The effect of lipid level on the growth and reproductive performance of female orange mud crab, Scylla olivacea (Herbst, 1796), during the fattening period
Abstract
This experiment aimed to evaluate the efficiency of formulated feeds with different lipid levels (T1 = 60 g/kg, T2 = 80 g/kg, T3 = 100 g/kg and T4 = 120 g/kg) in the biochemical composition of female orange mud crab, Scylla olivacea, during the fattening period. Experiments were conducted on 120 crabs with BW and CW of 186.42 ± 37.90 g and 10.24 ± 0.66 cm, respectively, for 90 days. The crabs were reared in tanks, and dissections were performed in 10 individuals per treatment every 30 days. Overall assessments disclosed that mud crabs fed the T4 showed the highest BWG (1.80 ± 0.78 g, 13.78 ± 1.81 g and 14.51 ± 3.81 g), SGR (0.03 ± 0.02%day−1, 0.19 ± 0.01%day−1 and 0.28 ± 0.02%day−1), GSI (9.90 ± 1.05%, 11.31 ± 1.68% and 17.30 ± 2.13%) and HSI (4.17 ± 0.52%, 5.22 ± 0.40% and 5.06 ± 0.45%) during fattening periods. Meanwhile, EPA and DHA were the most important fatty acids required for maturing ovaries in mud crabs. During 60- and 90-day culture period, higher EPA and DHA were noted in the ovary of mud crabs fed with T4 (EPA: 4.79 mg/g and 6.69 mg/g; DHA: 4.55 mg/g and 6.85 mg/g) compared with hepatopancreas (EPA: 1.84mg/g and 2.47 mg/g; DHA: 3.28 mg/g and 3.71 mg/g) (p < .05). The low fatty acid composition in the hepatopancreas indicates a nutrient transfer from the hepatopancreas to the ovaries for maturation. Two-way ANOVA suggested that 120 g/kg of feed with 60–90 days of duration would be the best for crab rearing.
1 INTRODUCTION
Mud crab genus Scylla belongs to the family Portunidae, is commonly associated with the mangrove wetlands and is grown well in the muddy habitats subjected to tidal changes. Compared with other portunid species, mud crab genus Scylla grows faster and attains larger size over a short period (Viswanathan & Raffi, 2015). This species signifies a highly valued candidate for coastal fisheries with a high price in the export market owing to their high nutritional value and delicious taste. There are four species of mud crabs worldwide, namely Scylla serrata (Forsskal, 1755), S. paramamosain (Estampador, 1949), S. tranquebarica (Fabricius, 1798) and S. olivacea (Herbst, 1796). Out of the four species, S. olivacea is the target for aquaculture in Malaysia because of its hardy nature, high fecundity and ease of capture (Waiho et al., 2015). However, most of the mud crab seed stock in the hatchery are obtained from the wild. This highlights the need for improved broodstock performance in captivity to get quality ovigerous females (Shelley & Lovatelli, 2011). It is well established that the nutritional content of crustacean diets directly affects the growth and reproduction of the crustaceans (Aaqillah-Amr et al., 2021).
It has been acknowledged that formulated feed can overcome the problems of inadequate nutrition in broodstock in captivity, plus their ability to accelerate the development of ovarian maturation (Azra & Ikhwanuddin, 2016). The advantages of formulated feeds have beaten the lack of fresh feeds to solve the water deterioration problems, with consistent nutrient contents such as total lipid and protein (Suresh, 2012). The advances in feeding technology formulation will eventually help sustain natural resources such as fish in pelagic areas from overexploitation for feeding purposes in aquaculture industries (Abdul-Kader et al., 2017). Besides that, the formulated feeds also are readily available throughout the culture period and only require minimal time for preparation (D’Abramo, 2002). Djunaidah et al. (2003) mentioned that there were only slight differences in dietary contents between natural diets and formulated feeds in relation to the reproductive performance of mud crabs. Azra and Ikhwanuddin (2016) reviewed that combining both formulated feeds and raw diets in alternate feedings resulted in better reproductive performances in Scylla broodstock such as growth, survival, fecundity and maturation processes.
Lipids are among the most important nutrient components required in crabs for energy expenditures, maturation and growth (García-Guerrero et al., 2003). The dietary lipid requirement for gonadal development and spawning is well documented for certain crustaceans such as penaeid shrimp (Adloo et al., 2020), freshwater prawn (Santander-Avenceña et al., 2020), crayfish (Díaz-Jiménez et al., 2018) and mud crabs (Alava et al., 2007; Thien & Annita, 2017). The ovarian development in matured crabs generally requires a high-lipid diet during their maturation (Azmie et al., 2017). In hatcheries, farmers practically provide the crabs with feed that contains high lipid levels to increase their growth performance and shorten the length of the feeding period. Zhao et al. (2015) experimented with dietary lipid levels (range 29.1–143.2 g/kg) on the growth performance of juvenile mud crab, Scylla paramamosain. The results showed that the lipid levels at a range of 85.2 g/kg and 116.3 g/kg provided a higher growth performance of mud crabs. Similarly, tests on juvenile swimming crab, P. trituberculatus, with various lipid levels (36.3 g/kg, 67.0 g/kg, 107.2 g/kg and 139.1 g/kg) observed the increase in crab BWG with increasing dietary lipid level by up to 107.2 g/kg, with the lowest BWG in crabs fed the 36.3 g/kg lipid (Han et al., 2018). Among the different lipid constituents (i.e. phospholipid, fatty acids, sterols, cholesterols, carotenoids and vitamins), fatty acids are mostly studied in crustacean reproduction for their effects in promoting ovarian maturation. During the reproduction of mud crabs, diets containing highly unsaturated fatty acids (HUFA) such as arachidonic acid (C20:4n-6; ARA), eicosapentaenoic acid (C20:5n-3; EPA) and docosahexaenoic acid (C22:6n-3; DHA) are essential for their role as energy sources. In particular, these HUFA are important in cell membrane functioning as a precursor for the formation of eicosanoids (Kangpanich et al., 2016; Wijaya et al., 2021). On the contrary, carotenoids are crucial for tissue pigmentation. It has been noted that the concentration of carotenoids increased with dietary lipid (deCarvalho & Caramujo, 2017). The concentration of carotenoids is usually related to ovarian maturation stages, where the levels increase as the ovary matures (Aaqillah-Amr et al., 2018). Across the ovarian maturation stages, as the GSI increased, the carotenoid content also increased (Tantikitti et al., 2015). Similarly, the HSI in the crabs remained constant across the maturation stages, as well as the carotenoid content (Aaqillah-Amr et al., 2018).
As nutrition plays a vital role in the growth and development, at the same time, the length of the fattening period also determines the success of maturation in the mud crabs. Since feeding practices for hatchery management account for the highest operational cost to approximately 60–80% in intensive farming (Azra & Ikhwanuddin, 2016), it is accepted that the feeding period is essential to cover the feed cost of farming. To date, very little information is available on the dynamic changes in the biochemical composition during the different fattening periods on the adult crustacean. A recent experiment on the effects of the fattening period on the ovarian development of adult female Chinese mitten crab, Eriocheir sinensis, concluded that the optimum duration of the fattening period of 40–60 days could improve the total edible yield and nutritional values of female E. sinensis (Long et al., 2020). This was supported by Wu, Zhu, et al. (2020), where postpubertal moult E. sinensis reared in pond took approximately 40 days to achieve optimum gonadal development and nutritional quality. Other study on blue swimming crab, Portunus trituberculatus, suggested the appropriate fattening period to be 60 days for pond-reared females postpuberty moulting for optimized ovarian development and nutritional quality (Wu et al., 2014). Therefore, investigations into the appropriate dietary lipid levels, coupled with the length of the feeding period for female S. olivacea, can improve the feeding management. This experiment aimed to evaluate the efficiency of the formulated diets with different dietary lipid levels and the length of the feeding period in the ovarian development and biochemical composition of female S. olivacea to examine the effectiveness of nutritional strategies and feeding management approaches.
2 MATERIALS AND METHODS
2.1 Mud crab samples
Matured S. olivacea samples were collected from a series of biosamplings in Setiu Wetlands, Terengganu coastal waters, Malaysia (N 05°40.651’, E 102°43.113). The crabs were purchased in the Pasar Nelayan wet market. Experiments conducted consist of 120 individuals of healthy mature female orange mud crab, Scylla olivacea, with body weight (BW) and carapace width (CW) ranges of 186.42 ± 37.90 g and 10.24 ± 0.66 cm, respectively. In this study, only matured female S. olivacea samples were collected. The mature female crabs usually showed a wide and globular-shaped abdomen with darkened colour (U-shape) (Islam et al., 2010). As it is difficult to attain the GSI value to confirm the maturity stages, the size of the ovary can be determined following a method adapted from Quinitio et al. (2007) by carefully pressing and pushing forward the first abdominal segment.
Experiments were carried out at the AKUATROP hatchery of the Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu (UMT) (N 5° 24’ 32.9364", E 103° 5’ 20.3784"). The crabs were disinfected before experimentation with 25 ppm of formalin for 30 min to kill parasites (Herlinah & Septiningsih, 2015). After disinfection, the crabs were stabilized in a 1-tonne litre tank, under a simple recirculated water system, equipped with moderate aeration, water pump and UV light. Water salinity and water temperature in all tanks were maintained at 25 g/L and 26–29°C, respectively. The crabs were stabilized for two days prior to experimentation. During the two days of stabilization, the crabs were fed with chopped yellow scad fish, Selaroides leptolepis, at 5% of BW. All of the experimental crabs were used according to the general guidelines to research using animals. All experimental protocols for this research were following the Principles and Ethical Guidelines for the Use of Laboratory Animals (2019) (http://www.ccac.ca/Documents/Standards/Guidelines/Euthanasia.pdf).
2.2 Feed formulation
- T1 (60 g/kg) = 54.7 g/kg lipid from mangrove clam meat +5.3 g/kg FO
- T2 (80 g/kg) = 54.7 g/kg lipid from mangrove clam meat +25.3 g/kg FO
- T3 (100 g/kg) = 54.7 g/kg lipid from mangrove clam meat +45.3 g/kg FO
- T4 (120 g/kg) = 54.7 g/kg lipid from mangrove clam meat +65.3 g/kg FO
Agar was used as a binder. The fresh mangrove clam meat was ground in a meat mincer and electric blender to obtain very fine food particles and was weighed. The feed ingredients were mixed except probiotics and vitamins and minerals according to the formula. Water was added (50 ml/kg) to the mixtures and cooked for a while to activate the agar until they mixed well. The mixtures were left until they cooled down, and then, the probiotics and vitamins and minerals (1:1) were added once the mixture temperature reached below 50°C. The resultant pellets were shaped into an ice tray cube (3.0 × 1.5 × 1.5 cm) and stored in a sealed bag in the −20°C freezer until use to maintain the nutrient content. Overall formulation and the determination of proximate analysis are described in Table 1. The designed feeds were preceded with the determination of fatty acid analysis (Table 2).
| Ingredients composition (g/kg) | Diets (Treatments) | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Mangrove clams flesh mix | 526.8 | 526.8 | 526.8 | 526.8 |
| Wheat flour | 257.9 | 237.9 | 217.9 | 197.9 |
| Fish oil | 5.3 | 25.3 | 45.3 | 65.3 |
| Soy lecithin | 20 | 20 | 20 | 20 |
| Vitamin and mineral mix (1:1)a | 30 | 30 | 30 | 30 |
| Molasses | 50 | 50 | 50 | 50 |
| Probioticsb | 50 | 50 | 50 | 50 |
| Agar | 60 | 60 | 60 | 60 |
| 1000 | 1000 | 1000 | 1000 | |
| Proximate analysis (g/kg) | ||||
| Dry matter | 775.4 ± 7.7 | 789.8 ± 14.5 | 786.7 ± 4.4 | 760.8 ± 6.6 |
| Crude lipid | 66.7 ± 1.5 | 82.9 ± 4.7 | 105.7 ± 8.8 | 127.3 ± 3.1 |
| Crude protein | 428.9 ± 11.4 | 424.3 ± 15.0 | 423.9 ± 14.3 | 420.4 ± 10.6 |
| Crude fibre | 0.2 ± 0.1 | 0.5 ± 0.3 | 0.5 ± 0.2 | 0.8 ± 0.0 |
| Crude ash | 83.3 ± 4.6 | 82.3 ± 29.9 | 85.6 ± 14.2 | 80.9 ± 1.5 |
- a Vitamin and mineral mixtures: vitamin C (L-ascorbyl-2-monophosphate): 40, 000 mg/kg; vitamin B1: 4, 600 mg/kg; vitamin B6: 4, 100 mg/kg; nicotinic acid: 14, 800 mg/kg; choline: 34, 800 mg/kg; lysine: 80, 340 mg/kg; selenium: 18 mg/kg; BHT: 150 mg/kg; BHA: 100 mg/kg; propyl gallate: 1 mg/kg; citric acid: 50 mg/kg (Sano TOP-S: INVE Aquaculture).
- b Probiotics: Strains of Bacillus subtilis, Bacillus licheniformis and Bacillus pumilus (Sano-life Pro-2 Powder: INVE Aquaculture)
| Fatty acid | Concentration of fatty acids (mg/g) | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| ∑SAFA | 13.69 ± 1.20 | 37.80 ± 2.88 | 31.01 ± 0.97 | 41.23 ± 0.58 |
| C14:0 | 1.60 ± 0.05 | 2.78 ± 0.12 | 2.04 ± 0.23 | 5.78 ± 0.08 |
| C15:0 | 0.11 ± 0.05 | 0.82 ± 0.05 | 0.52 ± 0.04 | 0.48 ± 0.09 |
| C16:0 | 9.24 ± 0.81 | 26.14 ± 0.53 | 20.99 ± 0.70 | 27.83 ± 0.18 |
| C17:0 | 0.62 ± 0.00 | 2.43 ± 1.40 | 1.84 ± 0.00 | 1.74 ± 0.78 |
| C18:0 | 2.04 ± 0.16 | 5.31 ± 0.32 | 5.44 ± 0.25 | 5.39 ± 0.01 |
| C20:0 | 0.18 ± 0.00 | 0.66 ± 0.00 | 0.36 ± 0.00 | nd. |
| ∑MUFA | 10.20 ± 1.05 | 28.18 ± 4.22 | 25.11 ± 0.95 | 27.90 ± 0.35 |
| C16:1 | 2.13 ± 0.33 | 4.34 ± 0.32 | 3.57 ± 0.12 | 4.74 ± 0.14 |
| C17:1 | nd. | 0.19 ± 0.00 | 0.23 ± 0.02 | 0.11 ± 0.00 |
| C18:1 | 1.92 ± 0.22 | 6.41 ± 3.09 | 4.30 ± 1.62 | 4.51 ± 0.06 |
| C18:1n−9 | 5.87 ± 0.58 | 16.82 ± 0.92 | 16.75 ± 0.53 | 18.16 ± 0.51 |
| C20:1 | 0.28 ± 0.08 | 0.51 ± 0.02 | 0.26 ± 0.01 | 0.45 ± 0.00 |
| ∑PUFA | 6.64 ± 0.51 | 11.55 ± 0.65 | 11.09 ± 0.65 | 10.98 ± 0.19 |
| C18:2n−6 | 5.25 ± 0.50 | 7.89 ± 0.38 | 7.84 ± 0.44 | 7.06 ± 0.06 |
| C20:4n−3 | 0.08 ± 0.00 | nd | nd | 0.34 ± 0.00 |
| C20:4n−6 | 0.47 ± 0.05 | 1.01 ± 0.06 | 1.07 ± 0.10 | 0.81 ± 0.01 |
| C22:4n−6 | 0.11 ± 0.01 | 0.24 ± 0.02 | 0.23 ± 0.02 | 0.13 ± 0.01 |
| C20:5n−3 | 0.32 ± 0.02 | 0.88 ± 0.05 | 0.82 ± 0.04 | 1.27 ± 0.06 |
| C22:5n−3 | nd. | 0.24 ± 0.02 | 0.17 ± 0.02 | 0.33 ± 0.00 |
| C22:5n−6 | 0.09 ± 0.00 | 0.26 ± 0.02 | 0.17 ± 0.04 | 0.17 ± 0.02 |
| C22:6n−3 | 0.26 ± 0.02 | 1.03 ± 0.09 | 0.73 ± 0.08 | 1.20 ± 0.04 |
| ∑n−3 | 0.65 | 2.15 | 1.71 | 2.81 |
| ∑n−6 | 5.92 | 9.40 | 9.31 | 8.17 |
| n−3:n−6 | 0.11 | 0.23 | 0.18 | 0.34 |
2.3 Feeding experiments
One hundred and twenty individuals of mud crabs were cultured for 90 days for each treatment, and sampling was done every 30 days (Day 30, Day 60 and Day 90). The crabs were separated individually in a cage (33.0 × 45.0 × 11.0 cm). The mud crabs were fed once a day during dusk since mud crabs are active at night (Hill, 1978) with 5% of BW (Kar et al., 2017). 100% water exchange was done weekly. At the end of each fattening period, the crabs were subjected to dissection of hepatopancreas and ovaries for growth performance, and morphological and biochemical analyses. Only crabs with Stage 4 of ovarian maturation were taken into the study as the ovary at this stage is ripen, which indicated its readiness to spawn (Hidir et al., 2021). The subsequent analyses of the captive crabs after fattening periods were compared with the wild-caught carbs (control) from Stage 4 as reference.
2.4 Growth performance
- (i)
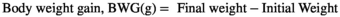
- (ii)
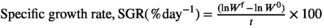
where
Wf = final weight (wet weight)
W0 = initial weight (wet weight)
t = day of culture.
2.5 The gonadosomatic index and hepatosomatic index

2.6 Biochemical analyses

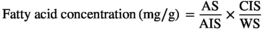
AS = peak area of fatty acid in the sample in chromatogram
AIS = peak area of internal standard in chromatogram
CIS = concentration of internal standard (mg)
WS = weight of sample (g)
2.7 Data collection and statistical analysis
All data from BWG, SGR, GSI, HSI, total carotenoid and fatty acids obtained were tested for normality by visual inspection (histogram) and measures of Shapiro–Wilk test and skewness and kurtosis. The normally distributed data were then tested with ANOVA tests, and the differences were compared between groups. Two-way ANOVA was used to determine the interactions between lipid levels (60 g/kg, 80 g/kg, 100 g/kg and 120 g/kg) during different fattening periods (30-, 60- and 90-day) in the BWG, SGR, GSI and HSI of S. olivacea. One-way ANOVA was used in the comparison of fatty acids of both hepatopancreas and ovary during different fattening periods. The difference in means was tested with Tukey's HSD multiple comparisons test at 95% confidence level where p < .05 was considered statistically significant. All analyses were conducted using the IBM SPSS Statistic Ver. 20. Pearson's correlation (r) and regression analyses (R2) were carried out using Microsoft Excel 2013 to determine the relationship between variables where necessary, and the strength of relationship was evaluated based on the r-values.
3 RESULTS
3.1 Growth performance, GSI and HSI
The data on BWG, SGR, GSI and HSI of S. olivacea fed different lipid levels during different fattening periods are presented in Table 3. Carapace width gain (CWG) was not recorded since no moulting occurred during the experimental period. All parameters were significantly influenced by both lipid levels and fattening periods, in which there was an increase in the BWG, SGR, GSI and HSI as the lipid levels increase, and the value increases at the extended fattening period (p = .000). Lower values of BWG, SGR, GSI and HSI were noted in the crabs when fed with lower lipid levels (60 g/kg and 80 g/kg) and during the first 30 days of fattening period. Analysis of the interaction between lipid levels with fattening periods revealed no significant difference in the BWG and SGR of crabs fed different lipid levels during the first 30 days of fattening period. Yet, the significant differences were noted during both 60 and 90 days of fattening period, in which there were increases in the BWG, SGR, GSI and HSI. Overall assessments disclosed that mud crabs fed the T4 showed highest body weight gain (BWG: 1.80 ± 0.78 g, 13.78 ± 1.81 g and 14.51 ± 3.81 g), specific growth rate (SGR: 0.03 ± 0.02%day−1, 0.19 ± 0.01%day−1 and 0.28 ± 0.02%day−1), gonadosomatic index (GSI: 9.90 ± 1.05%, 11.31 ± 1.68% and 17.30 ± 2.13%) and hepatosomatic index (HSI: 4.17 ± 0.52%, 5.22 ± 0.40% and 5.06 ± 0.45%) during 30-, 60- and 90-day fattening periods, respectively. Correlation and regression analyses in all parameters showed that BWG, SGR, GSI and HSI were strongly correlated with the length of fattening period (Table 4).
| BWG (g) | SGR (%day−1) | GSI (%) | HSI (%) | |
|---|---|---|---|---|
| Means for lipid levels | ||||
| T1 | 2.53 ± 0.72a | 0.03 ± 0.01a | 10.19 ± 0.64ab | 3.02 ± 0.27a |
| T2 | 3.31 ± 0.67a | 0.07 ± 0.01b | 8.38 ± 0.64a | 3.36 ± 0.27ab |
| T3 | 9.02 ± 0.62b | 0.06 ± 0.01b | 10.60 ± 0.69ab | 4.28 ± 0.28bc |
| T4 | 10.03 ± 0.2b | 0.16 ± 0.01c | 12.84 ± 0.67b | 4.82 ± 0.27c |
| F-value | 30.975 | 39.295 | 7.740 | 9.657 |
| p-Value | .000 | .000 | .000 | .000 |
| df | 3 | 3 | 3 | 3 |
| Means for fattening period | ||||
| 30-day | 3.29 ± 0.57a | 0.05 ± 0.01a | 7.67 ± 0.67a | 2.90 ± 0.28a |
| 60-day | 6.96 ± 0.57b | 0.08 ± 0.01b | 10.83 ± 0.50b | 4.58 ± 0.20b |
| 90-day | 8.42 ± 0.63b | 0.11 ± 0.01b | 13.00 ± 0.54b | 4.13 ± 0.22b |
| F-value | 19.923 | 16.522 | 19.383 | 12.298 |
| p-value | .000 | .000 | .000 | .000 |
| df | 2 | 2 | 2 | 2 |
| Interaction (Fattening period × lipid levels) | ||||
| 30-day ×T1 | 3.62 ± 1.18 | 0.06 ± 0.01 | 8.08 ± 3.32b | 1.87 ± 0.52a |
| 30-day ×T2 | 5.22 ± 0.98 | 0.07 ± 0.01 | 3.79 ± 0.76a | 1.82 ± 0.52a |
| 30-day ×T3 | 2.50 ± 0.33 | 0.03 ± 0.02 | 8.90 ± 0.68b | 3.74 ± 0.63b |
| 30-day ×T4 | 1.80 ± 0.78 | 0.03 ± 0.02 | 9.90 ± 1.05b | 4.17 ± 0.52b |
| 60-day ×T1 | 1.75 ± 0.86a | 0.02 ± 0.01a | 11.06 ± 2.06 | 4.00 ± 0.40a |
| 60-day ×T2 | 2.55 ± 0.62a | 0.06 ± 0.01b | 10.62 ± 2.31 | 4.43 ± 0.40ab |
| 60-day ×T3 | 9.77 ± 0.95b | 0.08 ± 0.02b | 10.34 ± 0.54 | 4.66 ± 0.40ab |
| 60-day ×T4 | 13.78 ± 1.81c | 0.19 ± 0.01c | 11.31 ± 1.68 | 5.22 ± 0.40b |
| 90-day ×T1 | 2.22 ± 1.38a | 0.01 ± 0.02a | 11.41 ± 3.43a | 3.20 ± 0.45a |
| 90-day ×T2 | 2.18 ± 1.82a | 0.08 ± 0.02b | 10.73 ± 2.87a | 3.82 ± 0.45ab |
| 90-day ×T3 | 14.77 ± 3.96b | 0.08 ± 0.02b | 12.55 ± 2.04a | 4.44 ± 0.40b |
| 90-day ×T4 | 14.51 ± 3.81b | 0.28 ± 0.02c | 17.30 ± 2.13b | 5.06 ± 0.45b |
| F-value | 15.713 | 15.157 | 2.321 | 0.774 |
| p-Value | .000 | .000 | .050 | .596 |
| df | 6 | 6 | 6 | 6 |
Note
- Within a column, values not sharing a common superscript are significantly different (p < .05).
- Abbreviation: df, degrees of freedom.
| T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
| BWG | y = −0.0233x + 3.93 | y = −0.0507x + 6.3567 | y = 0.2045x - 3.2567 | y = 0.212x - 2.6933 |
| R² = .5178 | R² = .8398 | R² = .9887 | R² = .7929 | |
| r = −.72 | r = −.92 | r = .99 | r = .89 | |
| SGR | y = −0.0008x + 0.0767 | y = 0.0002x + 0.0567 | y = 0.0007x + 0.0267 | y = 0.0042x - 0.0833 |
| R² = .75 | R² = .1071 | R² = .75 | R² = .9745 | |
| r = −.87 | r = .33 | r = .87 | r = .99 | |
| GSI | y = 0.0555x + 6.8533 | y = 0.1005x + 2.2967 | y = 0.0608x + 6.9467 | y = 0.1232x + 5.45 |
| R² = .8279 | R² = .5668 | R² = .9854 | R² = .8861 | |
| r = .91 | r = .75 | r = .99 | r = .92 | |
| HSI | y = 0.0222x + 1.6933 | y = 0.0332x + 1.3633 | y = 0.0117x + 3.58 | y = 0.015x + 3.9133 |
| R² = .382 | R² = .5324 | R² = .5308 | R² = .6202 | |
| r = .62 | r = .73 | r = .73 | r = .79 |
3.2 Biochemical analysis
3.2.1 Total carotenoid
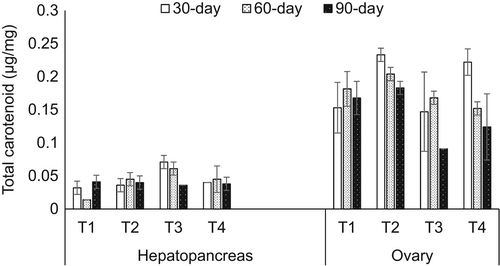
The total carotenoid content of mud crabs fed the formulated diet with different lipid levels during fattening periods in both hepatopancreas and ovaries is illustrated in Figure 1. As shown in the figure, inconsistent trends were observed in the carotenoid content of both tissues during all fattening periods (p < .05). Highest carotenoid contents were detected in the ovaries compared with the hepatopancreas during all fattening periods.
3.2.2 Fatty acid
The fatty acid compositions in hepatopancreas (Tables 5–7) and ovaries (Tables 8–10) during the 30-, 60- and 90-day fattening periods fed different dietary lipid levels were compared. From the tables, the concentrations of fatty acids were dominated by ∑SAFA, with half of the total fatty acid concentrations. This high percentage of ∑SAFA were contributed majorly from the palmitic acid, C16:0. Meanwhile, higher ∑MUFA concentrations were contributed by oleic acid (C18:1n-9; OA). For PUFA, linoleic acid (C18:2n-6; LA) was the most abundant fatty acid in the PUFA group that contribute approximately half of the ∑PUFA in all treatments. Of the major PUFA, particular interests were in the composition of HUFA such as ARA, EPA and DHA. Results revealed that ARA showed higher concentration than EPA and DHA did in both hepatopancreas and ovaries in all treatments throughout the fattening period. However, an exception was noticed in the mud crabs fed the T4 where EPA and DHA were shown considerable higher concentration than ARA in both hepatopancreas and ovaries.
| Fatty acids | Concentration of fatty acids (mg/g) | ||||
|---|---|---|---|---|---|
| Wild | T1 | T2 | T3 | T4 | |
| ∑SAFA | 170.72 | 102.31±8.87a | 123.26±4.42a | 104.48 ± 4.06a | 161.39±11.85b |
| C14:0 | 17.98 | 7.56 ± 0.81a | 8.49 ± 0.24a | 7.40 ± 0.22a | 15.72 ± 1.66b |
| C15:0 | 9.39 | 2.90 ± 0.29a | 2.78 ± 0.37a | 2.73 ± 0.03a | 3.81 ± 0.54a |
| C16:0 | 87.82 | 68.64 ± 6.46a | 76.39 ± 2.54a | 68.70 ± 3.24a | 106.07 ± 1.27b |
| C17:0 | 15.66 | 5.04 ± 0.29a | 14.39 ± 0.40a | 6.58 ± 3.47a | 10.40 ± 5.67a |
| C18:0 | 36.32 | 16.16 ± 1.65a | 18.18 ± 1.40a | 14.05 ± 0.04a | 23.47 ± 0.77b |
| C21:0 | 1.12 | 1.17 ± 0.75a | 2.26 ± 0.19a | 1.15 ± 0.92a | 1.54 ± 1.40a |
| C22:0 | 2.43 | 0.83 ± 0.12a | 0.78 ± 0.01a | 3.54 ± 0.00b | 0.75 ± 0.00a |
| ∑MUFA | 74.95 | 68.92 ± 7.18a | 84.00± 1.79ab | 81.22 ± 0.45ab | 120.50±18.42b |
| C16:1 | 34.62 | 11.25 ± 1.41a | 12.12 ± 0.01a | 11.75 ± 1.01a | 24.14 ± 2.52b |
| C17:1 | 3.35 | 0.53 ± 0.05a | 0.71 ± 0.04a | 0.66 ± 0.03a | 1.07 ± 0.11b |
| C18:1 | 0.52 | 16.20 ± 9.27a | 27.46 ± 0.67a | 17.80 ± 8.63a | 26.62 ± 12.30a |
| C18:1n−9 | 25.14 | 34.42 ± 2.81a | 41.72 ± 0.90a | 49.17 ± 9.89ab | 66.66 ± 3.59b |
| C20:1 | 11.32 | 5.51 ± 5.39a | 1.85 ± 0.04a | 1.66 ± 0.09a | 1.86 ± 0.13a |
| C22:1 | n.d. | 1.00 ± 0.98a | 0.30 ± 0.00a | 0.37 ± 0.00a | 0.31 ± 0.00a |
| ∑PUFA | 48.70 | 23.04 ± 2.49 | 27.54 ± 0.88 | 23.92 ± 0.39 | 50.64 ± 4.10 |
| C18:2n−6 | 4.11 | 14.72 ± 1.63a | 17.40 ± 0.42a | 15.64 ± 0.24a | 23.30 ± 1.43b |
| C22:2 | 0.93 | 0.12 ± 0.03 | 0.14 ± 0.00 | 0.15 ± 0.00 | n.d. |
| C18:3n−6 | 0.61 | 0.14 ± 0.00 | n.d. | 0.09 ± 0.07 | 0.31 ± 0.00 |
| C20:4n−3 | n.d. | 0.54 ± 0.08 | 0.58 ± 0.00 | n.d. | 1.26 ± 0.11 |
| C20:4n−6 | 15.24 | 2.21 ± 0.22a | 3.22 ± 0.35a | 2.66 ± 0.00ab | 4.31 ± 0.39b |
| C22:4n−6 | 1.57 | 0.59 ± 0.06 | 0.60 ± 0.00 | n.d. | 0.61 ± 0.17 |
| C20:5n−3 | 5.60 | 1.88 ± 0.20a | 2.51 ± 0.04a | 1.92 ± 0.01a | 7.11 ± 0.61b |
| C22:5n−3 | 1.44 | 0.59 ± 0.06a | 0.72 ± 0.02a | 0.64 ± 0.01a | 1.93 ± 0.13b |
| C22:5n−6 | 1.89 | 0.60 ± 0.15a | 0.76 ± 0.01a | 0.61 ± 0.01a | 1.22 ± 0.16b |
| C22:6n−3 | 17.32 | 1.71 ± 0.22a | 2.27 ± 0.01a | 2.28 ± 0.17a | 10.75 ± 1.09b |
| ∑n−3 | 24.36 | 4.73 | 5.80 | 4.84 | 21.05 |
| ∑n−6 | 23.42 | 18.19 | 21.68 | 19.01 | 29.59 |
| n−3:n−6 | 1.04 | 0.26 | 0.27 | 0.25 | 0.71 |
Note
- n.d. = not detected. No statistics are computed for groups with n.d. result.
- a,b,cMeans with different superscripts indicate significant differences (p < .05).
| Fatty acids | Concentration of fatty acids (mg/g) | ||||
|---|---|---|---|---|---|
| Wild | T1 | T2 | T3 | T4 | |
| ∑SAFA | 170.72 | 84.18 ± 16.38a | 106.93±2.96ab | 108.91±0.72ab | 134.84 ± 7.83b |
| C14:0 | 17.98 | 2.53 ± 0.67a | 4.66 ± 0.09a | 7.18 ± 0.36b | 8.84 ± 0.48b |
| C15:0 | 9.39 | 2.53 ± 0.28a | 2.42 ± 0.01a | 2.95 ± 0.06a | 2.95 ± 0.15a |
| C16:0 | 87.82 | 56.93 ± 10.80a | 75.18 ± 2.26ab | 71.58 ± 1.87ab | 88.93 ± 4.81b |
| C17:0 | 15.66 | 7.72 ± 4.19a | 4.94 ± 0.06a | 10.58 ± 0.18a | 13.07 ± 0.72a |
| C18:0 | 36.32 | 10.71 ± 7.39a | 16.18 ± 0.38a | 15.20 ± 0.53a | 18.43 ± 1.08a |
| C21:0 | 1.12 | 0.62 ± 0.08a | 2.68 ± 0.32b | 0.61 ± 0.00a | 1.83 ± 0.51ab |
| C22:0 | 2.43 | 0.73 ± 0.01a | 0.87 ± 0.04a | 0.82 ± 0.03a | 0.80 ± 0.07a |
| ∑MUFA | 74.95 | 54.51 ± 4.92a | 102.64±26.35a | 81.81 ± 3.04a | 81.95 ± 4.20a |
| C16:1 | 34.62 | 10.57 ± 0.27a | 9.26 ± 0.03a | 13.92 ± 0.49a | 12.96 ± 0.31a |
| C17:1 | 3.35 | 0.90 ± 0.10b | 0.98 ± 0.06b | 0.80 ± 0.04b | 0.51 ± 0.00a |
| C18:1 | 0.52 | 19.64 ± 2.18ab | 27.32 ± 1.01c | 13.74 ± 0.63a | 24.12 ± 0.95bc |
| C18:1n−9 | 25.14 | 22.04 ± 2.09a | 62.67 ± 27.11a | 51.14 ± 1.70a | 42.81 ± 2.92a |
| C20:1 | 11.32 | 1.35 ± 0.27a | 2.24 ± 0.05b | 1.86 ± 0.10ab | 1.64 ± 0.10ab |
| C22:1 | n.d. | n.d. | 0.33 ± 0.00 | 0.36 ± 0.08 | 0.34 ± 0.00 |
| ∑PUFA | 48.70 | 19.52 ± 1.00a | 23.69 ± 0.58ab | 27.05 ± 0.14b | 24.26 ± 1.87b |
| C18:2n−6 | 4.11 | 7.70 ± 0.56a | 12.11 ± 0.17b | 16.87 ± 0.42c | 13.59 ± 0.77b |
| C20:4n−6 | 15.24 | 4.74 ± 0.17c | 2.88 ± 0.08b | 2.50 ± 0.00ab | 2.18 ± 0.22a |
| C22:4n−6 | 1.57 | 2.65 ± 0.00d | 0.72 ± 0.00c | 0.58 ± 0.00b | 0.43 ± 0.02a |
| C20:5n−3 | 5.60 | 3.65 ± 0.41a | 1.97 ± 1.70a | 2.51 ± 0.02a | 1.84 ± 1.62a |
| C22:5n−3 | 1.44 | n.d. | 1.93 ± 1.54 | 3.42 ± 0.00 | 1.62 ± 1.07 |
| C22:5n−6 | 1.89 | 0.50 ± 0.18a | 0.95 ± 0.05b | 0.65 ± 0.09ab | 0.72 ± 0.04ab |
| C22:6n−3 | 17.32 | 1.60 ± 0.37a | 3.13 ± 0.12b | 2.85 ± 0.08b | 3.28 ± 0.30b |
| ∑n−3 | 24.36 | 5.25 | 7.02 | 7.70 | 7.34 |
| ∑n−6 | 23.42 | 14.27 | 16.66 | 19.35 | 16.92 |
| n−3:n−6 | 1.04 | 0.37 | 0.42 | 0.40 | 0.43 |
Note
- n.d. = not detected. No statistics are computed for groups with n.d. result.
- a,b,cMeans with different superscripts indicate significant differences (p < .05).
| Fatty acids | Concentration of fatty acids (mg/g) | ||||
|---|---|---|---|---|---|
| Wild | T1 | T2 | T3 | T4 | |
| ∑SAFA | 170.72 | 71.26 ± 7.50a | 109.98 ± 14.67b | 102.74 ± 4.57ab | 120.97 ± 4.39b |
| C14:0 | 17.98 | 4.53 ± 0.45a | 6.27 ± 0.97ab | 6.92 ± 0.18bc | 8.62 ± 0.13c |
| C15:0 | 9.39 | 2.33 ± 0.24a | 3.11 ± 0.46a | 2.96 ± 0.08a | 2.91 ± 0.02a |
| C16:0 | 87.82 | 40.54±4.05a | 68.89 ± 8.66b | 70.18 ± 4.94b | 83.87 ± 4.52b |
| C17:0 | 15.66 | 9.31 ± 1.06b | 12.02 ± 1.52b | 4.07 ± 0.39a | 4.88 ± 0.16a |
| C18:0 | 36.32 | 11.38±1.38a | 16.04 ± 1.83ab | 15.69± 0.05ab | 18.25 ± 0.31b |
| C21:0 | 1.12 | 2.28 ± 0.27a | 3.13 ± 0.51a | 2.12 ± 0.02a | 2.45 ± 0.10a |
| C22:0 | 2.43 | 0.88 ± 0.05 | 1.02 ± 0.00 | 0.79 ± 0.00 | n.d. |
| ∑MUFA | 74.95 | 38.33±4.69a | 66.54 ± 6.79b | 80.43 ± 0.33b | 83.53 ± 1.30b |
| C16:1 | 34.62 | 7.87 ± 1.07a | 12.17 ± 2.39ab | 12.37±0.69ab | 13.92 ± 0.88b |
| C17:1 | 3.35 | 0.64 ± 0.12a | 0.97 ± 0.22a | 0.83 ± 0.02a | 0.91 ± 0.10a |
| C18:1 | 0.52 | 9.56 ± 1.24a | 12.38 ± 0.96ab | 12.25±0.33ab | 13.66 ± 0.35b |
| C18:1n−9 | 25.14 | 18.59±2.05a | 39.37 ± 4.79b | 52.95 ± 0.88c | 52.75 ± 0.66c |
| C20:1 | 11.32 | 1.47 ± 0.18a | 1.65 ± 0.35a | 1.83 ± 0.07a | 2.28 ± 0.21a |
| ∑PUFA | 48.70 | 16.36±1.85a | 29.25 ± 7.78a | 31.83 ± 0.09a | 24.43 ± 1.37a |
| C18:2n−6 | 4.11 | 8.62 ± 0.92a | 15.83 ± 2.24b | 19.00 ± 0.44b | 14.47 ± 0.03b |
| C22:2 | 0.93 | 0.51 ± 0.13 | 6.61 ± 0.00 | n.d. | n.d. |
| C20:4n−6 | 15.24 | 2.04 ± 0.16a | 3.67 ± 0.54a | 3.45 ± 0.86a | 1.58 ± 0.44a |
| C22:4n−6 | 1.57 | 0.50 ± 0.06 | n.d. | 0.71 ± 0.11 | 0.60 ± 0.00 |
| C20:5n−3 | 5.60 | 1.65 ± 0.13a | 2.34 ± 0.33ab | 2.72 ± 0.04b | 2.47 ± 0.09b |
| C22:5n−3 | 1.44 | 0.42 ± 0.11a | 0.61 ± 0.04a | 0.68 ± 0.18a | 0.85 ± 0.00a |
| C22:5n−6 | 1.89 | 0.59 ± 0.06a | 0.78 ± 0.00ab | 0.97 ± 0.07b | 0.86 ± 0.04b |
| C22:6n−3 | 17.32 | 2.02 ± 0.31a | 3.10 ± 0.50ab | 4.25 ± 0.29b | 3.71 ± 0.10b |
| ∑n−3 | 24.36 | 4.10 | 6.06 | 7.65 | 7.22 |
| ∑n−6 | 23.42 | 11.75 | 19.89 | 24.18 | 17.22 |
| n−3:n−6 | 1.04 | 0.35 | 0.30 | 0.32 | 0.42 |
Note
- n.d. = not detected. No statistics are computed for groups with n.d. result.
- a,b,cMeans with different superscripts indicate significant differences (p < .05).
| Fatty acids | Concentration of fatty acids (mg/g) | ||||
|---|---|---|---|---|---|
| Wild | T1 | T2 | T3 | T4 | |
| ∑SAFA | 30.09 | 91.70 ± 4.99c | 52.35 ± 7.97ab | 74.54 ± 7.25bc | 41.47 ± 4.91a |
| C14:0 | 1.03 | 3.88 ± 0.33b | 3.44 ± 0.38ab | 3.49 ± 0.53ab | 2.17 ± 0.26a |
| C15:0 | 0.87 | 1.40 ± 0.01b | 0.95 ± 0.03a | 1.13 ± 0.08ab | 0.88 ± 0.13a |
| C16:0 | 16.17 | 55.81 ± 3.30b | 32.05 ± 3.99a | 48.65 ± 4.62b | 26.06 ± 3.15a |
| C17:0 | 2.30 | 5.60 ± 0.44a | 3.56 ± 2.21a | 3.17 ± 0.33a | 1.66 ± 0.22a |
| C18:0 | 9.52 | 24.10 ± 1.49c | 11.65 ± 1.40ab | 17.68 ± 2.05b | 10.69 ± 1.16a |
| C21:0 | 0.12 | 0.23 ± 0.10 | 0.71 ± 0.04 | 0.41 ± 0.36 | n.d. |
| ∑MUFA | 24.38 | 84.77 ± 4.33c | 54.56 ± 5.08ab | 70.36 ± 7.50bc | 37.44 ± 5.29a |
| C16:1 | 6.41 | 9.43 ± 0.79b | 11.52 ± 1.08b | 8.33 ± 1.03ab | 5.14 ± 0.98a |
| C17:1 | 0.93 | 0.95 ± 0.06ab | 1.05 ± 0.09b | 0.83 ± 0.16ab | 0.56 ± 0.05a |
| C18:1 | 1.44 | 8.74 ± 0.40a | 10.95 ± 4.38a | 9.47 ± 1.13a | 4.88 ± 0.46a |
| C18:1n−9 | 13.23 | 63.61 ± 2.66c | 28.26 ± 2.51a | 48.78 ± 4.87b | 22.10 ± 2.60a |
| C20:1 | 2.30 | 1.96 ± 0.30a | 2.79 ± 0.00a | 2.95 ± 0.32a | 4.51 ± 1.18a |
| ∑PUFA | 7.75 | 53.43 ± 1.59c | 27.90 ± 2.94ab | 38.46 ± 4.76b | 22.78 ± 3.15a |
| C18:2n−6 | 2.92 | 29.52 ± 0.46d | 13.62 ± 1.52b | 21.03 ± 1.95c | 6.14 ± 0.80a |
| C20:4n−3 | n.d. | n.d. | 0.56 ± 0.16 | n.d. | 0.34 ± 0.01 |
| C20:4n−6 | 1.57 | 7.41 ± 0.58b | 3.72 ± 0.05a | 4.67 ± 0.55a | 3.07 ± 0.40a |
| C22:4n−6 | n.d. | 1.27 ± 0.09c | 0.53 ± 0.00a | 0.91 ± 0.14b | 0.26 ± 0.03a |
| C20:5n−3 | 3.91 | 8.99 ± 0.03b | 4.44 ± 0.50a | 5.56 ± 0.81a | 4.77 ± 0.66a |
| C22:5n−3 | 0.18 | 1.51 ± 0.29a | 0.98 ± 0.12a | 1.04 ± 0.32a | 0.96 ± 0.15a |
| C22:5n−6 | n.d. | 0.56 ± 0.08a | 0.58 ± 0.08a | 0.70 ± 0.11a | 0.53 ± 0.09a |
| C22:6n−3 | 0.77 | 3.96 ± 0.36a | 3.75 ± 0.46a | 4.55 ± 0.88a | 6.71 ± 1.01a |
| ∑n−3 | 1.89 | 14.45 | 9.71 | 11.16 | 12.78 |
| ∑n−6 | 5.86 | 38.98 | 18.19 | 27.30 | 10.00 |
| n−3:n−6 | 0.32 | 0.37 | 0.53 | 0.41 | 1.28 |
Note
- n.d. = not detected. No statistics are computed for groups with n.d. result.
- a,b,c,dMeans with different superscripts indicate significant differences (p < .05).
| Fatty acids | Concentration of fatty acids (mg/g) | ||||
|---|---|---|---|---|---|
| Wild | T1 | T2 | T3 | T4 | |
| ∑SAFA | 30.09 | 51.93 ± 15.43a | 39.81 ± 0.28a | 45.86 ± 2.42a | 50.43 ± 0.11a |
| C14:0 | 1.03 | 1.95 ± 0.12b | 1.56 ± 0.03a | 1.87 ± 0.07ab | 2.95 ± 0.04c |
| C15:0 | 0.87 | 0.92 ± 0.08a | 0.83 ± 0.08a | 0.80 ± 0.15a | 0.68 ± 0.04a |
| C16:0 | 16.17 | 35.72 ± 14.75a | 22.69 ± 0.15a | 30.34±1.65a | 31.76 ± 0.93a |
| C17:0 | 2.30 | 3.21 ± 1.64a | 3.51 ± 0.12a | 1.40 ± 0.13a | 2.60 ± 1.25a |
| C18:0 | 9.52 | 9.87 ± 1.06a | 10.43 ± 0.15a | 11.15±0.42a | 11.97 ± 0.17a |
| C21:0 | 0.12 | 0.53 ± 0.00bc | 0.60 ± 0.04c | 0.31 ± 0.01a | 0.47 ± 0.04b |
| ∑MUFA | 24.38 | 21.59±11.26a | 31.59 ± 2.18a | 42.65 ± 1.99a | 40.74 ± 1.71a |
| C16:1 | 6.41 | 5.97 ± 0.46ab | 5.84 ± 0.09ab | 4.90 ± 0.13a | 6.23 ± 0.22b |
| C17:1 | 0.93 | 1.15 ± 0.66a | 0.99 ± 0.03a | 0.45 ± 0.05a | 0.50 ± 0.09a |
| C18:1 | 1.44 | 5.07 ± 3.03a | 2.47 ± 0.00a | 4.61 ± 0.44a | 5.96 ± 0.00a |
| C18:1n−9 | 13.23 | 16.47 ± 0.00a | 21.16 ± 0.28b | 32.06±1.73d | 26.91 ± 0.58c |
| C20:1 | 2.30 | 0.31 ± 0.07a | 1.13 ± 0.13a | 0.64 ± 0.36a | 1.15 ± 1.01a |
| ∑PUFA | 7.75 | 10.30 ± 3.09a | 14.74±0.14ab | 23.80±2.74bc | 26.57 ± 2.81c |
| C18:2n−6 | 2.92 | 4.16 ± 2.21a | 7.75 ± 0.35ab | 12.17±0.89b | 9.79 ± 0.04b |
| C20:4n−3 | 0.00 | n.d. | 2.60 ± 0.00 | 0.38 ± 0.00 | 1.06 ± 0.00 |
| C20:4n−6 | 1.57 | 1.77 ± 0.20a | 2.69 ± 0.00b | 2.87±0.24bc | 3.35 ± 0.05c |
| C22:4n−6 | n.d. | 0.20 ± 0.03a | 0.83 ± 0.02b | 0.38 ± 0.12a | 0.38 ± 0.00a |
| C20:5n−3 | 0.00 | 2.07 ± 0.17a | 2.21 ± 0.01a | 4.06 ± 0.71b | 4.79 ± 0.35b |
| C22:5n−3 | 0.18 | 0.33 ± 0.06a | 0.33 ± 0.02a | 0.81 ± 0.14b | 2.86 ± 0.00c |
| C22:5n−6 | n.d. | 0.21 ± 0.06ab | 0.14 ± 0.01a | 0.37 ± 0.08bc | 0.51 ± 0.04c |
| C22:6n−3 | 0.77 | 1.44 ± 0.28a | 0.83 ± 0.14a | 2.96 ± 0.29b | 4.55 ± 0.52c |
| ∑n−3 | 1.89 | 3.84 | 4.67 | 8.01 | 12.73 |
| ∑n−6 | 5.86 | 6.46 | 10.07 | 15.79 | 13.84 |
| n−3:n−6 | 0.32 | 0.63 | 0.48 | 0.51 | 0.92 |
Note
- n.d. = not detected. No statistics are computed for groups with n.d. result.
- a,b,cMeans with different superscripts indicate significant differences (p < .05).
| Fatty acids | Concentration of fatty acids (mg/g) | ||||
|---|---|---|---|---|---|
| Wild | T1 | T2 | T3 | T4 | |
| ∑SAFA | 30.09 | 89.06 ± 13.84 | 69.93 ± 10.05 | 57.19 ± 30.68 | 60.94 ± 0.84 |
| C14:0 | 1.03 | 4.88 ± 1.51a | 3.28 ± 0.58a | 3.22 ± 1.73a | 3.67 ± 0.16a |
| C15:0 | 0.87 | 1.75 ± 0.36a | 1.74 ± 0.31a | 0.98 ± 0.53a | 0.81 ± 0.01a |
| C16:0 | 16.17 | 52.97 ± 3.01a | 42.95 ± 6.40a | 35.68±19.18a | 38.99 ± 1.05a |
| C17:0 | 2.30 | 6.64 ± 4.01a | 2.74 ± 0.27a | 4.48 ± 2.40a | 2.16 ± 0.01a |
| C18:0 | 9.52 | 22.02 ± 3.82a | 18.69 ± 2.14a | 12.14 ± 6.50a | 15.32 ± 0.38a |
| C21:0 | 0.12 | 1.59 ± 0.00 | 0.33 ± 0.06 | 0.68 ± 0.34 | n.d. |
| ∑MUFA | 24.38 | 79.70 ± 3.43 | 57.78 ± 5.67 | 56.34 ± 30.17 | 59.09 ± 0.78 |
| C16:1 | 6.41 | 15.76 ± 2.58a | 11.46 ± 0.36a | 9.27 ± 4.90a | 9.05 ± 0.10a |
| C17:1 | 0.93 | 1.93 ± 0.10a | 1.47 ± 0.21a | 0.99 ± 0.44a | 0.85 ± 0.07a |
| C18:1 | 1.44 | 14.49 ± 1.73a | 6.88 ± 0.64a | 7.51 ± 3.82a | 7.88 ± 0.20a |
| C18:1n−9 | 13.23 | 46.46 ± 2.02a | 36.91 ± 3.52a | 36.50±20.32a | 40.70 ± 0.59a |
| C20:1 | 2.30 | 0.88 ± 0.40a | 0.93 ± 0.75a | 2.08 ± 0.69a | 0.61 ± 0.02a |
| C22:1 | 0.07 | 0.36 ± 0.00 | 0.26 ± 0.00 | n.d. | n.d. |
| ∑PUFA | 7.75 | 38.98 ± 15.28 | 24.05 ± 3.92 | 27.86 ± 15.15 | 34.72 ± 0.67 |
| C18:2n−6 | 2.92 | 21.69 ± 6.49a | 13.74 ± 1.74a | 14.40 ± 7.96a | 13.48 ± 0.32a |
| C18:3n−6 | 0.05 | 0.36 ± 0.04 | 0.65 ± 0.00 | n.d. | 0.28 ± 0.06 |
| C20:4n−3 | 0.00 | 0.44 ± 0.00 | n.d. | 0.12 ± 0.00 | 0.34 ± 0.05 |
| C20:4n−6 | 1.57 | 5.23 ± 2.09a | 4.27 ± 0.47a | 3.62 ± 2.02a | 4.29 ± 0.10a |
| C22:4n−6 | n.d. | n.d. | n.d. | 0.33 ± 0.13 | 0.80 ± 0.00 |
| C20:5n−3 | 0.00 | 4.56 ± 1.69a | 3.08 ± 0.48a | 4.20 ± 2.35a | 6.69 ± 0.19a |
| C22:5n−3 | 0.18 | 0.95 ± 0.60a | 0.42 ± 0.10a | 0.72 ± 0.40a | 1.22 ± 0.07a |
| C22:5n−6 | n.d | 0.96 ± 0.54a | 0.39 ± 0.00a | 0.26 ± 0.00a | 0.78 ± 0.06a |
| C22:6n−3 | 0.77 | 5.01 ± 4.21a | 2.02 ± 0.39a | 4.39 ± 2.47a | 6.85 ± 0.13a |
| ∑n−3 | 1.89 | 10.74 | 5.52 | 9.37 | 15.10 |
| ∑n−6 | 5.86 | 28.24 | 18.53 | 18.48 | 19.62 |
| n−3:n−6 | 0.32 | 0.36 | 0.30 | 0.50 | 0.77 |
Note
- n.d. = not detected. No statistics are computed for groups with n.d. result.
- a,b.cMeans with different superscripts indicate significant differences (p < .05).
3.3 Analysis of biochemical changes of fatty acids in hepatopancreas and ovaries during different fattening periods
The general analysis of the fatty acids in the hepatopancreas and ovaries throughout the fattening periods is summarized in Tables 11 and 12, respectively. Throughout the fattening period, inconsistent levels of ∑SAFA, ∑MUFA and ∑PUFA were noted in all treatments with a ‘low–high–low’ pattern despite the degree of lipid levels. In comparison with the fatty acid concentration in the hepatopancreas, lower concentrations of ∑SAFA, ∑MUFA, OA and ∑n-6 were discovered in the ovaries of mud crabs in all treatments throughout the fattening periods. In contrast, higher concentrations of ARA, EPA and DHA, as well as the n-3 and n-3: n-6, were noted in the ovary compared with the hepatopancreas of mud crabs fed all diets. Highest HUFA concentrations were apparent in mud crabs fed the T4 compared with other treatments. During the 60- and 90-day fattening period, higher EPA and DHA were noted in the ovary of mud crabs fed with T4 (EPA: 4.79 mg/g and 6.69 mg/g; DHA: 4.55 mg/g and 6.85 mg/g) than in the hepatopancreas (EPA: 1.84 mg/g and 2.47 mg/g; DHA: 3.28 mg/g and 3.71 mg/g). Significant differences were detected in both EPA and DHA between treatments (p < .05).
| Fatty Acids | Wild (Control) | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||||||||
| Day 30 | Day 60 | Day 90 | Day 30 | Day 60 | Day 90 | Day 30 | Day 60 | Day 90 | Day 30 | Day 60 | Day 90 | ||
| ∑SAFA | 170.72 | 102.31 | 84.18 | 71.26 | 123.26 | 106.93 | 109.98 | 104.48 | 108.91 | 102.74 | 161.39 | 134.84 | 120.97 |
| ∑MUFA | 74.95 | 68.92 | 54.51 | 38.33 | 84.00 | 102.64 | 66.54 | 81.22 | 81.81 | 80.43 | 120.50 | 81.95 | 83.53 |
| ∑PUFA | 48.70 | 23.04 | 19.52 | 16.36 | 27.54 | 23.69 | 29.25 | 23.92 | 27.05 | 31.83 | 50.64 | 24.26 | 24.43 |
| OA | 25.14 | 34.42 | 22.04 | 18.59 | 41.72 | 62.67 | 39.37 | 49.17 | 51.14 | 52.95 | 66.66 | 42.81 | 52.75 |
| ARA | 15.24 | 2.21 | 4.74 | 2.04 | 3.22 | 2.88 | 3.67 | 2.66 | 2.50 | 3.45 | 4.31 | 2.18 | 1.58 |
| EPA | 5.60 | 1.88 | 3.65 | 1.65 | 2.51 | 1.97 | 2.34 | 1.92 | 2.51 | 2.72 | 7.11 | 1.84 | 2.47 |
| DHA | 17.32 | 1.71 | 1.60 | 2.02 | 2.27 | 3.13 | 3.10 | 2.28 | 2.85 | 4.25 | 10.75 | 3.28 | 3.71 |
| ∑n−3 | 24.36 | 4.73 | 5.25 | 4.10 | 5.80 | 7.02 | 6.06 | 4.84 | 7.70 | 7.65 | 21.05 | 7.34 | 7.22 |
| ∑n−6 | 23.42 | 18.19 | 14.27 | 11.75 | 21.68 | 16.66 | 19.89 | 19.01 | 19.35 | 24.18 | 29.59 | 16.92 | 17.22 |
| n−3:n−6 | 1.04 | 0.26 | 0.37 | 0.35 | 0.27 | 0.42 | 0.30 | 0.25 | 0.40 | 0.32 | 0.71 | 0.43 | 0.42 |
- Abbreviations: ARA, arachidonic acid, C20:4n-6; DHA, docosahexaenoic acid, C22:6n-3; EPA, eicosapentaenoic acid, C20:5n-3; n-3, omega-3; n-6, omega-6; OA, oleic acid, C18:1n-9.
| Fatty acids | Wild (control) | Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||||||||||
| Day 30 | Day 60 | Day 90 | Day 30 | Day 60 | Day 90 | Day 30 | Day 60 | Day 90 | Day 30 | Day 60 | Day 90 | ||
| ∑SAFA | 30.09 | 91.70 | 51.93 | 89.06 | 52.35 | 39.81 | 69.93 | 74.54 | 45.86 | 57.19 | 41.47 | 50.43 | 60.94 |
| ∑MUFA | 24.38 | 84.77 | 21.59 | 79.70 | 54.56 | 31.59 | 57.78 | 70.36 | 42.65 | 56.34 | 37.44 | 40.74 | 59.09 |
| ∑PUFA | 7.75 | 53.43 | 10.30 | 38.98 | 27.90 | 14.74 | 24.05 | 38.46 | 23.80 | 27.86 | 22.78 | 26.57 | 34.72 |
| OA | 13.23 | 63.61 | 16.47 | 46.46 | 28.26 | 21.16 | 36.91 | 48.78 | 32.06 | 36.50 | 22.10 | 26.91 | 40.70 |
| ARA | 1.57 | 7.41 | 1.77 | 5.23 | 3.72 | 2.69 | 4.27 | 4.67 | 2.87 | 3.62 | 3.07 | 3.35 | 4.29 |
| EPA | 0.00 | 8.99 | 2.07 | 4.56 | 4.44 | 2.21 | 3.08 | 5.56 | 4.06 | 4.20 | 4.77 | 4.79 | 6.69 |
| DHA | 0.77 | 3.96 | 1.44 | 5.01 | 3.75 | 0.83 | 2.02 | 4.55 | 2.96 | 4.39 | 6.71 | 4.55 | 6.85 |
| n−3 | 1.89 | 14.45 | 3.84 | 10.74 | 9.71 | 4.67 | 5.52 | 11.16 | 8.01 | 9.37 | 12.78 | 12.73 | 15.10 |
| n−6 | 5.86 | 38.98 | 6.46 | 28.24 | 18.19 | 10.07 | 18.53 | 27.30 | 15.79 | 18.48 | 10.00 | 13.84 | 19.62 |
| ∑n−3:n−6 | 0.32 | 0.37 | 0.63 | 0.36 | 0.53 | 0.48 | 0.30 | 0.41 | 0.51 | 0.50 | 1.28 | 0.92 | 0.77 |
- Abbreviations: and n-6: omega-6; ARA: arachidonic acid, C20:4n-6; DHA: docosahexaenoic acid, C22:6n-3; EPA: eicosapentaenoic acid, C20:5n-3; n-3: omega-3; OA: oleic acid, C18:1n-9.
4 DISCUSSION
The present study formulated semi-moist pellets with graded lipid levels to examine the dietary lipid requirement and the effect of the fattening period on the biochemical composition in the hepatopancreas and ovary of the adult S. olivacea. Based on the previous experiments carried out by Ahamad-Ali et al. (2011), the optimum protein requirement for mud crabs was 420 g/kg; therefore, authors try to find the optimum level for lipid in the maturation of mud crab with varying lipid levels. From the experiment, the crabs showed positive BWG and SGR during the 90-day fattening period for all treatments. The highest BWG and SGR during the fattening period were dominated by mud crabs fed the T4. The notable increase in the rate of BWG in the crabs fed with T4, especially during the Day 30 (1.80 g) to Day 60 (13.78 g) (p = .001) of the fattening period would indicate the body's highest need for nutrients. This can be evidenced based on data from SGR, where the highest value in mud crab fed the T4 was significant during Day 30 (0.03%day−1) to Day 60 (0.19%day−1) of the culture period.
In the meantime, the increase in the rate of BWG from 60-day (13.78 g) to 90-day fattening period (14.51 g) was not aggressive compared with during the earlier fattening period, probably because the crabs have been supplied with the same diet throughout the feeding experiment. Herlinah and Septiningsih (2015) suggested a mixed diet commenced during feeding exercise of mud crabs in the hatchery to improve the broodstock reproduction performance. Even though there are no moulting occurrences during experiments, the adult female S. olivacea used for fattening in this study showed a noticeable increase in their BWG throughout the fattening period, attributed to the higher ovary weight. The GSI of adult female S. olivacea increased significantly over the 90-day fattening period with crabs fed the T4 that showed the highest GSI value (17.30 ± 2.13%) compared with other treatments. The increasing rate of GSI indicates that the speed of ovarian development in adult S. olivacea was highest during the 90-day fattening period.
Meanwhile, lower HSI values compared with the GSI were a result of the transfer of nutrients from the hepatopancreatic reserve to the developing ovaries. This aligned with a study conducted by Sui et al. (2011), where the crabs’ HSI decreased significantly from 9.4% to 5.5–6.3%, while GSI recorded a significant increasing value from 0.7 to 8.8–9.5% after three months of the culture period. According to Kari-Woll and Marit-Berge (2007), crabs with a GSI between 15 and 18% with no increase in HSI were considered close to spawning. Overall, experiments showed that mud crabs fed with T4 had the best performance in BWG, SGR, GSI and HSI compared with other diets. The high growth performance in mud crabs fed with T4 may be credited to the high lipid levels compared with other diets.
On the contrary, analysis of total carotenoids showed no significant effect in the intake of lipid levels and length of the fattening period to the carotenoid readings. yet, higher carotenoid contents were noted in the ovary than in the hepatopancreas in all fattening periods. These results were contributed by the bright red colour observed in the ovary due to modifications in carotenoid content during oogenesis (Aaqillah-Amr et al., 2018). At the same time, lower carotenoid content in the hepatopancreas of mud crabs resulted from the mobilization of carotenoids to the ovary for oocyte maturation (Ravi & Manisseri, 2010; Wu, Khor, et al., 2020).
The proximate composition on feeds in this study revealed higher crude lipid in T4 (120 g/kg) with higher EPA and DHA values (1.27 mg/g and 1.20 mg/g, respectively). As lipid and fatty acid contents in diets directly affect the reproductive performance in the crustacean, they received considerable research attention, aiming to optimize reproduction by manipulating the lipid and HUFA levels in diets. Both EPA and DHA are believed to play an important role in reproduction in many cultured crustaceans, and crustaceans cannot synthesize these fatty acids de novo, so the requirements for these HUFA must be supplied through the diet (Azmie et al., 2017). According to Sheen and Wu (2002), the increasing BWG in juvenile S. serrata was a result from diets supplemented with HUFA, which concluded the roles of these fatty acids, majorly in maximizing growth. A recent study on juvenile S. paramamosain revealed the highest BWG and SGR in crabs fed the diets with optimal dietary DHA/EPA ratio of 1.2 at 120 g/kg lipid (Wang et al., 2021), whereas a study conducted on P. monodon showed that the accumulation of ARA in the ovary of P. monodon was as a result of provision of ARA from the diet. At the same time, high GSI in the ovary of S. olivacea reflected high levels of fatty acids in the ovary, where the increasing levels at subsequent fattening period resulted from the accumulation of HUFA levels in the ovary. Comprehensively, the present study showed that the appropriate fattening period would best be carried out for 60 – 90 days of duration, with the lipid levels best recorded at 120 g/kg.
SAFA has been the predominant fatty acid at all fattening periods, with the highest concentration noted in the hepatopancreas. These findings were comparable to a study conducted by Tantikitti et al. (2015). It is interesting to note that the high concentrations of ∑SAFA are contributed by the high levels of C16:0, which functions as a key metabolite that provides the crabs with energy during embryonic development (Persia-Jothy et al., 2019). In contrast, analysis of individual MUFA showed that OA had a higher concentration in the hepatopancreas than in the ovary in all treatments throughout the fattening periods. In particular, OA will be utilized as an energy source (Long et al., 2020), which explains the high OA values in the hepatopancreas. These results were in accordance with those of Persia-Jothy et al. (2019), where the high concentration detected in OA was probably used for energy metabolism during reproduction in anomuran crab, Emerita asiatica. Many studies have highlighted the importance of OA in the maturation of mud crabs in the desaturation of LA and ALA (Monroig & Kabeya, 2018) besides their roles in complex signalling (Liu et al., 2021). The present investigation revealed that both LA and ALA were highly concentrated in the ovary of all treatments compared with the hepatopancreas, which supported this statement.
Moreover, results revealed that mud crabs fed the T4 had higher EPA and DHA concentrations than ARA in both hepatopancreas and ovaries. Even though ARA is considered one of the HUFA, since ARA was derived from the n-6 fatty acids, their importance was less significant than the n-3 EPA and DHA. To be precise, ARA shared the same functions as EPA and DHA as the precursor to eicosanoids (Heckmann et al., 2008). Nonetheless, the n-6 LC PUFA will compete with the n-3 LC PUFA for the same desaturation enzyme as the limiting factor (Abedi & Sahari, 2014) . The eicosanoids are necessary for mud crabs that control against the inflammatory responses. The derivation of eicosanoids from n-6 has stronger inflammatory signals than n-3-derived eicosanoids, which are anti-inflammatory (Calder, 2013). From the present study, the high provision of lipid in T4 has resulted in the higher concentration of both EPA and DHA, especially in the ovary compared with other experimental diets although the levels of these fatty acids fluctuated throughout the fattening period. The fluctuation concentrations of both EPA (4.77 mg/g, 4.79 mg/g and 6.69 mg/g) and DHA (6.71 mg/g, 4.55 mg/g and 6.85 mg/g) in the ovary during 30-, 60- and 90-day fattening period highlight their importance in the reproduction of mud crab, suggesting that these fatty acids should be given more attention during the formulation. As such, the crabs were supplemented with exogenous EPA and DHA in the diet of S. olivacea. In the present study, both EPA and DHA supplied from T4 (120 g/kg lipid) were 1.27 mg/g and 1.20 mg/g, respectively, in which these supplementations have increased twice the EPA (4.77 mg/g, 4.79 mg/g and 6.69 mg/g) and DHA (6.71 mg/g, 4.55 mg/g and 6.85 mg/g) in the ovary. However, the experiment carried out by Wu et al. (2007) showed that diet containing 7.8 – 10.0 mg/g EPA and 10.5 – 13.0 mg/g DHA had the highest reproductive performance, with EPA and DHA in the egg of E. sinensis being 97.6–112.1 mg/g and 77.6–91.0 mg/g, respectively, in which the supplementation has caused ten times increment in HUFA. The differences in HUFA requirements may be due to different species, and the requirement of an in-depth study to confirm the actual requirement of HUFA in each species. The high lipid provision in T4 has resulted in the higher concentration of both EPA and DHA in the ovary during all experimental feedings compared with other diets, with the highest increments noted in the 90-day fattening period. Ying et al. (2006) explained the variation in the fatty acid content in the ovary and hepatopancreas is because the crabs at this stage are in a critical reproduction stage, where lipid is the only energy for embryonic development. In crustacean, lipids are synthesized and stored in the hepatopancreas before being deposited to the ovary during the ovarian cycle (Chansela et al., 2012), which explained these occurrences. The mechanism of lipid deposition in crustaceans can be observed in the histological changes of both hepatopancreas and ovaries where the oocyte size increases during maturation, and increasing amounts of lipid droplets was found to be observed with intense eosinophilic staining (Aaqillah-Amr et al., 2018; Chansela et al., 2012). In comparison, the hepatopancreatic tubule had an abundance of B and R cell, which function in the intracellular digestion and storage of lipids (Hidir et al., 2018). The lower proportions of ARA in the ovaries than both EPA and DHA signify that the high concentration of both EPA and DHA has lowered the chances in the competition for the same desaturation enzymes.
∑n-3 from the present study recorded higher level in the ovary where the concentrations were almost twice from the hepatopancreas in all fattening periods. As a result, the n-3: n-6 ratio recorded higher level in the ovary (0.30–1.28) than in the hepatopancreas (0.26–0.71), depicting the occurrence of nutrients transferred from the hepatopancreas to the ovary for reproduction purposes. These findings were similar to what has been reported in the wild-caught mud crabs (Aaqillah-Amr et al., 2018; Azmie et al., 2017). This coincides with the fact that hepatopancreas functions are mainly as a storage organ of nutrients where the nutrients that have undergone complete digestion will be transported to other parts of the organ such as muscle and gonads (Xu et al., 2020). Present data were evident in the study by Xu et al. (2014) where the fatty acid levels in paddle crabs, Charybdis japonica, were lower in hepatopancreas but increasing in the ovary along the course of ovarian maturation. This lipid mobilization from the hepatopancreas to the ovary suggested that the lower fatty acid levels would probably take part in the vitellogenesis process (Alava et al., 2007). It can be generalized that the inclusion of lipid levels affects the concentration of specific fatty acids such as PUFA, which is vital in the reproduction of the mud crabs where the demand for fatty acids gradually increases during ovarian development (Holme et al., 2009). Thus, this result emphasized the necessity of lipid in the diet formulation for the reproductive organs.
Compared with the fatty acids of mud crabs from the wild (control), the highest levels of ∑PUFA were detected in the hepatopancreas of wild-caught mud crab compared with crabs in captivity. The high fatty acid composition in the wild-caught S. olivacea is probably driven by the wide accessibilities to various food sources from the wild compared with the crabs kept in captivity (Aaqillah-Amr, 2017; Cartes, 1994; Gibson & Barker, 1979). The high percentage of ∑n-3 in the hepatopancreas of wild-caught S. olivacea may likely be due to the supplementation from diet sources from other organisms such as plants and other eukaryotes. It is well known that crabs are described as omnivores where they consume almost everything in the wild based on the gut content analysis (Hidir et al., 2018), which supported this evidence. Nevertheless, analysis of the fatty acids in the ovary of S. olivacea fed the T4 was higher than the wild-caught carbs, indicating a significant transfer of n-3 fatty acids from hepatopancreas during the fattening periods. This finding shared similarity to the Alava et al. (2007) studies, where biochemical analysis of S. serrate found that HUFA in the ovary was higher in pond-reared than in the wild-caught crabs. At the same time, the HUFA content in ovaries from pond-reared was higher than in hepatopancreas from pond-reared mud crabs. The increase in several fatty acids such as OA and DHA during the 90th day of fattening periods suggests that the appropriate fattening period would best be carried out for 60–90 days of duration with the lipid levels best recorded at 120 g/kg.
This study validates that diets containing 120 g/kg lipid provided a more remarkable performance in fatty acid composition in the hepatopancreas and ovaries of the mud crabs. These results suggested that the optimal dietary lipid requirement in the mud cab broodstock would be 120 g/kg in S. olivacea. Previously, an experiment conducted on S. paramamosain recorded an optimum diet of 95 g/kg (Zhao et al., 2015), whereas studies on the S. serrate recorded several different results from different studies, which were 53–138 g/kg (Sheen & Wu, 1999), 86 g/kg (Sheen, 2000), 60 g/kg and 120 g/kg (Catacutan, 2002), 100 g/kg (Alava et al., 2007) and 97.9 g/kg (Unnikrishnan et al., 2010). The lipid requirement differences may likely be because different crabs live in different environments with different salinity preferences. This was supported by a study carried out by Long et al. (2019) where Chinese mitten crab (Eriocheir sinensis) showed high total lipid content after being reared for 40 days in high salinity as a result of long-term salinity adaptation. Although current study recorded different results from both S. serrate and S. paramamosain, the lipid requirement in S. olivacea remains within the optimal range recorded in crustaceans for good reproduction, which is 20–120 g/kg (Zhao et al., 2015). Such results indicated that feeding the adult S. olivacea with 120 g/kg lipid levels within 60-day to 90-day fattening period could effectively improve the reproductive performance in the crabs. The results will be helpful for the rearing of mud crabs with suitable lipid levels and a fattening period to obtain ovigerous females at a faster rate.
5 CONCLUSION
A proper level of dietary protein (420 g/kg) and lipid (120 g/kg) could maintain solid growth and maturation of adult mud crabs, S. olivacea. In particular, overall assessments disclosed that mud crabs fed the T4, which contained 120 g/kg lipid, showed highest BWG (1.80 g, 13.78 g and 14.51 g), SGR (0.03%day−1, 0.19%day−1 and 0.28%day−1), GSI (9.90%, 11.31% and 17.30%) and HSI (4.16%, 5.22%, 5.06%) during 30-, 60- and 90-day fattening period, respectively. Higher fatty acids were noted in the ovary (EPA: 4.79 mg/g and 6.69 mg/g; DHA: 4.55 mg/g and 6.85 mg/g) in comparison with the hepatopancreas (EPA: 1.84 mg/g and 2.47 mg/g; DHA: 3.28 mg/g and 3.71 mg/g) during 60- and 90-day fattening period, indicating the occurrences of nutrient transfer from the hepatopancreas to ovaries during maturation. Two-way ANOVA suggested that feed formulation containing 120 g/kg lipid levels was ideal as feeding practice in aquaculture with the appropriate fattening period would best be carried out for 60–90 days of duration.
ACKNOWLEDGEMENTS
The Ministry of Higher Education funded this research under the Higher Institution Center of Excellence (HICoE) grant for the development of future food through sustainable shellfish aquaculture (HICoE AKUATROP Trust Account No. 66955) and Golden Goose Research Grant (GGRG) on Marine amphipod potential as an alternative feed resource for aquaculture (Vot. 55189). We acknowledge all the staff at the Institute of Tropical Aquaculture and Fisheries, Institute of Marine Biotechnology and Central Laboratory of Universiti Malaysia Terengganu (UMT) who involved directly or indirectly to this research article.
CONFLICT OF INTERESTS
The authors declare that there is no competing or financial interests.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article.




