Growth performance, immune response, antioxidant capacity and disease resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss) as influenced through singular or combined consumption of resveratrol and two-strain probiotics
Abstract
In the current study, the possible synergistic properties of resveratrol (Res) and two-strain probiotics (Pro; Lactobacillus acidophilus and Bifidobacterium bifidum) on growth performance, innate immune responses, antioxidant defence and disease resistance against Yersinia ruckeri were assayed in rainbow trout. Accordingly, fish (10.42 ± 0.26 g) were fed with a basal diet as control and diets supplemented with R1 (400 mg/kg Res), R2 (800 mg/kg Res), P1 (0.5 g/kg Pro), P2 (1 g/kg Pro), R1P1 (400 mg/kg Res + 0.5 g/kg Pro) and R2P2 (800 mg/kg Res + 1g/kg Pro) for 8 weeks. The final weight, weight gain and feed conversion rate significantly improved in fish fed the P1, P2, R1P1 and R2P2 (p < .05), after 8 weeks. Diets containing R1P1 and R2P2 significantly elevated the total leucocyte count, and percentages of monocyte and neutrophil at the end of the 8th week (p < .05). Serum complement activity, lysozyme activity and bactericidal activity (BA) of fish fed the diets containing P1, P2, R1P1 and R2P2 were higher than the control group. Oral administration of R2P2 remarkably increased mucus immune parameters (lysozyme, BA, alkaline phosphatase and total immunoglobulin) (p < .05). Moreover, the highest activity of catalase, superoxide dismutase and glutathione peroxidase, and the survival rate were recorded in the R2P2 group. In conclusion, R2P2 remarkably elevated growth performance, innate immune and antioxidant responses, and disease resistance in rainbow trout.
1 INTRODUCTION
The global demand for seafood just keeps growing, and the only way to fulfil this demand is through aquaculture (Pradeepkiran, 2019). Despite the progress of fish production technologies, the fish is constantly exposed to diverse stressors (e.g. sorting, handling and poor water quality) during the rearing period in the intensive and super-intensive production methods (Dawood et al., 2018; Galina et al., 2009; Nayak, 2010). Hence, the fish farmers may be persuaded to use antibiotics extensively to control or treat infectious diseases on a commercial scale (Galina et al., 2009; Hoseinifar et al., 2015; Oliva-Teles, 2012). However, there has been great concern about the use of antibiotics in animal production due to their side effects on the host, environment and humans (Dawood et al., 2019; Elumalai et al., 2020). Recently, the use of diets fortified by functional feed additives with natural origins such as phytochemicals, probiotics, prebiotics and acidifiers has been strongly recommended to boost the innate immune system and control infectious diseases in aquafarming (Awad & Awaad, 2017; Dawood & Koshio, 2016; Hoseinifar et al., 2018; Stratev et al., 2018). Moreover, most studies have stated that natural supplements alleviate the effects of stress on the host by improving its physiological capacity (Aydın & Barbas, 2020; Dawood et al., 2018).
Phytochemicals are a promising solution for limiting antibiotic use in aquaculture. These compounds are usually inexpensive, eco-friendly and accessible (Elumalai et al., 2020; Reverter et al., 2014). In this context, resveratrol is a polyphenolic phytoalexin derived from different herbs such as grape seed, strawberries and blueberries, which is produced by the enzymatic stilbene synthase in the plant cells in response to stressful conditions and infection diseases (Athar et al., 2009; Averilla et al., 2019). Pharmacological studies have reported the therapeutic effects of resveratrol against senescent, cancer, obesity and diabetes in animals (Candelario-Jalil et al., 2007; Vervandier-Fasseur & Latruffe, 2019). Moreover, several studies have found beneficial effects of resveratrol on the physiological functions of aquatic animals such as inflammatory responses, growth and skeletal muscle physiology, and that resveratrol increases nutrient digestibility and attenuates oxidative stress (Castro et al., 2008; Tan et al., 2019; Torno et al., 2018; Wilson et al., 2015). However, there is a lack of information about the role of dietary resveratrol as an immunostimulant in aquatic animals.
Probiotics are another prominent candidate for antibiotic replacement and disease control in the aquaculture industry (Gatesoupe, 1999). These microorganisms exert beneficial effects on the host through a variety of mechanisms such as the production of biomolecules (short-chain fatty acids, vitamins, enzymes, antimicrobial substances, etc.) and competing with pathogenic bacteria for binding sites in the gastrointestinal tract (Dawood et al., 2019; Lazado & Caipang, 2014; Ringø et al., 2010). In this regard, Lactobacillus acidophilus and Bifidobacterium bifidum are well-known probiotic genera that have been widely used in aquaculture nutrition in recent years (Panigrahi et al., 2010; Ringø et al., 2018). It was pointed out that the combination of lactobacilli and bifidobacteria has stronger effects on the host health than their single use, because lactobacilli are important producers of lactic acid, whereas bifidobacteria are generators of short-chain fatty acids as the major end product (Tojo et al., 2014). The beneficial effects of both probiotic species on immune parameters and disease resistance have been reported in several fish species such as B. animalis and B. lactis in rainbow trout (Oncorhynchus mykiss; Sahandi et al., 2019), L. acidophilus in African catfish (Clarias gariepinus; Al-Dohail et al., 2009), L. acidophilus in Nile tilapia (Oreochromis niloticus; Hassaan et al., 2021) and L. acidophilus in black swordtail (Xiphophorus helleri; Hoseinifar et al., 2015).
It seems that the combination of herbal compounds and probiotics in aquafeed can lead to a wide range of biological activities, synergistic effects and faster induction of physiological reactions in animals. Therefore, the current study was carried out to evaluate the possible effects of resveratrol alone or in combination with a mixture of probiotics (L. acidophilus and B. bifidum) on growth performance, immune system parameters in mucus and serum, antioxidant defence and resistance against Yersinia ruckeri in rainbow trout fingerlings for 8 weeks in two time intervals. Indeed, the measurements of the physiological parameters in the 4th and 8th weeks reveal to us whether the experimental diet containing probiotics and resveratrol, especially in the combined form, can lead to faster induction of host physiological responses or not.
2 MATERIALS AND METHODS
2.1 Experimental diets
A commercial probiotic (Pro) mixture (L. acidophilus; 3 × 109 CFU/g +B. bifidum; 3 × 109 CFU/g) and resveratrol (Res) were purchased from TakGene (Iran, Tehran) and Sigma-Aldrich (purity > 99%), respectively. The experimental diets were prepared using the feedstuffs presented in Table 1. In summary, after mixing the dry components of the diet, 300 ml/kg of water was added to the mixture. The produced dough was turned into pellets by a meat grinder with a 3 mm die diameter. The pellets were dried at room temperature for 48 h. Also, the diets containing Res (400 and 800 mg/kg) were produced by adding Res to the dough. After dehumidifying the pellets, the commercial probiotic mixture was suspended in phosphate buffer saline (PBS) and supplemented to the diets by the spraying method using 3% gelatine as a coating agent. Finally, the biochemical composition of the diet was measured according to the AOAC (2016) and is presented in Table 1. Moreover, during the period of the feeding trial, the viability of the probiotic count in the diets was carried out using the method of spread plate on TSA (tryptic soy agar). In this study, the basal diet was considered as a control feed and other experimental diets were produced by supplementing the basal diet using the Pro or Res as follows: C (basal diet), R1 (400 mg Res), R2 (800 mg Res), P1 (0.5 g Pro), P2 (1 g Pro), R1P1 (0.5 g Pro + 400 mg Res) and R2P2 (1g Pro + 800 mg Res). The concentrations of the Pro and Res in this study were selected based on their positive effects in previous reports (Miandare et al., 2016; Soltani et al., 2017; Torno et al., 2017, 2018).
| Ingredients | (g/kg in dry basis) | Proximate composition | (g/kg) |
|---|---|---|---|
| Fishmeala | 320 | Crude protein | 425 |
| Soybean meal (defatted)b | 260 | Crude lipid | 164 |
| Wheat flour | 168 | Crude fibre | 32 |
| Meat mealc | 100 | Crude ash | 96 |
| Fish oil | 60 | ||
| Soybean oil | 50 | ||
| Mineral mixd | 16 | ||
| Vitamin mixd | 10 | ||
| Cellulose (Res) | 10 | ||
| Phytasee | 3 | ||
| DL-methioninef | 3 | ||
| Total | 1000 |
- a 67% protein, 10% lipid and 13% ash (Khazar Company, Mazandaran Province, Iran).
- b Gorgan Soya Co., Gorgan, Iran (46% protein).
- c 60% protein and 18% lipid.
- d The premixes were provided followed by Hosseini Shekarabi et al. (2021).
- e Golbid Co., Tehran, Iran (10,000 IU).
- f Mad Tiour Co., Sanandaj, Iran.
2.2 Experimental procedure
Seven hundred fingerlings of rainbow trout (O. mykiss) with an average weight of 10.02 ± 0.10 g were purchased from a local fish farm (Mirshekarloo village, Urmia City, West Azerbaijan Province, Iran). All fish were regulated to the laboratory conditions (water temperature of 17 ± 1°C, dissolved oxygen of 7.5–8.5 mg/L, pH: 7.2–7.8) for 14 days in 1000-L tanks and were fed with the basal diet before starting the experiment. After the period of adaptation, to design seven experimental groups with triplicate, six hundred and thirty fingerlings (mean weight: 10.42 ± 0.26 g) were randomly distributed into 21 fibreglass 300-L tanks (30 fingerlings in each tank). The water quality was maintained at a high level via constant ventilation of the tanks by a blower (STREAM-HG-1500B, China), and the removal of the wastes twice a day. The rainbow trout were fed based on ad libitum three times per day (8:00, 13:00 and 18:00) for 8 weeks. The biomass in each replicate was weighed biweekly.
2.3 Sample collection
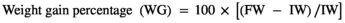
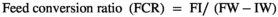
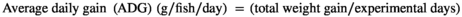
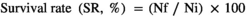
FW; final weight, IW; initial weight, Nf; fish number at the end of trial feeding, Ni; fish number at the beginning of trial feeding.
To collect blood samples, three fish from each replicate tank (9 fish per treatment) were anaesthetized using clove powder (200 mg/L). The blood was withdrawn from the caudal vein using a syringe (2.0 ml). The first part of the blood sample was transferred to tubes containing heparin to measure the number of white blood cells (WBC). Another part of the blood was used for serum isolation by leaving them for 3 h at 4°C to clot followed by centrifuging at 3,000 g for 20 min at 4°C. The supernatants were retained as serum and kept frozen at −80°C until immunological and antioxidant assays (Webb et al., 2007).
The fish skin mucus was sampled based on the methods described by Ross et al. (2000). Briefly, three fish from each tank were randomly selected and individually placed into polyethylene bags, and 10 ml of 50 mM NaCl was added to the bags. Epidermal mucus was collected by rubbing the fish for 2–3 min. Then, the mucus samples were transferred to sterile vials and centrifuged at 2500 g (for 10 min at 4°C), and the supernatants were kept at −80°C for subsequent analysis.
2.4 Total and differential WBC counts
Total leucocyte count was determined by haemocytometer slide based on the protocol described by Martins et al. (2004). Besides, differential leucocyte count was performed using blood smear preparation with Giemsa staining under an electron microscope (Borges et al., 2004).
2.5 Immunological assays in the serum and mucus samples
The lysozyme activity in mucus and serum samples was measured based on the lysis of Micrococcus lysodeikticus cells (Ellis, 2001) using a spectrophotometer (Biophotometer Eppendorf) at 450 nm for 6 min in 30-s intervals. One unit of lysozyme activity was considered as a reduction in optical density at 0.001 min−1. Alternative complement pathway activity (ACH50) was estimated in the serum samples using lysis of rabbit red blood cells (RaRBC) based on the protocol described by Yano (1992).
The serum and mucus immunoglobulin (Ig) levels were determined according to the Siwicki (1993) method by the difference between the protein concentrations before and after the addition of 12% polyethylene glycol.
Skin mucus alkaline phosphatase (ALP) was determined by a commercial kit (Pars Azmoon; Tehran, Iran) using a spectrophotometer (Biophotometer, Eppendorf) at 405 nm (Oroji et al., 2021) based on the manufacturer's protocol.
Serum and skin mucus bactericidal activity (BA) against Y. ruckeri was measured in vitro by the method of Fazelan et al. (2020). Briefly, Y. ruckeri (KC291153) was harvested from the cultivation plate and then washed twice with sterile PBS. The absorbance of the bacterial suspension at 600 nm was read by the spectrophotometer. Then, the bacterial suspension was diluted five times with PBS (1:10). In the next step, 200 µl serum was added to 2 ml of the bacterial suspension. To commencement of the reaction, the samples were stored at 22°C for 1 h, and the mixture was cultured on tryptic soy agar (TSA) medium and incubated at 22°C for 24 h. Also, the control sample was prepared using 20 ml of PBS. After the incubation period, the number of grown colonies was counted.
2.6 Serum antioxidant enzyme activity
The activity of serum antioxidant enzymes including catalase (CAT), glutathione peroxidase (GPx) and superoxide dismutase (SOD) was estimated using a commercial kit (ZellBio GmbH, Germany) according to the manufacturer's protocols. Serum malondialdehyde (MDA) value was evaluated according to reaction with thiobarbituric acid at 95°C for 1 h using the method of Rajabiesterabadi et al. (2020).
2.7 In vivo fish infection model
2.8 Statistical analysis
Statistical analysis of data was done using software SPSS (version 20). At first, the normal distribution and homogeneity of the data were verified by the Kolmogorov–Smirnov test and Levene's test, respectively. All results are expressed as the mean ± standard deviation (SD). Analysis of results was performed by one-way ANOVA, and Tukey's test was used to evaluate significant variation (p < .05) among the treatments.
3 RESULTS
3.1 Growth performance and feed utilization
The growth indices and feed utilization of O. mykiss fed with levels of Res and Pro on the 4th and 8th weeks are presented in Table 2. On the 4th week, no significant difference was observed between the experimental groups (p > .05); however, growth performance was slightly increased in R2P2 treatment. On the contrary, growth performance and feed utilization were significantly affected by the experimental diets on the 8th week (p < .05). FW and WG in rainbow trout remarkably responded to diets supplemented P1, P2, R1P1 and R2P2, when compared to the control group (p < .05), and the highest values were obtained in R1P1 and R2P2. Fish fed with the diet containing R2P2 significantly increased the ADG compared with those receiving the basal diet. Besides, the FCR value was significantly lower in fish fed the P1, P2, R1P1 and R2P2 (p < .05). However, there was no significant difference in SR among the experimental groups (p > .05).
| Parameter | Different experimental diets | ||||||
|---|---|---|---|---|---|---|---|
| Control | R1 | R2 | P1 | P2 | R1 + P1 | R2 + P2 | |
| IW(g) | 10.37±0.23a | 10.40±0.32a | 10.32±0.29a | 10.52±0.22a | 10.28±0.35a | 10.48±0.34a | 10.50±0.18a |
| FW (g) | |||||||
| 4 weeks | 30.75±1.26a | 31.50±0.91a | 31.12±2.01a | 31.20±2.66a | 31.87±1.93a | 32.37±2.13a | 32.50±2.88 a |
| 8 weeks | 65.00±1.37c | 67.00±1.82bc | 71.25±1.50ab | 73.67±1.29a | 75.25±2.21a | 75.00±2.94a | 74.25±2.62a |
| ADG (g) | |||||||
| 4 weeks | 0.72±0.04a | 0.73±0.03a | 0.74±0.06a | 0.75±0.10a | 0.77±0.07a | 0.78±0.09a | 0.79±0.08a |
| 8 weeks | 0.97±0.02b | 1.01±0.03ab | 1.13±0.14ab | 1.12±0.03ab | 1.13±0.03ab | 1.17±0.05ab | 1.18±0.06a |
| WG (g) | |||||||
| 4 weeks | 20.37±1.21a | 21.09±0.87a | 20.79±1.91a | 20.67±2.82a | 21.59±1.95a | 21.89±2.41a | 22.0±2.75a |
| 8 weeks | 54.62±1.37c | 56.59±1.82bc | 60.92±1.43ab | 63.15±1.51a | 64.96±2.29a | 64.51±3.27a | 63.75±2.73a |
| FCR | |||||||
| 4 weeks | 2.05±0.16a | 1.88±0.15a | 1.93±0.24a | 1.95±0.33a | 1.74±0.16a | 1.82±0.21a | 1.78±0.26a |
| 8 weeks | 1.58±0.06a | 1.47±0.12ab | 1.36±0.09abc | 1.31±0.07bc | 1.28±0.6bc | 1.26±0.16bc | 1.20±0.09c |
| SR (%) | |||||||
| 4 weeks | 98.0 ±2.30a | 98±2.30a | 97.25±3.40a | 98±2.30a | 97.25±3.40a | 98.0±2.30a | 97.25±3.40a |
| 8 weeks | 96.5±4.04a | 98±2.30a | 97.25±3.40a | 98±2.30a | 97.25±3.43a | 97.25±3.40a | 98±2.30a |
Note
- Different letters in each column show significant differences among the experimental groups (p < .05). Values are shown as mean ± SD (n = 3).
- Abbreviations: ADG: average daily gain; FCR: feed conversation rate; FW, final weight (g); IW, initial weight (g); SR: survival rate (%); WG, weight gain (g).
3.2 Total and differential WBC counts
The results of the leukogram profile in rainbow trout fed with the experimental diets are illustrated in Table 3. The WBC count in the treated groups significantly changed after the 4th and 8th weeks. Accordingly, WBC count in the R1P1 and R2P2 groups significantly increased compared with the fish fed with P1, R1, R2 and control diets, after the 4 and 8 weeks (p < .05). At the end of the 4th and 8th weeks, the fish fed with a diet containing R2P2 had a higher neutrophil percentage than those that fed with diets supplemented with R1, R2 and the control diet (p < .05). Besides, there was no significant difference in lymphocyte percentage in different experimental groups after the 4th and 8th weeks (p > .05). Finally, the highest monocyte percentage was observed in rainbow trout fed with R1P1, following by R2P2, after the 4th and 8th weeks (p < .05).
| Parameter | Different experimental diets | ||||||
|---|---|---|---|---|---|---|---|
| Control | R1 | R2 | P1 | P2 | R1+P1 | R2+P2 | |
| WBC × (103/µl) | |||||||
| 4 weeks | 12.09 ± 2.20cd | 11.65 ± 1.12d | 12.50 ± 0.72cd | 13.15 ± 1.09cd | 13.71 ± 0.87bc | 15.48 ± 1.21ab | 16.05 ± 0.75a |
| 8 weeks | 12.19 ± 0.62e | 12.62 ± 0.05e | 12.67 ± 0.04e | 13.19 ± 0.06d | 15.86 ± 0.27c | 19.30 ± 0.07b | 21.63 ± 0.56a |
| Lym (%) | |||||||
| 4 weeks | 82.11 ± 2.16a | 81.88 ± 1.78a | 81.16 ± 2.11a | 80.16 ± 1.82a | 79.53 ± 1.43a | 78.89 ± 2.06a | 77.97 ± 2.29a |
| 8 weeks | 80.60 ± 2.23a | 81.01 ± 4.18a | 78.14 ± 2.49a | 77.29 ± 2.48a | 76.95 ± 2.77a | 75.28 ± 2.51a | 75.16 ± 3.04a |
| Neu (%) | |||||||
| 4 weeks | 13.94 ± 1.99b | 13.72 ± 1.99b | 13.62 ± 2.41b | 14.19 ± 2.53ab | 14.33 ± 1.99ab | 16.47 ± 1.53ab | 17.14 ± 2.01a |
| 8 weeks | 14.61 ± 0.66c | 14.52 ± 0.75c | 15.09 ± 1.11c | 15.27 ± 1.13c | 15.71 ± 1.11bc | 17.16 ± 1.12ab | 17.71 ± 1.14a |
| Mono (%) | |||||||
| 4 weeks | 2.51 ± 0.23c | 2.74 ± 0.18bc | 2.48 ± 0.21c | 3.50 ± 0.16ab | 3.50 ± 0.19ab | 3.71 ± 0.11a | 3.26 ± 0.69abc |
| 8 weeks | 3.75 ± 0.10d | 3.31 ± 0.11d | 4.77 ± 0.17c | 4.98 ± 0.20bc | 5.53 ± 0.21ab | 5.74 ± 0.24a | 5.50 ± 0.47ab |
Note
- Different letters in each column show significant differences among the experimental groups (p < 0.05). Values are shown as mean ± SD (n = 3).
- Abbreviations: Lym, lymphocytes; Mono, monocytes; Neu, Neutrophils; WBC, white blood cell.
3.3 Serum immune parameters
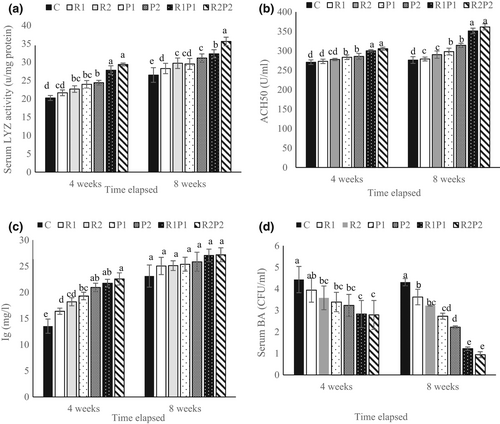
Dietary supplementation of the combined form of Pro and Res (R1P1 and R2P2 diets) showed a significant increase in lysozyme activity when compared to the other groups on the 4th week (p < .05; Figure 1a). At the end of the 8th week, a peak lysozyme activity was observed in the fish fed a diet containing R2P2 (Figure 1a). ACH50 activity was also significantly higher in rainbow trout fed a combined form of Res and Pro than in the fish fed the other experimental diets at the end of the 4th and 8th weeks (p < .05; Figure 1b). Besides, all treatments revealed a significant improvement in the serum Ig level after 4 weeks (p < .05), while there was no significant difference at the end of the feeding trial (p > .05; Figure 1c). The diets enriched with R2, P1, P2 and the combined form of Pro and Res resulted in a significant improvement in the serum bactericidal activity at the end of the 4th and 8th weeks (p < .05; Figure 1d).
3.4 Immunological parameters in the skin mucus samples
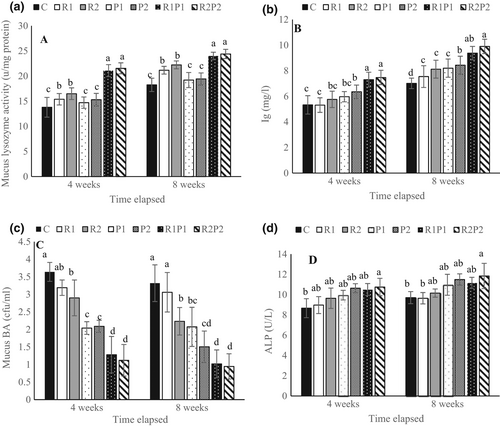
The mucosal immune parameters of rainbow trout were affected by the treated diets and are presented in Figure 2. After the 4th and 8th weeks of the feeding trial, the mucus lysozyme activity and BA were remarkably increased in the fish fed with diets containing R2, R1P1 and R2P2 (p < .05; Figure 2a,c). The mucus Ig and ALP values were significantly improved in the fish fed with the R2P2 diet compared with the fish fed the basal diet throughout the experiment period (p < .05; Figure 2b,d).
3.5 Serum enzymatic antioxidant assay
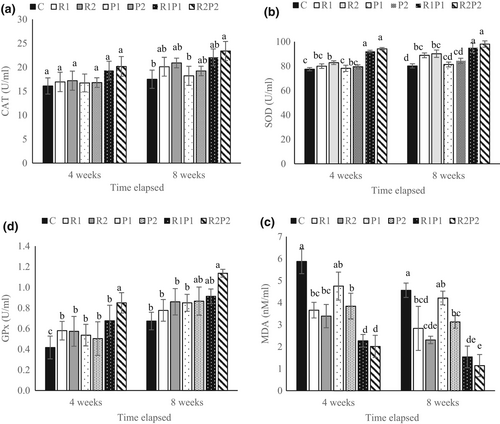
The activity of serum antioxidant enzymes of the fish treated with the commercial Pro mixture and Res in the 4th and 8th weeks is shown in Figure 3. The present study showed that the level of CAT activity was similar in different experimental groups at the end of the 4th week, while it was higher in the groups fed with R2P2 than the control group at the end of the 8th week (p < .05; Figure 3a). SOD activity was significantly higher in the fish fed with R1P1 and R2P2 than in those receiving the other diets at the end of the 4th and 8th weeks (p < .05; Figure 3b). In the case of GPx activity, the findings strongly showed an improvement in the serum GPx activity in the specimens fed with a diet supplemented with R2P2 after the 4th and 8th weeks (p < .05; Figure 3c). On the contrary, single or combined administration of Res significantly reduced the serum MD level after the 4th or 8th weeks (p < .05; Figure 3d).
3.6 Experimental infection
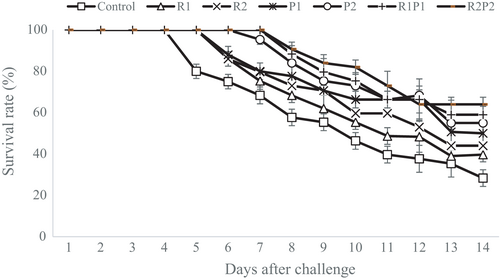
The SR of rainbow trout during 14-day challenge with Y. ruckeri is shown in Figure 4. Accordingly, the SR in different days of the experimental infection was notably higher in rainbow trout fed with P1, P2 R1P1 and R2P2 than in those receiving the basal diet (p < .05). After the challenge period, the highest SR was observed in rainbow trout fed with R2P2 (64.04%) and R1P1 (59.08%). Moreover, the lowest SR was observed in the control group (28.33%). Also, the highest RPS and lowest RPS were 63.72% and 15.81% in R2P2- and R1-fed groups, respectively (p < .05) (Table 4).
| Treatments | SR (%) | MR (%) | RPS (%) |
|---|---|---|---|
| C | 28.33±4.04e | 71.66 | – |
| R1 | 39.66±6.50de | 60.33 | 15.81 |
| R2 | 44.00±3.46cde | 56 | 21.86 |
| P1 | 50.00±10.14bcd | 50 | 30.23 |
| P2 | 55.01±6.33abc | 45 | 37.20 |
| R1P1 | 59.08±6.66ab | 31 | 56.74 |
| R2P2 | 64.04±4.02a | 37.20 | 63.72 |
Note
- Different letters in each column show significant differences among the experimental groups (p < .05). Values are shown as mean ± SD (n = 3).
- Abbreviations: MR, mortality rate; RPS, relative percentage survival; SR, survival rate.
4 DISCUSSION
Probiotics and plant derivatives are popular immunostimulants that provide other benefits such as growth enhancement (Mostafavi et al., 2022; Shekarabi et al., 2020). In this study, growth performance and nutritional efficiency in the groups treated by Pro or Res were positively affected at the end of the period. However, no significant difference was recorded among the different experimental groups in the 4th week. Our findings revealed that growth indices and feed utilization were significantly influenced by diets containing both Pro and Res. In line with our results, the simultaneous use of probiotics and other plant derivatives such as fenugreek +Lactobacillus plantarum (Bahi et al., 2017) and Spirulina platensis +Bacillus amyloliquefaciens (Sahandi et al., 2019) improved growth performance in gilthead seabream and O. niloticus, respectively. Interaction between Pro and Res in enhancing the growth performance of rainbow trout may be related to their effects on improving the population of useful bacteria in the host GIT (Dawood & Koshio, 2020; Soni et al., 2017). The role of intestinal microbial flora is to facilitate the digestion and absorption of nutrients through the epithelial cells (Dawood et al., 2018). Besides, lactic acid bacteria in the intestine inhibit the growth of harmful bacteria in GIT (Elsabagh et al., 2018). Res also has known properties such as antibacterial or antioxidant that can indirectly promote growth by stimulating digestive enzymes or modulating the intestinal flora (Torno et al., 2018). Moreover, Magrone et al. (2016) reported that Res in the sea bass (Dicentrarchus labrax) diet enhanced intestinal integrity by reducing intestinal inflammatory cytokines. Other studies have also reported that phytochemicals may affect molecular signal pathways associated with nutrient metabolism and uptake (Torno et al., 2018; Zang et al., 2006). Given these explanations, both PRO and RES have synergistically affected the growth performance of rainbow trout. Previous studies have also shown that diets supplemented with probiotics or plant derivatives have beneficial effects on the length, width and the number of intestinal goblet cells that subsequently lead to nutrient uptake (Guardiola et al., 2016; Sahandi et al., 2019; Zheng et al., 2017). Therefore, improving intestinal health and efficiency can play a significant role in improving growth performance and nutritional functions in the treated rainbow trout in our study.
According to previous studies, dietary supplementations of probiotics or herbal compounds can positively affect fish health by boosting innate immune responses and antioxidant defence systems (Amaretti et al., 2013; Aydın & Barbas, 2020; Elumalai et al., 2020). Leucocytes are one of the main elements in non-specific immunity that play a notable role in regulating immunological activities such as phagocytose, respiratory burst and inflammatory processes (Ellis, 1999). In the present study, leucocyte count significantly increased along with phagocytic cells, that is neutrophils and monocytes/macrophages in the groups fed with dietary R1P1 and P2R2. However, no significant improvement in the lymphocyte count was observed in the fish fed with treated diets, which may be associated with an increase in the number of neutrophils. Consistent with previous reports, plant derivatives can promote the production of phagocytic cells in rainbow trout by stimulating lymphoid tissues such as the thymus and spleen (Rashmeei et al., 2020; Yılmaz & Ergün, 2018; Yonar, 2019). On the contrary, Naseri et al. (2015) and Mohammadian et al. (2020) documented, respectively, that Bacillus licheniformis and B. subtilis and L. bulgaricus actively induced the proliferation of WBC, especially phagocytic cells in rainbow trout. Our findings reveal that the combined form of the Pro and Res has beneficial effects on leucocyte proliferation and anti-infection properties in rainbow trout fingerlings.
Serum immune parameters of rainbow trout were enhanced with increasing the dose of Res and Pro supplements (single or combined form) in the diet. In this study, the highest lysozyme, BA and ACH50 activities were recorded in the fish fed with the R2P2 diet on the 4th and 8th weeks. Besides, Ig value significantly increased in the fish fed the diets containing R2P2 compared with those receiving diets containing R1, R2, P1 and control on the 4th week. However, on the 8th week, no significant difference was recorded between different experimental groups. The effect of a mixture of Pro and Res on the humoral immunity of fish is not yet available. However, a few studies have well documented the improvement in serum immune responses following diets supplemented with the mixtures of probiotics and plant products in several fish species, such as date palm (Phoenix dactylifera) and Shewanella putrefaciens on European seabass (D. labrax; Guardiola et al., 2016), Fenugreek (T. foenum graecum) and B. subtilis on gilthead seabream (Sparus aurata; Bahi et al., 2017), and Spirulina platensis and Bacillus amyloliquefaciens on Nile tilapia (O. niloticus; Al-Deriny et al., 2020). Lysozyme is an antibacterial enzyme that breaks down the glycosidic bonds of Gram-positive bacterial walls (Magnadóttir, 2006). It is widely accepted that an increase in lysozyme activity level is associated with an increase in the number of lysozyme-producing cells, that is neutrophils (Abdel-Tawwab & Abbass, 2017). Therefore, a possible reason for the high serum lysozyme level in this study was the increase in the neutrophil count. The complement system is the acute-phase proteins of the immune system that play an active role in initiating inflammatory, chemotactic and phagocytic responses (Sunyer et al., 2005; Xie et al., 2006). The level of serum complement activity depends to a large extent on the health and efficiency of the liver (Adineh et al., 2021; Jia et al., 2019). Therefore, this improvement may be due to the properties of hepatoprotective and the reduction in oxidative stress by the Pro and Res, which has previously been suggested by Gabriel et al., 2015; Kuebutornye et al., 2019. The level of serum BA indicates the immune status of the serum. Interestingly, the present study illustrated a higher level of serum BA in the trout fed the diet supplemented with R1P1 and R2P2; an increase in some non-specific humoral factors was the possible explanation for the serum bactericidal efficiency.
Fish skin mucus is a biological reservoir of various immune molecules (lysozyme, proteins, immunoglobulins, enzymes, lectins, etc.), and it is a defence barrier against foreign particles, pathogens and toxins (Esteban & Cerezuela, 2015; Salinas & Magadán, 2017). Our experimental findings showed that diets supplemented with a combined form of Pro and Res (R1P1 and R2P2 diets) significantly increased mucosal levels of lysozyme, BA and Ig for the 4th and 8th weeks. It seems that synergistic reactions between the Pro and Res have led the fish to have strong mucus immune responses. Indeed, probiotics stimulate epidermal goblet cells by producing nutrients such as short-chain fatty acids, vitamins and minerals, which lead to the greater secretion of antibacterial agents (Hoseinifar, Esteban, et al., 2015). Also, earlier studies have well demonstrated the enhancement of mucosal immunity by consuming a diet containing L. acidophilus or B. bifidum. For instance, Hoseinifar, Roosta, et al. (2015) reported that dietary supplementation of L. acidophilus elevated the mucus antibacterial activity of black swordtail. Also, Mirghaed et al. (2018) indicated that dietary L. acidophilus and B. bifidum significantly increased the mucosal lysozyme activity of Caspian white fish (Rutilus frisii kutum). In general, there is a close relationship between the diets containing some compounds with antioxidant properties and the stimulation of fish immune system (Gatlin et al., 2007). In this study, Res had positive effects on the mucosal immune responses of rainbow trout. Similarly, previous reports have found that the diets fortified with antioxidant agents such as grape seed extract, black mulberry and beta-carotene positively affected the lysozyme activity and Ig value in X. maculatus (Abdollahi et al., 2019), Cyprinus Carpio (Mehrinakhi et al., 2021) and rainbow trout (Shekarabi et al., 2020), respectively.
Probiotics and herbal extracts are known for their effectiveness in reducing oxidative stress in fish and shellfish (Dawood et al., 2016; Hamed et al., 2021; Tang et al., 2019). In fact, reactive oxygen species (ROS) levels will be raised and lead to cell destruction through the process of lipid peroxidation when animals are exposed to stress. In this mode, the fish antioxidant defence system fights with the produced free radical by secreting antioxidant enzymes such as SOD, CAT and GPX. These enzymes play a vital role in responding to uncontrolled oxidative stress in fish rearing conditions and protect the integrity of the cellular components (Aliko et al., 2018). The results of the current study showed that these three enzymes were notably increased in P2R2 on the 8th week compared with the control group. Also, the lowest level of MDA was recorded on the 4th and 8th weeks in the R2P2 group. The final product of the lipid peroxidation process is MDA, which is a valid indicator of intracellular component damage. Previous findings also confirm the role of probiotics or natural compounds with antioxidant properties in increasing intracellular antioxidant enzymes. For instance, Mostafavi et al. (2022) found a notable improvement in the activity of CAT, GPX and SOD in rainbow trout fed a diet supplemented with 3 g/kg common dandelion (Taraxacum officinaleon) extract. Also, Yonar (2019) stated that diet incorporated with curcumin at 2% led to a significant elevation in the activity of SOD, CAT and GPX and an obvious reduction in MDA level in the liver of rainbow trout. On the contrary, the levels of SOD and GPX activity were increased in the serum of O. mossambicus and Labeo rohita by a dietary B. licheniformis at 107 CFU/g (Abarike et al., 2018) and L. plantarum at 108 CFU/g (Gobi et al., 2018), respectively. The antioxidant activity of probiotics is likely via chelating ions, reducing reactive oxygen metabolites, preventing the production of oxidant compounds, reducing the ascorbate autoxidation and ROS scavenging (Amaretti et al., 2013; Martarelli et al., 2011). It seems that the combined form of Pro and Res in the rainbow trout diet can increase the antioxidant capacity of the fish and protect the cells against the dangerous effects of oxidative stress.
The main purpose of boosting the immune system by immunostimulants is to increase resistance to infectious diseases. Y. ruckeri, the causative agent of yersiniosis, is one of the most common diseases with high economic losses in the rainbow trout industry (Ingerslev et al., 2014). In this study, single or combined administration of Pro or Res increased rainbow trout SR against Y. ruckeri. Indeed, the diets containing RP protected up to 65% of the fish against yersiniosis infection. The combination of herbal plants and probiotics has not yet been tested to assess disease resistance in rainbow trout, although previous studies have demonstrated the beneficial effects of diets containing different probiotics such as L. plantarum (Soltani et al., 2019), B. subtilis (Yılmaz & Ergün, 2018) or herbal products such as d-limonene (Gültepe, 2020) and Ellagic acid (Yonar, 2019) on disease resistance against Y. ruckeri in rainbow trout. These findings suggest that supplementing aquafeed with probiotics and plant derivatives in combination form could be a potential alternative to antibiotics.
5 CONCLUSIONS
Overall, the dietary inclusion of two-strain probiotics (L. acidophilus and B. bifidum) and resveratrol improved the growth performance, innate immune system, antioxidant defence and resistance against Y. ruckeri in rainbow trout fingerlings. Moreover, the combined form of these two supplements in the diets, especially R2P2 diet, resulted in a faster induction of innate immune responses and antioxidant capacity in rainbow trout after 4 weeks. The results of a study showed that the combination of probiotics and resveratrol (probiotic 1g+ resveratrol 800 mg/kg) can be a beneficial option to improve the growth rate and health status of rainbow trout.
ACKNOWLEDGEMENTS
The authors acknowledge the financial assistance for Saeed Meshkini provided by the Urmia University, Urmia, Iran (grant number d10/968). The authors would like to thank the staff and management of the faculty of Veterinary Medicine (Urmia University, Iran) for their cooperation in conducting this research.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL STATEMENT
This study is a part of a Ph.D. thesis. All international principles for the care and use of laboratory animals were followed according to the National Research Council, and all procedures were approved by the committee of Urmia University, Urmia, Iran.
Open Research
DATA AVAILABILITY STATEMENT
The data are available from the corresponding author upon reasonable request.




