | 6.36 | 6.60 |
| Histidine | 1.88 | 1.90 | 2.13 | 3.02 | 1.93 | 2.60 |
| Isoleucine | 3.63 | 3.64 | 2.94 | 4.41 | 4.32 | 4.60 |
| Leucine | 6.36 | 6.43 | 7.06 | 7.72 | 6.32 | 7.70 |
| Lysine | 6.88 | 6.84 | 7.93 | 9.28 | 8.41 | 7.90 |
| Methionine | 2.75 | 2.79 | 3.02 | 1.83 | 2.45 | 2.90 |
| Phenylalanine | 3.77 | 3.78 | 3.59 | 4.36 | 3.52 | 4.40 |
| Threonine | 4.41 | 4.46 | 4.00 | 4.95 | 4.13 | 4.70 |
| Tryptophan | 0.76 | 0.85 | 1.04 | 0.93 | 1.66 | 1.10 |
| Valine | 4.03 | 4.05 | 3.43 | 5.09 | 5.22 | 5.10 |
| Taurine1 | 1.95 | 0.87 | 2.45 | – | 0.82 | – |
| Non-essential amino acids | ||||||
| Cystine | 3.42 | 3.33 | 0.97 | 0.95 | 2.45 | 1,00 |
| Tyrosine | 2.64 | 2.63 | 2.99 | 3.50 | 2.87 | 3.20 |
| Serine | 4.26 | 4.34 | 4.73 | 4.61 | 3.69 | 4.40 |
| Glycine | 11.0 | 11.3 | 11.1 | 7.41 | 8.35 | 7.30 |
| Aspartic acid | 8.68 | 9.10 | 9.04 | 9.92 | 8.10 | 9.80 |
| Glutamic acid | 13.0 | 13.1 | 13.3 | 14.3 | 13.4 | 15.3 |
| Proline | 6.17 | 6.18 | 6.98 | 4.64 | 5.04 | 4.70 |
| Alanine | 7.66 | 7.67 | 6.77 | 6.52 | 6.61 | 6.60 |
Note
- –, not reported.
- a Classified as conditionally essential amino acid in present study for dusky grouper.
- b Present study, values from fish fed AquaMar and fresh fish diets.
- c Tesser et al. (2014).
- d Wilson and Cowey (1985).
- e Raggi et al. (2019).
- f Mean data from several cultured fish species (Kaushik & Seiliez, 2010).
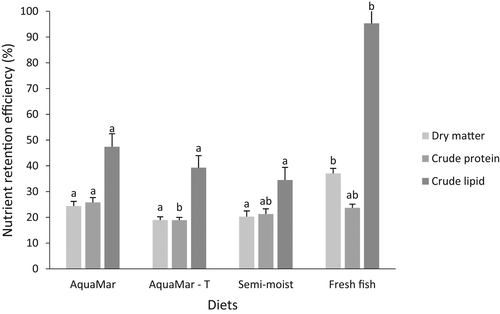
The retention efficiency of juvenile dusky grouper was higher for lipid compared with dry matter and crude protein in all dietary treatments (Figure 1). The comparison among diets showed significantly increased retention efficiency of dry matter and lipid (>90%) in FF in relation to other tested diets (p < .05). Crude protein retention efficiency was significantly lower in fish fed diet AQ (no taurine supplementation) compared with AQT (p < .05). In contrast, the retention efficiency was higher in fish fed AQT compared with FF for the majority of amino acids (Figure 2). Data showed retention efficiencies varying from −0.06% (taurine, diet FF) to 73.2% (cystine, diet AQT). Average retention efficiency of amino acids of juvenile grouper fed diets AQT and FF was ca. 34.8% and ca. 23.3%, respectively. Grouper retention was over 100% higher in treatment AQT compared with FF for histidine (172%) isoleucine (106%), and aspartic acid (103%), and over 50% for leucine (61%), lysine (93%) methionine (68%), valine (76%), cystine (65%) and tyrosine (80%). The negative retention efficiency of taurine in diet FF contrasted with the positive value of fish fed AQT. The higher amino acid retention with diet AQT was checked for cystine, followed by lysine and aspartic acid.
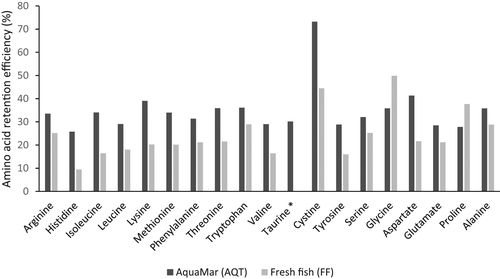
The dietary amino acid requirement of juvenile dusky grouper was estimated, and the quantitative value observed depended on the method of calculation employed (Table 5). Lysine requirement-based estimations resulted in lower overall amino acid requirement values for dusky grouper compared with calculation methods based on the sum of amino acids in the whole-body and crude protein requirement. The requirement range coincided with previous requirements reported for grouper species in six out of ten individual amino acids, including arginine, histidine, leucine, lysine, methionine and threonine. In addition, present estimates were found lower or higher if compared to reported to other marine fish species as Plata pompano (Trachinotus marginatus) and cobia (Rachycentron canadum), respectively (Table 5).
| Epinephelus marginatus | Trachinotus marginatus b | Rachycentron canadum c | Grouper sp.d | |||
|---|---|---|---|---|---|---|
| Method 1f | Method 2a | Min. | Max. | |||
| Arginine | 2.74 | 3.39 | 4.01 | 1.76 | 3.09 | 3.29 |
| Histidine | 0.76 | 0.94 | 1.29 | 0.53 | 0.83 | 1.23 |
| Isoleucine | 1.47 | 1.82 | 1.78 | 1.2 | 2.19 | 2.19 |
| Leucine | 2.58 | 3.19 | 4.27 | 1.75 | 3.17 | 3.37 |
| Lysine | 2.8 | 3.45 | 4.8 | 2.33 | 2.84 | 3.04 |
| Methionine | 1.12 | 1.38 | 2.33 | 1.36 | 1.37 | 1.37 |
| Phenylalanine | 1.53 | 1.89 | 3.99 | 1.78 | 1.20 | 1.20 |
| Threonine | 1.79 | 2.21 | 2.42 | 1.14 | 1.77 | 1.99 |
| Tryptophan | 0.31 | 0.38 | 0.63 | 0.46 | 1.39 | 1.79 |
| Valine | 1.64 | 2.03 | 2.08 | 1.45 | 2.09 | 2.29 |
| Taurine | 0.78 | 0.98 | – | – | – | – |
Note
- –, not determined.
- a Based on whole-body composition and lysine requirement (2.8% of dry diet NRC, 2011) as suggested by Kaushik (1998).
- b Based on sum of amino acids in whole-body, crude protein requirement and sum of essential amino acids + tyrosine + cystine, as suggested by Tacon (1989).
- c Based on whole-body composition and lysine requirement (Tesser et al., 2014). Methionine requirement represented by sum of methionine plus cystine, and phenylalanine requirement represented by sum of phenylalanine plus tyrosine.
- d Based on whole-body composition and lysine requirement (Raggi et al., 2019). Methionine requirement represented by sum of methionine plus cystine, and phenylalanine requirement represented by sum of phenylalanine plus tyrosine.
- e Data obtained in minimum and maximum values for essential amino acids for grouper species (Cai-Juan et al., 2016).
4 DISCUSSION
4.1 Use of practical diets within recirculated-water tank production systems
The bulk of literature on dusky grouper farming, Epinephelus marginatus, reports experiments on species adaptation to farming and management, reproduction and feeding (Kerber et al., 2012; López & Castello-Orvay, 2003; Sousa et al., 2019; Yoshimini et al., 2019). These studies often use generic commercial diets, formulated for other marine fish species and include testing fish, crustacean- and mollusk-based moist diets (Araújo et al., 2018; Kerber et al., 2012; López & Castello-Orvay, 2003; Ramos et al., 2012; Sanches et al., 2007; Sousa et al., 2019). In addition, these studies mostly employed fish collected from the wild which may have affected grouper performance. Though specific studies on nutrient requirements of dusky grouper are still scarce, information related to other grouper species may be used as a baseline for the formulation of practical grouper diets (NRC, 2011).
Adequate growth of farmed grouper may be achieved with animals fed high protein diets (ca. 50% protein) using conventional feed ingredient sources (Luo, Liu, Mai, & Tian, 2005). The ideal dietary lipid level in studies with Malabar grouper (Epinephelus malabaricus) using fish and corn oil was reported at 9% for maximum growth and feed efficiency (Lin & Shiau, 2002). Accordingly, studies with juveniles orange-spotted grouper (Epinephelus coioides) cultured in floating net cages with increasing dietary lipid concentration (5.16% to 16.04%, fish oil as the main source), provided an optimum lipid level of 10.0% for maximum growth (Luo, Liu, Mai, Tian, Liu, et al., 2005). In the present study, diets AQT and AQ, based on fish meal and oil, had nutrient levels similar to the above-mentioned recommendations for grouper species (Luo, Liu, Mai, & Tian, 2005). In particular, the current study showed that fish fed the dry pelleted diet AquaMar (AQT) diet (a marine reference diet formulation tested previously for other marine carnivorous fish species (Cobia; Raggi et al., 2019)) was capable of sustaining good performance in juvenile dusky grouper with no significant difference compared with a fresh fish or semi-moist pelleted diet. Similar results were observed with juvenile humpback grouper (Cromileptes altivelis), with best performance observed with fish fed a commercial diet compared with fresh fish (Sardinella sp.; Shapawi et al., 2011). Present FCR values observed in treatments fed the reference fish meal diet (AQT) were lower compared with previously reported values for dusky grouper using dry or moist diets (López & Castello-Orvay, 2003; Ramos et al., 2012; Sanches et al., 2007; Sousa et al., 2019) and were similar to other consolidated farmed marine fish species (Aas et al., 2019; Company et al., 1999; Raggi et al., 2019; Rosenlund et al., 2004; Tacon & Metian, 2015). As expected, FCR was lower for dry diets compared with semi-moist and fresh fish diets, which is in agreement with previously reported for tropical carnivorous marine fish (Bunlipatanon et al., 2014; Ma et al., 2020; Raggi et al., 2019), including grouper species (Muyot et al., 2021; Nugraha & Rozi, 2020; Ramos et al., 2012; Shapawi et al., 2011; Sim et al., 2005; Tacon et al., 1991).
The semi-moist diet showed performance as good as AquaMar diet, but its use may be constrained with issues related to fresh fish use, including competition with humans for consumption, and difficulties with sourcing, storage, pathogens and potential negatives effects upon water quality and consequent elevated FCR (Lee et al., 2016; Tacon & Metian, 2009). During the present trial, daily feeding observations confirmed the increase in water turbidity due to fractioning and solid suspension in treatments semi-moist and fresh fish, which is in agreement with verified in Japanese flounder, Paralithicys olivaceus, fed a semi-moist diet resulting in increased turbidity, ammonium level and total dissolved phosphorus affecting survival and growth (Lee et al., 2016). In the present survey, the dry pelleted diet had the advantage of sustaining grouper performance with proper feed efficiency and water quality within a water-recirculation system.
The absence of mortality during the trial confirms the suitability of juvenile dusky grouper for management in culture (tank system). The species is known to be docile, sedentary and having low energy expenditure as previously showed (Sanches et al., 2014; Sousa et al., 2019; Souza et al., 2014). Dusky grouper is an emerging species in Brazil with high market value (Lobo, 2021), whose grow-out cycle in Southeast region comprises 1.5 year to reach 1 kg fish. The proper performance of dusky grouper fed diet AquaMar corroborates recent observations of local farmers (Ilhabela, Brazil) on the adequacy of this diet formulation for grow-out of the species (Kerber, 2020).
4.2 Taurine supplementation
Taurine is considered a conditionally essential amino acid in fish nutrition (El-Sayed, 2014; Espe et al., 2012). However, some fish species such as Japanese flounder (Paralichthys olivaceus), yellowtail (Seriola quinqueradiata) and red sea bream (Pagrus major) have limited taurine synthesis, especially during larval and juvenile stages, and therefore require exogenous supply via food/feed (El-Sayed, 2014; Kuzmina et al., 2010; Matsunari et al., 2008; Salze & Davis, 2015; Takagi et al., 2008; Takeuchi et al., 2001; Wei et al., 2018). Several studies report beneficial effects of taurine supplementation upon growth and feed efficiency in fish fed low fish meal inclusion diets (Chatzifotis et al., 2008; Gaylord et al., 2006; Koven et al., 2016; Lunger et al., 2007; Rossi & Davis, 2012; Takagi et al., 2011). In spite of being considered an excellent protein source and rich in taurine compared with most feed ingredients, the taurine content of fish meal may be dependent on the raw material (including if produced from whole fish or filleting by-product, the species and life stage) and the processing strategy employed (Gormley et al., 2007; Salze & Davis, 2015). Although the high fish meal content in dry pelleted diets tested in the present study, the supplementation of taurine at 0.5% (diet AQT) resulted in increased grouper performance and feed efficiency in comparison with the similarly formulated but non-supplemented diet AQ. Previous studies have reported positive effects of taurine supply even in fish meal-based diets, as verified in juvenile cobia, Rachycentron canadum (better final weight and FCR with 0.5% taurine inclusion, 50% fish meal diet—Raggi et al., 2019), and juvenile Japanese flounder, Paralithicys olivaceus (improved weight gain and FCR with 1% taurine supplementation, 62% fish meal diet – Kim et al., 2005). In a recent study, juvenile orange-spotted grouper (Epinephelus coioides) fed diets with different taurine contents (0.0%, 0.5%, 1.0% and 1.5%) showed positive effects of taurine supplementation upon the fish metabolome, improving energy utilization and amino acid uptake, and accelerating fish growth (Shen et al., 2019). A recent review on the topic suggests benefit of taurine at levels between 0.5% and 1.5% and estimated 0.91% as a mean dietary content for optimal growth and metabolism of some fish species (Sampath et al., 2020). The total dietary taurine content in diet AquaMar (AQT) was close to level recommended in the review (1.04%) and may be related to the proper performance of fish checked in the present study.
The amino acid analysis of fresh fish used in the present trial revealed a reduced taurine content, and the fresh fish used was predominantly composed by one species: chub mackerel (Scomber japonicus). However, when analysing the whole-body amino acid composition of experimental dusky grouper, no remarkable difference was found in fish fed diets AquaMar (AQT) versus fresh fish (FF), with exception to taurine content, >100% higher whole-body content in treatment AQT (taurine supplemented diet). This fact combined with the null taurine retention efficiency checked in treatment FF may suggest beyond the poor taurine content of fresh fish, and juvenile dusky grouper may have limited capacity for taurine synthesis, as previously reported for other marine fish species (Kim et al., 2005; Park et al., 2002; Takagi et al., 2008). Therefore, dietary taurine supplementation to minimum 1.0% diet is recommended for the grow-out of this species.
4.3 Estimation of essential amino acid requirement
Ten amino acids have been considered to date as being essential for grouper species: arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine (Luo, Liu, Mai, & Tian, 2005; Muhammadar et al., 2011). However, amino acid imbalanced diets may negatively affect health and performance of farmed fish (Bicudo & Cyrino, 2014; Lall & Anderson, 2005) impairing the utilization of other dietary nutrients and resulting in increased faecal nutrient loads and pollution (Bicudo & Cyrino, 2014; Li et al., 2009). The determination of amino acid requirements by dose-response using semi-purified diets has been widely reported in fish species (NRC, 2011), despite the relatively long experimental time of evaluation this method usually takes for individual amino acids. In view of the importance of quantifying the requirement for essential amino acids under practical farming conditions, the whole-body profile has been used as reference as it holds a good correlation with dietary amino acid requirements (Cowey & Tacon, 1983; Peres & Oliva-Teles, 2008; Wilson & Poe, 1985), with comparatively lower cost. Different methodologies have also been reported for the estimation of amino acids requirement in fish species based on whole-body composition (Kaushik, 1998; Meyer & Fracalossi, 2005; Tacon, 1989). Despite limited in determining amino acid expenditure during maintenance metabolism (Wilson & Poe, 1985), methods based on body nutrient profiles are considered as an important and practical tool by industry players and researchers, especially in the lack of previous dose-response studies (Bicudo & Cyrino, 2014).
With exception to tryptophan, present study found individual amino acid requirements similar to those previously reported for grouper species farmed in Asia (Cai-Juan et al., 2016). In addition, these estimates are comparable to the dietary nutrient content of commercial diets currently used for feeding juvenile tiger grouper, Epinephelus fuscoguttatus (Muhammadar et al., 2011). Dose-response assessment of dietary amino acid requirements is not abundant and has been mainly focused on Asian farmed grouper species. Optimum dietary methionine levels for hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) were determined by dose-response studies at 1.45% (Li et al., 2020), while the requirement for arginine (Wu et al., 2018), leucine (Zhou et al., 2019) and isoleucine (Zhou et al., 2020) for this hybrid was reported as 3.65%, 3.25% and 1.98% of diet, respectively. Despite determined for other grouper species, these requirement values are comparable to those estimated in the present study for dusky grouper, especially values obtained according to Tacon (1989), confirming the potential of whole-body composition for quantitative determination of amino acid requirement.
5 CONCLUSIONS
Present results demonstrate the satisfactory performance of dusky grouper reared within water recirculating tank systems fed a dry pelleted diet, with fish production equal or better to that obtained with fish fed a fresh fish diet. Moreover, results with taurine supplementation suggest that the supplementation with this amino acid results in improved growth and feed efficiency within a fishmeal-based diet. Moreover, the results of this study indicate that the estimated amino acid requirements of dusky grouper based on whole-body composition could be used as a good starting point for the formulation of practical diets for this species, until more precise dietary nutrient determinations become available.
ACKNOWLEDGEMENTS
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 303259/2017-5) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2019/11828-6). R. Coelho acknowledge fellowship support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 169299/2017-1).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, R. Coelho, upon reasonable request.




