Yeast culture supplementation alters the performance and health status of juvenile largemouth bass (Micropterus salmoides) fed a high-plant protein diet
First author: Zhicheng Xv, Address: College of Fisheries, Southwest University, Chongqing 400715, P.R. China, Tel and Fax: +86 23 68366066
Abstract
The present study was conducted to investigate the effects of yeast culture on the growth, hepatic antioxidant capacity, intestinal barrier and microflora of fish fed a high-cottonseed protein diet. The largemouth bass (initial body weight: 12.17 ± 0.02 g) were randomly allotted into three treatments, fish meal-based diet (FM), cottonseed protein concentrate-based diet (CPC, 36% of the fish meal replaced by cottonseed protein concentrate) and CPC diet with yeast culture (CPCY, CPC+3% yeast culture) for 8 weeks, respectively. The results indicated fish fed 3% yeast culture diet could enhance specific growth rate (SGR), protein efficiency ratio (PER) and feeding rate (FR), while the hepatosomatic index (HSI) and liver glycogen content were significantly decreased compared with the CPC group (p < .05). Compared with the FM group, the CPC diet resulted in liver tissue damage accompanied by upregulation of pro-inflammatory factor (IL-1β and TNFα) expression, while adding yeast culture significantly increased the relative expression of liver anti-inflammatory factor (IL-10) and antioxidant enzyme (SOD, CAT and GSH-Px) (p < .05). Meanwhile, yeast culture can reduce the accumulation of liver glycogen and improve the disorder of liver glucose metabolism caused by CPC. Furthermore, compared with CPC group, CPCY group significantly up-regulated the expression of ZO-1, Claudin and Occludin and the activities of antioxidant enzymes (SOD, CAT) in intestine (p < .05) as well as down-regulated diamine oxidase (DAO) activity, D-lactate (D-lac) and lipopolysaccharide (LPS) contents in serum (p < .05). In addition, the abundance of probiotics (Lactobacillus) increased significantly, and the abundance of intestinal potential pathogenic bacteria (Vibrio, Brevundimonas) decreased in CPCY group (p < .05). In conclusion, yeast culture can alleviate the adverse effects of concentrated cottonseed protein on the growth of largemouth bass and might be used as an effective probiotic for farmed fish.
1 INTRODUCTION
Recently, plant proteins are increasingly being used as an economical and versatile alternative replacing animal source in fish nutrition. Animal protein presents growing costs and limited supply, and has been highly associated with resources depletion, biodiversity loss and environmental pollution. Therefore, reducing fishmeal in aquafeeds is becoming necessary for ensuring aquaculture sustainability, being particularly relevant in the production of carnivorous fish. As one of the most important aquaculture species in China, the largemouth bass (Micropterus salmoides) has received great attention, and several studies have been focused on alternative protein sources but have led to reduced feed intake, growth and impaired health status when included at high levels (He, Li, et al., 2020; He, Yu, et al., 2020; Jiang et al., 2018; Wu et al., 2021).
Proteins are essential macronutrients for fish nutrition, and the nutritional quality of a protein source varies substantially depending upon their bioavailability, digestibility, amino acid profile, purity, antinutritional factors and processing effects. Plant proteins are often recognized as incomplete or nutritionally inferior to animal proteins (Hughes et al., 2011). However, plant proteins certainly play a very valuable role in fish nutrition. The suitability of the plant protein sources in terms of growth performance has resulted to be highly variable among fish species and experimental conditions. Generally, carnivorous fish showed a much lower ability to accept high-plant protein diets even with balanced essential nutrients (Krogdahl et al., 2010). How to improve the utilization of plant protein sources in aquafeeds remains to be an important aspect. Therefore, specific strategies and techniques to increase the use of plant feedstuffs in aquafeeds and limit potentially adverse effects of bioactive compounds on farmed largemouth bass are worth research.
At present, yeast culture (YC) as synbiotics is widely used in aquaculture (Ayiku et al., 2020; Barnes et al., 2009). Studies have shown that yeast culture can not only be used as a protein source to replace fish meal (Barnes et al., 2006; Zhang et al., 2018) but also can be used as an immune modulator for fish due to it contains immunomodulatory components such as yeast polysaccharides (β-glucan and mannooligosaccharide), peptides and nucleotides (Choudhury et al., 2005; Jensen et al., 2008; Selvaraj et al., 2005; Torrecillas et al., 2007). Furthermore, it was also documented that yeast culture can alleviate intestinal tissue damage caused by high-plant protein in yellow catfish (Pelteobagrus fulvidraco) (Sun, 2020) and turbot (Scophthalmus maximus) (Zhao, 2008). In addition, yeast cultures can alleviate gossypol induced inflammatory response in liver tissue of Ussuri catfish (Pseudobagrus ussuriensis) (Bu et al., 2020). Up to now, whether dietary yeast culture supplementation can mitigate detrimental effects of plant proteins and modulate antioxidant status still remains to be elucidated. Therefore, we aimed to explore the effect of yeast culture dietary intervention in largemouth bass fed a high-cottonseed protein diet for an 8-week randomized clinical trial. It provides valuable reference for the daily use of yeast culture in aquaculture.
2 MATERIALS AND METHODS
2.1 Experimental diets
Three isonitrogenous (crude protein 480 g/kg) and isolipidic (crude lipid 120 g/kg) diets included a fish meal-based diet (named as FM group, control), a diet that FM with 36% fish meal protein replaced by cottonseed protein concentrate (named as CPC group), and a diet supplementing 3% yeast culture (Beijing Enhalor International Tech Co., Ltd) into the CPC diet (named as CPCY group) (Table 1). The CPC and CPCY diets were supplemented with methionine and lysine to approximate the amino acid composition of FM. The ingredients were weighed and mixed in a commercial food mixer YGH-150 (Guibao Group), then blended with the oils. Pellets were prepared using a dry power press MUZL180 (Muyang Group), then air-dried and stored at 4°C until used.
| Ingredient (g kg−1) | Experiment diets | ||
|---|---|---|---|
| FM | CPC | CPCY | |
| Fish meala | 400 | 256 | 256 |
| Domestic chicken powder | 100 | 100 | 100 |
| Cottonseed protein concentrate | 0 | 157.8 | 157.8 |
| Yeast culture | 0 | 0 | 30 |
| Soybean meal | 70 | 70 | 70 |
| Concentrated soy protein | 110 | 110 | 95 |
| Wheat gluten | 30 | 30 | 30 |
| Cassava starch | 90 | 90 | 90 |
| Fish oil | 20 | 31 | 31 |
| Soy lecithin | 15 | 15 | 15 |
| Soybean oil | 40 | 40 | 40 |
| Vitamin premixb | 15 | 15 | 15 |
| Mineral premixc | 15 | 15 | 15 |
| Choline chloride | 5 | 5 | 5 |
| Ca(H2PO4)2 | 15 | 15 | 15 |
| Microcrystalline fibre | 75 | 47.8 | 32.8 |
| L-lysine·HCL | 0 | 1.7 | 1.5 |
| DL-methionine | 0 | 0.7 | 0.9 |
| Chemical composition (g/kg, dry matter basis) | |||
| Crude protein | 481 | 482 | 484.2 |
| Crude lipid | 120.6 | 120.6 | 121.2 |
| Ash | 115.8 | 105.1 | 105.1 |
- Abbreviations: CPC, 36% of the fish meal replaced by cottonseed protein concentrate; CPCY, CPC diet with 3% yeast culture; FM, fish meal-based diet.
- a Imported from Peru.
- b Vitamin premix(mg kg−1 of diet): VA, 18; VD3, 5; VE, 150; VC (350 g kg−1), 500; VB1, 16; VB6, 20; VB12, 6; VK3, 18; riboflavin, 40; inositol, 320; calcium-D-pantothenate, 60; niacinamide, 80; folic acid, 5; biotin, 2; ethoxyquin, 100.
- c Mineral premix (mg kg−1 of diet): Na, 30; K, 50; Mg, 100; Cu, 4; Fe, 25; Zn, 35; Mn, 12; I, 1.6; Se, 0.2; Co, 0.8.
2.2 Feeding trial
Largemouth bass juveniles were obtained from a commercial farm (Changshou, Chongqing, China) and acclimated to the experimental conditions with a commercial feed (Guangzhou Jieda Feed Co. LTD) for 2 weeks. Two hundred and seventy juveniles of largemouth bass (initial average weight: 12.17 ± 0.02 g) were randomly distributed into 9 cylindrical plastic tanks (capacity: 180 L) for the growth trial (30 fish per tank). Each dietary treatment was randomly assigned to three tanks. Fish were fed to apparent satiation by hand three times (08:30, 13:00 and 18:00) daily for 8 weeks. The water source for aquaculture is fully aerated tap water. During the experiment, water temperature ranged from 23.2 to 28.5°C, ammonia-N was below 0.1 mg L−1, dissolved oxygen was above 6.5 mg L−1, and pH was around 7.5. The photoperiod was 12 L:12 D, with the light period from 08:00 to 20:00 h. Water is exchanged every 2 days and is a quarter the size of the aquarium.
2.3 Sample collection
At the end of the experiment, fish were fasted for 24 h before harvest, and then weighed and counted separately after being anaesthetized with 0.1 g L−1 MS-222 (Sigma). The growth characteristics and survival rate of largemouth bass in each experimental tank were calculated. Twenty-one animals per treatment (7 fish per tank) were individually used for determining hepatosomatic index (HSI), viscera somatic index (VSI), condition factor (CF), and for collecting Liver and intestinal tissues for histology and function assays. All the experiments were conducted under the standard code of protocol for the Care and Use of Laboratory Animals in China. The study protocol and all experimental procedures were approved by the Experimental Animal Ethics Committee of Southwest University.
Six hours after feeding, four fish from each tank were randomly selected and anaesthetized with 0.1 g L−1 MS-222 (Sigma), and blood sample was obtained from the caudal vein using the 1 mL syringe. After standing for 12 h, serum was obtained by centrifugation. The bloodless fish were then dissected to obtain liver and intestinal tissues for RNA isolation, and samples with the protector (RNA/DNA, TaKaRa, Type 9750) were fast frozen in liquid nitrogen. At the same time, the digesta from the whole intestine of each fish was taken. The digesta samples were mixed, collected in sterilized tubes and immediately frozen in liquid nitrogen for microbiome sequencing.
2.4 Chemical analysis
Chemical composition analysis of diets and whole body were conducted by standard methods (AOAC, 2005). Moisture was determined by oven drying to a constant weight at 105°C; crude protein was determined by the micro-Kjeldahl method using the N × 6.25 factor; lipid was determined by ether extraction (without acid hydrolysis) using FOSS Soxtec 2050 (Foss Analytical Instruments Co. Ltd.), liver lipid was determined by chloroform–methanol (2:1); ash was determined by combustion at 550°C for 12 h in a muffle furnace. Liver glycogen content was assayed using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Serum D-lactate, diamineoxidase and endotoxin were assayed using attached kit (Shanghai Youxuan Biotechnology Co., Ltd.). The activities of superoxide dismutase (SOD) and catalase (CAT), glutathione peroxidase(GSH-Px) and malondialdehyde (MDA) contents in liver and intestine were assayed with a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The activities of glucokinase (GK), phosphofructokinase (PFK), fructose-1,6-bisphosphatase (FBPase) and glucose-6-phosphatase (G6Pase) in liver were determined using a commercial kit (SinoBest Biological Technology Co., Ltd.). All enzyme assays were performed in triplicate. The liver and intestinal samples were stained with haematoxylin and eosin (H&E). Subsequently, the liver and intestinal morphology was observed under a light microscope (OLYMPUS, DP73, Nikon Corporation).
2.5 Quantitative real-time PCR analysis
Total RNA in liver and intestine was extracted using RNAISO Plus reagent (Takara) according to the manufacturer's instructions. The RNA quality was assessed by formaldehyde agarose gel electrophoresis, and the concentration of RNA was quantified with a spectrophotometer Nano Drop 2000 (Thermo Scientific). Complementary DNA (cDNA) was synthesized from RNA with a NovoScrip Plus All-in-one 1st Strand cDNA Synthesis Supersis Super Mix (TaKaRa) and stored at −80°C for later use. Quantitative real-time PCR (qPCR) was carried out on the Bio-Rad CFX96 (Bio-Rad) in a total volume of 20 μL with the NovoStart SYBR qPCR Super Mix Plus (Novoprotein) following the manufacturer's protocol. The specific primers of the β-actin gene and target genes were synthesized by Beijing Qingke Biotech Co., Ltd. (shown in Table 2). The relative mRNA expression of target genes was normalized to the β-actin mRNA and determined using the 2−ΔΔCT method (Livak & Schmittgen, 2001).
| Genes | Primers | Sequence 5′−3′ | TM (℃) |
|---|---|---|---|
| SOD | Fa | CCACCAGAGGTCTCACAGCA | 60 |
| Ra | CCACTGAACCGAAGAAGGACT | ||
| CAT | F | TGGTGTTCACGGATGAGATGG | 60 |
| R | GGAGAAGCGGACAGCAATAGG | ||
| GSH-Px | F | ATACCAAGTCTCCTTCCCTCTGT | 59 |
| R | CGTCCACCACTTTGCCATT | ||
| IL−8 | F | CGTTGAACAGACTGGGAGAGATG | 64.9 |
| R | AGTGGGATGGCTTCATTATCTTGT | ||
| IL−10 | F | CGGCACAGAAATCCCAGAGC | 62.1 |
| R | CAGCAGGCTCACAAAATAAACATCT | ||
| IL−1β | F | CGTGACTGACAGCAAAAAGAGG | 59.4 |
| R | GATGCCCAGAGCCACAGTTC | ||
| TGF-β1 | F | GCTCAAAGAGAGCGAGGATG | 59 |
| R | TCCTCTACCATTCGCAATCC | ||
| TNF-α | F | CTTCGTCTACAGCCAGGCATCG | 63 |
| R | TTTGGCACACCGACCTCACC | ||
| GK | F | CATTCGGAGACAACGGGGAG | 60.46 |
| R | ATTGAGGTCTCGTCCACCAC | ||
| PFK | F | ACAACACACCAACTGACACCT | 59.39 |
| R | GCAGCATCGAGCAGAACATGA | ||
| G6Pase | F | GCCTACTGCGTAACATGGGT | 60.11 |
| R | ATCCTGCCTTGAAACTGGCA | ||
| FBPase | F | CACCATGACCCGGTTCGTTA | 60.04 |
| R | CAGGAAATTGCCTTCACCGC | ||
| ZO1 | F | ATCTCAGCAGGGATTCGACG | 59.61 |
| R | CTTTTGCGGTGGCGTTGG | ||
| claudin | F | CCAGGGAAGGGGAGCAATG | 60.08 |
| R | GCTCTTTGAACCAGTGCGAC | ||
| Occludin | F | GATATGGTGGCAGCTACGGT | 59.61 |
| R | TCCTACTGCGGACAGTGTTG | ||
| β-actin | F | AAAGGGAAATCGTGCGTGAC | 60 |
| R | AAGGAAGGCTGGAAGAGGG |
- Abbreviations: CAT, catalase; FBPase, fructose-1,6-bisphosphatase; G6Pase, glucose-6-phosphatase; GK, glucokinase; GSH-Px, glutathione peroxidase; IL-10, interleukin-10; IL-8, interleukin-8; PFK, phosphofructose kinase; SOD, superoxide dismutase; TGF-β1, transforming growth factor-β1; TNF-α, tumour necrosis factor-α; ZO1, zonula occludens-1.
- a F, forward primer; R, reverse primer.
2.6 Intestinal microbiota
Intestinal microorganism index entrusts biological company (Biomarker Technologies Co, LTD) to determine, and the data results were analysed on the BMKCloud. Total bacterial community DNA was extracted using a PowerFecal™ DNA Isolation kit (MO BIO Laboratories), and then quality and quantity of extracted genomic DNA were estimated by agarose gel electrophoresis and a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific). The V3-V4 region of bacterial 16S rRNA gene was amplified by PCR and purified. Then, the high-throughput sequencing was performed using a HiSeq platform (Illumina) at Biomarker Technologies Corporation. Sequences with similarity ≥97% were assigned to the same OTUs. The taxonomic annotation of OTU sequences with reference database to obtain the species classification information corresponding to each OTU and then the community composition of each sample can be counted at each level (phylum, class, order, family, genus and species). The table of species abundance at different taxonomic levels was generated Using a QIIME software (Zaura et al., 2009), and the alpha diversity index of samples was evaluated Using a QIIME2 software (Grice et al., 2009). Meanwhile, phenotypic predictive analysis was performed using the BugBase tool (Ward et al., 2017).
2.7 Calculations and statistical methods
All data were presented as Mean ±S.E.M. The level of significance was determined by one-way analysis of variance (ANOVA) with SPSS 18.0 (IBM) for Windows. Multiple comparisons were performed using Tukey multiple range tests. The statistical significance level was set at p < .05. The graphics were drawn using GraphPad Prism6.0 (GraphPad Software Inc. USA).
3 RESULTS
3.1 Growth performance
The Lower WG and SGR, and higher FCR were observed in fish fed the FM diet (control) (p < .05, Table 3). Compared with CPC group, the CPCY diet significantly enhanced the WG, SGR, PER and FR (p < .05), while no significant differences were observed between the CPCY group and the FM group. Furthermore, the VSI and HSI values, liver lipid and glycogen content of CPCY group were significantly lower than CPC group (p < .05, Table 4). No significant differences were found in the whole body moisture, protein, lipid and ash contents among dietary treatments (p > .05, Table 4).
| Items | Experiment diets | ||
|---|---|---|---|
| FM | CPC | CPCY | |
| Initial body weight (g) | 12.17 ± 0.04 | 12.25 ± 0.12 | 12.08 ± 0.01 |
| Final body weight (g) | 83.55 ± 0.32b | 75.35 ± 1.07a | 86.30 ± 1.06b |
| WG (%)a | 586.34 ± 4.78b | 511.02 ± 3.99a | 614.15 ± 9.07b |
| SGR (%)b | 3.21 ± 0.01b | 3.02 ± 0.11a | 3.28 ± 0.02b |
| PERc | 2.10 ± 0.04a | 1.99 ± 0.17a | 2.42 ± 0.06b |
| FR (% per day)d | 2.14 ± 0.05ab | 1.98 ± 0.18a | 2.50 ± 0.04b |
| FCRe | 0.86 ± 0.04a | 1.04 ± 0.04b | 0.99 ± 0.02ab |
| Survival (%)f | 100 | 100 | 100 |
Note
- Data are expressed as mean ±S.E.M (n = 3). Values in each row with different superscripts have significant differences (p < .05). FM, fish meal-based diet; CPC, 36% of the fish meal replaced by cottonseed protein concentrate; CPCY, CPC diet with 3% yeast culture.
- aWeight gain (WG) = [final weight (g) - initial weight (g)] × 100/initial weight (g).
- bSpecific growth rate (SGR%) = [ln (mean final weight) - ln (mean initial weight)/56 days] × 100.
- cProtein efficiency ratio (PER) = total weight gain (g)/protein intake (g).
- dFeeding ratio (FR) = 100 × total feed intake in dry basis (g)/[final weight (g) + initial weight (g)] × 2/56 days.
- eFeed conversion ratio (FCR) = total feed intake in dry basis (g)/weight gain (g).
- fSurvival rate (SR) = 100 × final fish number/initial fish number.
| Items | Experiment diets | ||
|---|---|---|---|
| FM | CPC | CPCY | |
| Morphological measurements | |||
| CF (%)a | 2.27 ± 0.02a | 2.19 ± 0.02a | 2.46 ± 0.03b |
| VSI (%)b | 8.80 ± 0.42ab | 9.92 ± 0.15b | 8.34 ± 0.30a |
| HSI (%)c | 2.24 ± 0.02b | 2.38 ± 0.06b | 1.69 ± 0.01a |
| Body composition | |||
| Moisture (%) | 70.27 ± 0.12 | 71.17 ± 0.00 | 71.11 ± 0.06 |
| Crude lipid (%) | 6.27 ± 0.04 | 6.10 ± 0.03 | 6.16 ± 0.02 |
| Crude protein (%) | 18.25 ± 0.30 | 17.99 ± 0.08 | 18.24 ± 0.04 |
| Crude ash (%) | 4.32 ± 0.02 | 4.29 ± 0.07 | 4.24 ± 0.02 |
| Liver content | |||
| Liver lipid (%) | 3.76 ± 0.18a | 5.96 ± 0.33b | 4.25 ± 0.34a |
| Liver glycogen (mg/g) | 75.16 ± 0.15a | 85.00 ± 0.39b | 72.55 ± 0.45a |
Note
- Data are expressed as mean ±S.E.M (n = 3). Values in each row with different superscripts have significant differences (p < .05). FM, fish meal-based diet; CPC, 36% of the fish meal replaced by cottonseed protein concentrate; CPCY, CPC diet with 3% yeast culture.
- aCondition factor (CF) = [body weight (g)/standard length3 (cm)] × 100.
- bViscera somatic index (VSI) = 100 × viscera weight(g)/body weight(g).
- cHepatosomatic index (HSI) = 100 × hepatopancreas weight (g)/body weight (g).
3.2 Hepatic parameters
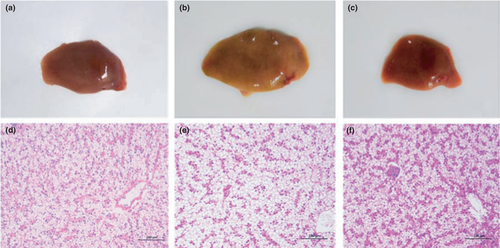
As indicated in Figure 1, the yellowing livers were observed in CPC group, and the hepatic tissue was also disordered including diffused lipid vacuolization in some hepatocytes. However, normal liver colour and liver structure were found when yeast culture was added (CPCY group).
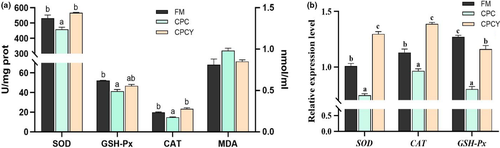
Furthermore, compared with the FM group, the activities of antioxidant enzymes (SOD and CAT) in fish liver of CPC group were significantly decreased (p < .05), but there was no significant difference between CPCY group and FM group (p > .05, Figure 2a). Meanwhile, the gene expression of SOD, CAT and GSH-Px in CPC group was significantly lower than the other group (p < .05, Figure 2b).
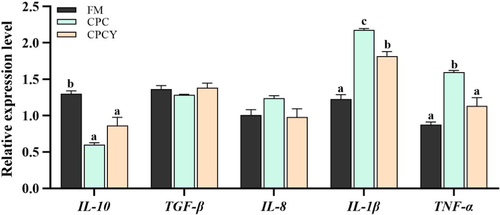
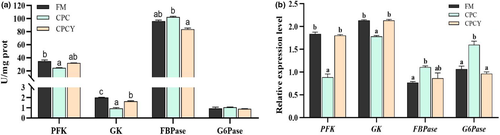
In addition, the expression of pro-inflammatory factors (IL-1β and TNF-α) in liver was significantly up-regulated in CPC group compared with FM group, while it was down-regulated in CPCY group (p < .05, Figure 3). Interestingly, this study found that glycolytic enzyme (PFK and GK) activity was significantly reduced in the CPC group compared with the FM group (p < .05, Figure 4a). However, adding YC to CPC diet significantly increased the activity of glycolysis enzymes (GK) and decreased the activity of gluconeogenesis enzymes (FBPase). At the same time, the expression of glycolysis-related genes (PFK and GK) was significantly up-regulated and the expression of gluconeogenesis related genes (G6Pase) was down-regulated in the CPCY group compared with the CPC group (p < .05, Figure 4b).
3.3 Intestinal health parameters
Compared with FM group, the relative gene expression of intestinal tight junction protein (ZO-1, Claudin and Occludin) in CPC group was significantly down-regulated (p < .05, Figure 5), while the contents of D-lactate (D-lac) and lipopolysaccharide (LPS) in serum were significantly increased as well as the activities of diamine oxidase (DAO) in serum were significantly enhanced (p < .05, Figure 6).
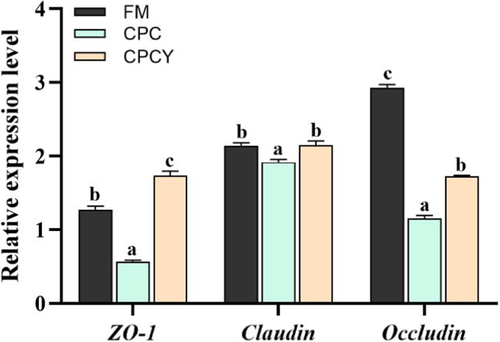
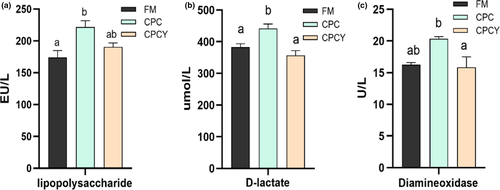
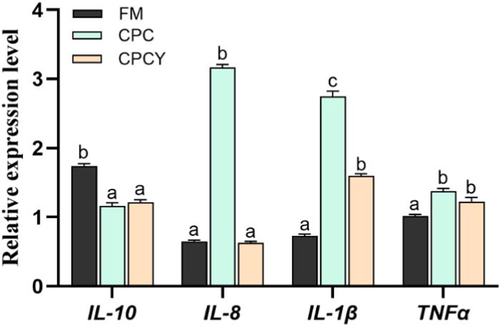
However, the addition of YC to the CPC diet significantly up-regulated intestinal tight junction protein-related gene (ZO-1, Claudin, Occludin) expression and reduced the content of LPS and the activity of DAO in serum (p < .05). Meanwhile, CPC diet significantly up-regulated the expression of pro-inflammatory factors (IL-1β, IL-8 and TNF-α) and down-regulated the expression of anti-inflammatory factors (IL-10) compared with FM group (p < .05, Figure 7), while adding YC in CPC diet significantly down-regulated the expression of pro-inflammatory factors (IL-1β and IL-8).
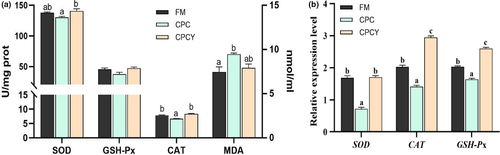
In addition, compared with FM group, the activity of antioxidant enzyme (CAT) in CPC group was significantly decreased, and the level of MDA was significantly increased (p < .05, Figure 8a), but there was no significant difference between CPCY group and FM group (p > .05). Meanwhile, the expression of antioxidant genes (SOD, CAT and GSH-Px) in CPC group was significantly lower than those in FM group and CPCY group (p < .05, Figure 8b).
3.4 Intestinal microbial
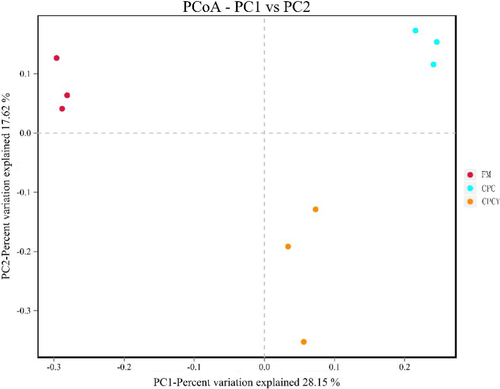
A total of 239,263.17 sequences were obtained from fish intestinal bacteria. All OTUs were from samples with 97% similarity and sample coverage ≥99.7%. Compared with CPC group, the Shannon index and Simpson index of CPCY group had no significant difference, while the Chao1 index increased significantly (p < .05; Table 5). Beta-diversity index of intestine microbiota was presented by principal coordinate analysis (PCoA) by comparing the microbiota similarity of samples. As shown in Figure 9, the clusters of FM, CPC and CPCY group samples separated from each other on the coordinate axis.
| Items | Experiment diets | ||
|---|---|---|---|
| FM | CPC | CPCY | |
| OTUs | 708.50 ± 0.87a | 697.33 ± 18.41a | 873.33 ± 23.34b |
| ACE | 1,363.17 ± 10.01c | 941.25 ± 7.63a | 985.25 ± 7.03b |
| Chao1 | 1,004.60 ± 19.01b | 899.38 ± 16.40a | 970.89 ± 19.23b |
| Simpson | 0.85 ± 0.01 | 0.86 ± 0.06 | 0.94 ± 0.03 |
| Shannon | 5.32 ± 0.28a | 5.72 ± 0.38ab | 7.05 ± 0.49b |
| Goods coverage | 99.82 ± 0.08 | 99.79 ± 0.11 | 99.81 ± 0.18 |
Note
- Data are expressed as mean ±S.E.M (n = 3). Values in each row with different superscripts have significant differences (p < .05). FM, fish meal-based diet; CPC, 36% of the fish meal replaced by cottonseed protein concentrate; CPCY, CPC diet with 3% yeast culture.
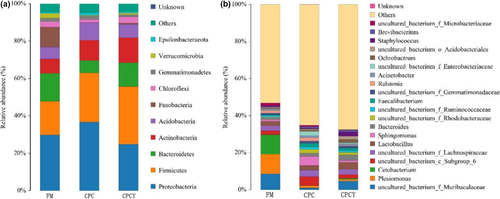
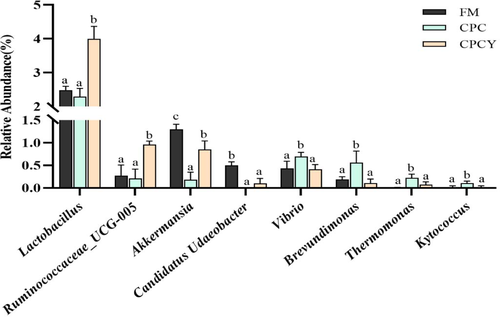
At the phylum level, the intestinal bacterial profiles of largemouth bass were dominated by Proteobacteria and Firmicutes, followed by Bacteroidetes and Actinobacteria (Figure 10a). Notably, the relative abundance of Proteobacteria in the CPC group increased from 29.85% to 36.73%, while that in the CPCY group decreased to 24.84% compared with the FM group. Meanwhile, the relative abundance of Bacteroidetes in the CPC group decreased from 14.99% to 6.68%, while that in the CPCY group increased to 12.81%. At the genus level, Plesiomonas and Cetobacterium were the dominant genera in the FM group, while Lactobacillus, Sphingomonas and Bacteroides were the dominant genera in the CPC group and the CPCY group (Figure 10b). Further Metastat analyses showed that the relative abundances of Lactobacillus, Ruminococcaceae UCG-005, Akkermansia and Candidatus Udaeobacter increased, and the relative abundance of Vibrio, Brevundimonas, Thermomonas and Kytococcus decreased significantly in the CPCY group compared with the CPC group (p < .05; Figure 11).
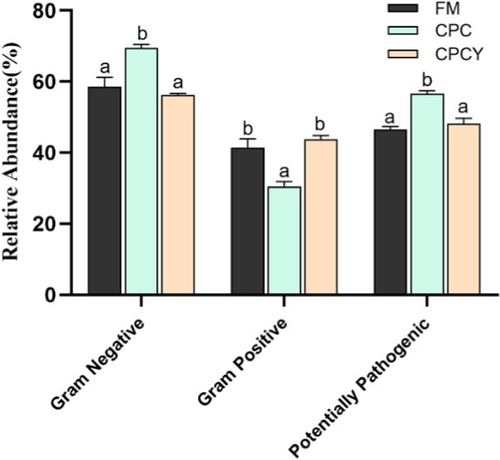
In addition, we used BugBase tools to predict microbial phenotypes (Figure 12). The results showed the relative abundance of intestinal gram-negative bacteria and potential pathogenic bacteria increased significantly in CPC group (p < .05), whereas decreased in CPCY group compared with FM group (p < .05).
4 DISCUSSION
The results of the present study clearly show that the plant proteins significantly affected the growth of largemouth bass. Fish fed the diet containing 15.8% CPC showed lower growth and PER compared with the control group, which indicate that incorporating more than 15.8% CPC in the diet has been shown to depress growth of largemouth bass. Similar results were also reported by He, Yu, et al. (2020); He, Li, et al. (2020), in which the substitution level of FM with SBM was only 15% (16.2% SBM) in largemouth bass diet. In recent years, the nutritive value and potential of plant proteins for fish feeds have been examined in numerous studies (Jiang et al., 2018; Lin et al., 2010; Xu et al., 2017).
A large number of studies have shown that high dietary inclusion levels of CPC result in decreased growth performance and feed efficiency in fish (Liu et al., 2020; Ye et al., 2020). Noteworthy, the present study shows that replacement of a part of fish meal by increasing levels of yeast culture in diets significantly results in growth improvement, indicating a better valorization of the feed as shown by the better PER observed with CPCY. Previous study also has demonstrated that yeast production is a suitable protein to elevate the nutritive values of diets for fish (Vidakovic et al., 2016). In fact, there are numerous studies that demonstrated that yeast and its derivatives may have beneficial effects on growth performance and health (Shurson, 2018). Accordingly, this suggested that the yeast culture supplementation can achieve balanced nutritional composition and improve the palatability to further reduce the use of fishmeal cost-effectively in aquafeeds (Guo et al., 2019; Wang et al., 2017).
Surprisingly, the results of the present study show that yeast incorporation in aquafeeds appears as a promising solution to decrease both lipid and glycogen content in liver. At the same time, the activities of hepatic glycolytic enzymes (GK and PFK) increased, whereas the hepatic gluconeogenic enzyme activities (FBPase and G6Pase) decreased significantly when fish fed with yeast culture, thus resulting in the decrease in glycogen content in the liver. High glycogen content in the liver will induce fat synthesis and increase hepatic fat deposition (Brauge et al., 1994). These investigations showed that the changes in HSI might result from the increase in lipid content and glycogen content in the liver. The results of this study suggested that endogenous glucose synthesis was particularly depressed by the yeast culture supplementation, even though further studies would be necessary to prove it.
The current study further found that dietary high-plant protein level significantly affected the activities of liver antioxidant defence enzymes (SOD, CAT and GSH-Px), which was also observed in tilapia (Lin et al., 2010). This suggests that the antioxidant system of largemouth bass is damaged because of certain constituents of plant proteins, and which could be partly responsible for the changes in hepatic morphology in this study. Previous studies have shown that anti-nutrition factors (gossypol) in cottonseed protein accumulate in the liver tissue of fish and cause inflammation (Jensen et al., 1982; Lee et al., 2002; Soares et al., 2012). However, adding yeast culture to feed can reduce gossypol induced liver inflammation of Ussuri catfish (Pseudobagrus ussuriensis) (Bu et al., 2020). Similarly, effects of YC supplementation on antioxidant capacity are observed in weaning pigs (Yang et al., 2016). Additionally, we also found that yeast culture down-regulated the expression of pro-inflammatory cytokines (IL-1β and TNFα) and increases the antioxidant capacity of liver in largemouth bass. Therefore, fish prefed diet with yeast culture supplementation had lower occurrences of histopathological symptoms in response to high-plant protein diet, suggesting the mitigative effect of yeast culture on the hepatic lesion induced by high-plant protein for largemouth bass. This may be related to the presence of β-glucans and mannan oligosaccharides in yeast cultures (Ganner & Schatzmayr, 2012). Studies have shown that β-glucans in yeast culture can inhibit the excessive activation of the inflammatory response during the later phase of infection (Sherif et al., 2019). Apart from increasing the anti-inflammatory cytokine transcripts, β-glucans can prevent the degradation of NF-κB inhibitor to minimize the expression of pro-inflammatory cytokines (tnf-α, il-1β and il-8) in the fish (Ren et al., 2019). Moreover, studies indicate that manna oligosaccharides can improve the antioxidant capacity of the liver of European sea bass and repair the liver tissue structure (Torrecillas et al., 2007). These results show that yeast cultures are effective against liver inflammation. Clearly, more research is needed in this area to elucidate the repeatability of these findings.
The intestine is one of the organs for fish to digest and absorb nutrients, which play an essential role in maintaining multiple physiological and pathological processes, and its health status is closely related to the growth of fish. Previous studies have shown that high-plant protein often causes intestinal damage in aquatic animals such as largemouth bass (He, Li, et al., 2020; He, Yu, et al., 2020) and amur sturgeon (Acipenser schrenckii) (Wei et al., 2019). In this investigation, fish fed the high CPC diet led to the increase in intestinal permeability and the upregulation of pro-inflammatory factor (IL-1β, IL-8 and TNFα). Unexpectedly, however, dietary yeast culture supplementation to a plant protein-based diet improved the health status of intestine in this study. On one hand, the yeast-containing diets can improve the integrity of intestinal tight junction proteins and inhibit the expression of pro-inflammatory factor (TNFα) (Wang et al., 2020). On the other hand, dietary supplementation of yeast culture could up-regulate the express of the intestinal barrier-related genes (Occludin and Claudin 1) (Zhang et al., 2020). Additionally, the current experiment also confirmed that yeast supplementation enhances the antioxidant capacity of the gut. These data suggest that yeast supplementation may be able to reduce inflammatory reactions induced by high-plant protein during the receiving period. The main reason evoked to explain the efficiencies of yeast culture is the presence of some yeast components, such as MOS, may have a strong beneficial effect on gut health condition in fish fed yeast-containing diets. Thus, it is possible to hypothesize that yeast culture supplementation in high-plant protein diets may have contributed to enhance the protective properties of the intestinal epithelium and facilitate nutrient absorption, which is consistent with the better growth of largemouth bass of this group. It seems that a period of adaptation is needed before the effects of yeast culture supplementation can be significant because the changes in intestinal morphology and immune responses take time.
Notably, the current study found that diet structure can modify intestinal bacterial communities, and high-plant protein diet decreased the abundance of intestinal microflora of largemouth bass. Interestingly, the supplementation of yeast culture could restore both ACE and Chao1 indices, which indicated that yeast culture can regulate the stability of intestinal microflora in this study, consistent with the result of Liu et al. (2018). In addition, the present results also pointed out that Proteobacteria and Firmicutes were the two dominant phyla of largemouth bass. This is in agreement with previous reports of the same species (Zhou et al., 2018). Meanwhile, we found that the abundance of Proteobacteria increased caused by feeding CPC diet. Previous studies have shown that an increase in Proteobacteria is often regarded as a marker of microbial community instability (Shin et al., 2015), which may lead to nutritional and metabolic disorders in the host. More importantly, the bacterial phylum Proteobacteria is closely correlated with expression of IL-10 and TNF-α, and trigger intestinal inflammatory responses (Larsen et al., 2010; Livak et al., 2001; Zhu et al., 2013). However, the supplementation of yeast culture in CPC diet not only decreased the abundance of Proteobacteria, but also increased the abundance of Bacteroidetes. It is of note that elevated Bacteroidetes conduced to the protection against gut inflammation (Ferreira et al., 2011). These results suggested that yeast cultures may be able to improve intestinal inflammation by regulating intestinal microbiota.
Further analysis at the genus level showed that CPCY diet increased the abundance of lactobacillus, Ruminococcaceae UCG-005 and Akkermansia. Studies indicate that lactobacillus could reduce the intestinal permeability and exert a protective effect on intestinal epithelium via inhibiting the pro-inflammatory factor (IL-6, TNF-α, TLR4 and NF-κB), and activating IL-10 expression (Liu et al., 2011). The Ruminococcaceae UCG-005 has been reported to be butyrate-producing bacteria that may protect healthy subjects from chronic intestinal inflammation (Mancabelli et al., 2017). The metabolites synthesized by Akkermansia muciniphila modulate intestinal barrier integrity in vitro by strengthening the pore pathway and regulating tight junction protein expression (Cruz-Lebron et al., 2021). These results suggested that yeast cultures may strengthen the intestinal barrier and enhance intestinal immunity by increasing the abundance of these beneficial bacteria. On the contrary, supplement the yeast culture in diet decreased the abundance of Vibrio, Brevundimonas and Kytococcus. Noteworthily, Vibrio is one of the most common bacterial infections in fish, which causing inflammation and mortality (Pan et al., 2017). Studies have shown that Vibrio anguillarum induces inflammation and apoptosis of silvery pomfret (Pampus argenteus) epithelial cells (Gao et al., 2016). Moreover, Brevundimonas and Kytococcus are increasingly identified as an opportunistic pathogen in infections leading to bacteremia, especially among immunosuppressed patients (Chi et al., 2004) (Blennow et al., 2012; Chi et al., 2004). Subsequently, the Bugbase phenotype analysis again demonstrated that the supplementation of yeast culture could reduce the abundance of harmful bacteria and increase the abundance of beneficial bacteria, thus improving the intestinal ecosystem. This suggested that the yeast culture has positive effects on key microbial populations and their metabolism, and consequently mediating intestinal immune function and stabilizing intestinal environment. However, the specific mechanisms and interactions between yeast culture and intestinal bacteria in largemouth bass remains unclear.
In conclusion, the present study successfully confirmed that dietary supplementation of yeast culture improved the performance of largemouth bass fed with high-plant protein diet. Furthermore, the positive effects of yeast culture on the performance of largemouth bass were associated with enhanced hepatic antioxidant capacity and specific immune-inflammatory biomarkers. Moreover, the improvement in the intestinal health by dietary yeast culture supplementation was related to the upregulation of intestinal barrier-related genes and positive effects on intestinal bacterial populations. Overall, these findings provide a potential explanation for the mechanisms that mediate the positive effects of yeast culture on largemouth bass. Thus, these findings will be beneficial for the nutritional management of largemouth bass.
ACKNOWLEDGEMENTS
This research was supported by Chongqing Technology Innovation and Application Development Project (cstc2020jscx-msxmX0046) and Chongqing Ecological Fishery Technology System, China (2021), the Special Fund for Beijing Enhalor Bio-Tech Co., Ltd. We thank W. M. Kuang and X. M. Zhou for their help during the experiment. We also thank H. Shun and J.X. Wu for their help with the chemical analysis.
CONFLICT OF INTEREST
The authors report no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data supporting the results of this study can be obtained from corresponding author upon reasonable request.




