Growth performance, haemato-immunological responses and antioxidant status of Pacific white shrimp Penaeus vannamei fed with turmeric powder, curcumin and curcumin nanomicelles
Abstract
Effects of dietary administration of turmeric (TUR), curcumin (CUR) and curcumin nanomicelles (NMC) were investigated on growth, haemato-immunology and antioxidant status of the Pacific white shrimp Penaeus vannamei. The shrimps (2.96±0.10 g) were fed with 10 diets: without supplementing TUR, CUR and NMC (control), supplemented with 2.5, 5 and 10 g TUR, 0.075, 0.150 and 0.300 g CUR and 0.075, 0.150 and 0.300 g NMC per kg of diet. CUR 0.075 and NMC 0.150 improved final weight, weight gain (%), specific growth rate and feed conversion ratio. Survival rate was more increased with NMC 0.150, which improved the biomass gain. The lowest levels of serum alanine transaminase and aspartate transaminase and the highest levels of acid phosphatase, total antioxidant capacity, glutathione, catalase and superoxide dismutase were detected in NMC 0.150 (p < .05). Malondialdehyde was significantly lower in NMC 0.150 compared with the control. NMC treatments showed higher levels of total protein, lysozyme and albumin than the other treatments. It is concluded that both the dietary CUR and NMC improved the antioxidant and immunity response of P. vannamei, however, NMC 0.150 induced a better overall shrimp performance. Application of nanocarriers for the delivery of dietary immunostimulants seems promising in shrimp nutrition.
1 INTRODUCTION
Noticeable decline in global fisheries catches and the need to expand food production industries owing to the rising human population have made marine aquaculture very important and beneficial. The expansion of intensive as well as stressful fish and shellfish culture systems makes the spread of pathogens and the outbreak of infectious diseases inevitable. These problems increase the use of chemotherapeutics and antibiotics and subsequently cause drug resistance, suppression of immune systems and the pollution of aquatic ecosystems as well as the health risks to consumers (Kohshahi et al., 2019; Sarkheil et al., 2017). The Pacific white shrimp Penaeus vannamei Boone, 1931 with characteristics suitable for intensive aquaculture, is the most important cultured penaeid species in many parts of the world (Chen et al., 2018; Khanjani et al., 2016).
In recent years, medicinal herbs and their compounds have been used as phytogenics and popular feed additives in aquaculture feeds to secure health and improve the growth and immune response of cultured animals. These phytogenic natural compounds act as non-antibiotic growth promoters and natural immunostimulants instead of antibiotics and chemicals (Alagawany et al., 2021; Giri et al., 2019; Moniruzzaman & Min, 2020). Turmeric (TUR), a well-known spice powdered from the rhizome of the tropical herb Curcuma longa from the Zingiberaceae family (Gupta et al., 2013), has displayed powerful medicinal properties such as anticarcinogenic, anti-inflammatory, antimicrobial and antioxidant activities (Dosoky & Setzer, 2018; Xu et al., 2018). About 235 natural and bioactive compounds, mainly phenolics and terpenoids, have been recognized from various species of TUR. Curcuminoids are amongst the major bioactive compounds of TUR. Curcumin (CUR), or diferuloylmethane, as a hydrophobic and polyphenolic compound, is one of the three major curcuminoids (curcumin: 60%–70%) found in TUR with medicinal values and immunomodulatory and antioxidant properties (Alagawany et al., 2021; Anand et al., 2007).
In aquaculture, different biological activities including immunomodulatory, growth enhancement, hepatoprotective, antioxidant, antiparasitic and bactericidal properties have been reported for dietary CUR (Cao et al., 2015; El-Barbary, 2018; Liu et al., 2017; Mahmoud et al., 2017; Sruthi et al., 2018). However, studies on the uptake, metabolism and excretion of CUR have shown its poor absorption, rapid metabolism and elimination because of its hydrophobicity and unstable chemical structures that severely limit its bioavailability and therapeutic usage (Alagawany et al., 2021; Anand et al., 2007; Douglass & Clouatre, 2015; Kotha & Luthria, 2019). Some approaches including the use of adjuvants and polymeric nanoparticles, encapsulation in liposomes and micelles, and the use of phospholipid complexes and hydrogels have been undertaken to overcome the CUR low biodistribution and bioavailability (Anand et al., 2007; Moniruzzaman & Min, 2020; Xu et al., 2018). The emphasis has been put on the nanocarrier delivery systems as an effective strategy to enhance the bioavailability of highly hydrophobic agents such as CUR (Li et al., 2017; Youssouf et al., 2019). The use of CUR along with tiny structures such as liposomes and micelles as carriers for the delivery of bioparticles has been suggested in this regard (Douglass & Clouatre, 2015; Moniruzzaman & Min, 2020). Nanomicelles are one of the eight types of nanocarriers used in nanobiotechnology for smart drug delivery systems (Hossen et al., 2019). These nano spherical structures that are formed by self-assembly of amphiphilic molecules have received tremendous interest in nanomedicine. They can deliver drugs that do not dissolve well in water to the target position and protect the drug molecules as well (Gong et al., 2012; Tawfik et al., 2020). Existing empirical evidence suggests nanomicelles as efficient and promising nanocarrier delivery systems for peptides, proteins and other hydrophobic components (Li et al., 2017; Pirani et al., 2021; Youssouf et al., 2019).
Feeding with CUR has led to promising findings about the growth and feed efficiency, haemato-immunological parameters and antioxidant status of various aquaculture species e.g. common carp Cyprinus carpio (Abdel-Tawwab & Abbass, 2017; Giri et al., 2019; Yonar, 2018), rainbow trout Oncorhynchus mykiss (Akdemir et al., 2017; Kohshahi et al., 2019; Yonar et al., 2019), Nile tilapia Oreochromis niloticus (Abd El-Hakim et al., 2020; Mahmoud et al., 2017; Mohamed et al., 2020) and crucian carp Carassius auratus (Jiang et al., 2016; Yang et al., 2020).
However, to our knowledge, the studies on the effects of TUR and CUR dietary administration on shrimp species are scarce, and no comparative study has addressed the potential effects of TUR and CUR alone or encapsulated in nanocarriers on the overall performance of cultured shrimps. Here, we tried to investigate the probable effects of TUR and its bioactive compound, CUR, alone and encapsulated in nanomicelles on the growth, haemato-immunology and antioxidant status of the cultured juvenile Pacific white shrimp.
2 MATERIALS AND METHODS
2.1 Experimental shrimps and conditions
A total number of 1200 healthy P. vannamei with an average initial weight of 2.96±0.10 g were received from a private nursery (Kesht-o-Sanat Tiab) in Hormozgan Province, southern Iran. The shrimps were transferred to Kolahi breeding center, Hormozgan Province and acclimated to the experimental conditions in 30 fibreglass tanks (300-L cylindrical tanks filled with 250 L seawater) with 40 shrimps randomly distributed in each and fed on a commercial diet for 2 weeks. The commercial diet was provided from Hatami Aqua Feed Co. The ingredients and proximate composition of the commercial diet are presented in Table 1. The chemical composition of diets was determined based on standard methods (AOAC, 2000). Sodium, potassium, calcium and magnesium were determined by atomic emission spectrometry and phosphorus by atomic absorption spectrometry. All analyses were conducted in triplicate.
| Ingredients | % | Composition | (Dry matter; g kg−1) |
|---|---|---|---|
| Fish meal | 27 | Crude protein | 400.2 |
| Squid liver powder | 2 | Crude lipid | 78.2 |
| Canola meal | 6 | HUFA | 7.5 |
| Cow gelatin | 5 | Phospholipid | 11.4 |
| Wheat flour | 27 | Cholesterol | 1.1 |
| Soybean meal | 16 | Fibre | 23.1 |
| Corn gluten | 6 | Ash | 144.2 |
| Soy lecithin | 1.5 | Calcium (Ca) | 34.8 |
| Kilka fish oil | 1.5 | Magnesium | 4.2 |
| Wheat gluten | 5 | Sodium | 7.9 |
| Yeast | 0.5 | Potassium | 8.0 |
| NaCl | 0.5 | Phosphorus (P) | 15.9 |
| Premix | 2 | P/Ca | 0.46 |
| Total | 100 |
Note
- Premix (Creve Tec shrimp feed concentrate 2%): wheat protein, vitamins minimum value: (inositol, biotin, folic acid, nicotinic acid, panthothenic acid, vit B2 (riboflavin), vit B1 (thiamine), vit B6 (pyridoxine), vit B12 (cyanocobalamine), vit A 1000, vit D3, vit K, vit C (L-ascorbic acid), choline, organic trace minerals: (Fe, Cu, Mn, Zn, Se, I), Phosphates, digestibility enhancer, cholesterol.
The shrimps were then assigned to experimental treatments. During the 63 days of the experiment, all the tanks contained filtered seawater with constant aeration supplied with an air stone linked to a central air compressor. The mean salinity, dissolved oxygen, temperature and pH of the water were 37.0 ± 0.4 g L−1, 6.8 ± 0.3 mg L−1, 32.0 ± 0.5°C and 7.9±0.2, respectively. The shrimp excreta were removed from each tank and about two-thirds of the water was replaced at an interval of three times weekly throughout the trial. During the culture, feeding was done three times daily at 7:00, 12:00 and 17:00 based on 3%–6% of the biomass of each tank according to the weekly biometric measurements. The photoperiod was 14 h light and 10 h dark.
2.2 Diet preparation
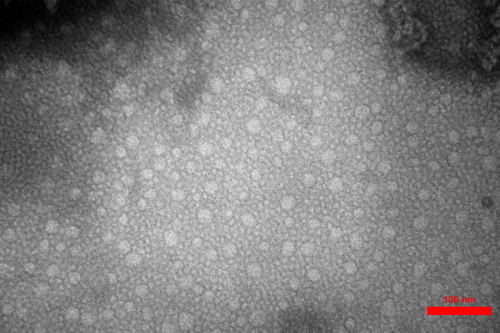
Freshly powdered TUR with proximate composition (% dry matter) of 69.2% carbohydrates, 7.35% protein, 3.55% lipid, 7.10% fibre and 12.8% ash was purchased from the local market. Curcumin (C21H20O6) consist of 75% curcumin, 20% demethoxycurcumin and 5% bisdemethoxycurcumin was obtained from Sigma-Aldrich. Curcumin nanomicelles (9.3 ± 0.2 nm) containing 6.65% curcuminoids (72% curcumin, 25% demethoxycurcumin and 3% bisdemethoxycurcumin) were provided by Exir Nano Sina Co (TEM image is shown in Figure 1).
Different dosages (g) of turmeric (TUR 2.5, TUR 5 and TUR 10), curcumin (CUR 0.075, CUR 0.150 and CUR 0.300) and curcumin nanomicelles (NMC 0.075, 0.150 and 0.300) per kg of diet were supplemented in the experimental diets. These nine treatments (experimental treatments) together with the control group (control), which contained no additive, made the 10 treatments of the present study each with three replicates.
To make the experimental diets, the diet ingredients based on the commercial diet formula were milled into powder using a hammer mill machine and sieved through mesh no. 80 (Tyler Sieve, mesh size 180 µ). The additives (TUR, CUR and NMC) were gradually added to the weighed ingredients to get the final concentrations in the diet. In this regard, wheat flour was used as the principal ingredient to balance the diets considering the different levels of the additives (Mahmoud et al., 2017). Ingredients were mixed in a mixer; then, fish oil, soy lecithin and distilled warm water (30%) were added and blended for 15 min. The wet diet was passed through a cold-pellet machine (pellet diameter: 1.5 mm). An electric oven was used to steam the pellets at 80°C for 45 min. The pellets were then air-dried to about 10% moisture and kept at −20°C. The levels of the additives were selected based on the range of levels used in previous nutrition studies in different aquaculture species (Alagawany et al., 2021; Moniruzzaman & Min, 2020). The only available reference on the use of CUR in shrimp species was the study by García-Pérez et al. (2020), in which 0.15, 0.2 and 0.3 g of CUR per kg of diet was used to protect the juvenile P. vannamei from aflatoxins. Therefore, after careful evaluation of all fish and shrimp studies, doses of 0.075, 0.150 and 0.300 g of CUR and NMC per kg of diet were selected. Based on the TUR analysis, it contained 3% CUR and therefore, doses of 2.5, 5 and 10 g TUR per kg of diet were selected for the present study.
2.3 Growth performance and proximate composition analysis
The shrimp feeding was terminated 24 h before the end of the experimental period. After the end of the trial, the remaining shrimps were counted, and biometric measurements were made. The mean weight gain percentage (WG %), specific growth rate (SGR), feed conversion ratio (FCR) and survival rate (SR) were calculated with the following equations (Chen et al., 2018):
WG (%) = {(final weight [g]−initial weight [g])/initial weight [g]} × 100.
SGR (% day−1) = {(Ln final weight [g]−Ln initial weight [g])/experimental days} × 100.
FCR = feed intake (g)/weight gain (g).
SR (%) = (final number of shrimps/initial number of shrimps) × 100.
Based on the biometric measurements at the beginning and at the end of the experiment, biomass gain (BG) was calculated for all the treatments through the following equation (Wang et al., 2015),
BG (g) = final biomass (g)−initial biomass (g).
Economical conversion ratio (ECR) was also determined for all the treatments based on the following equation (Skalli et al., 2004):
ECR = feed cost ($/kg) × FCR.
Five shrimps were randomly selected from each tank and kept at −20°C for the analysis of whole-body proximate composition. Standard procedures were used to determine the content of dry matter, crude protein, crude lipid and ash of the shrimp's whole body (AOAC, 2000).
2.4 Measurement of haematological parameters
Haemolymph was taken from the ventral sinus of five shrimps from each tank by a 1-ml sterile syringe having precooled anticoagulant (30 mM trisodium citrate, 388 mM sodium chloride, 115 mM glucose and 10 mM EDTA). The collected haemolymph was centrifuged at 4600 g for 10 min at 4°C, and the supernatant liquid was collected in a sterile tube and then, kept at −80°C until analysed for haemato-immunological and antioxidant parameters. The serum haemato-immunological parameters were determined applying commercial kits (Pars Azmun Company) following the company's protocol.
Briefly, serum alkaline phosphatase (ALP) was measured using a UV-2100 spectrophotometer at 405 nm based on the conversion of P-Nitrophenylphosphate to phosphate and P-Nitrophenol (Kind & King, 1954). Acid phosphatase (ACP) activity was estimated spectrophotometrically at 410 nm based on the P-Nitrophenylphosphate conversion to P-Nitrophenol (Fishman & Lerner, 1953). Aspartate transaminase (AST) was measured based on the aspartate conversion to glutamate and finally to malate. Alanine transaminase (ALT) was spectrophotometrically determined at 340 nm based on the alanine conversion to glutamate and pyruvate and finally to lactate (Reitman & Frankel, 1957).
2.5 Measurement of antioxidant parameters
Total antioxidant capacity (TAOC) was measured using commercial kits (ZellBio GmbH) based on the manufacturer's guidance. Malondialdehyde (MDA) concentration was determined through the thiobarbituric acid approach (Buege & Aust, 1978), glutathione (GSH) activity by the procedure explained by Inoue et al. (1987) and superoxide dismutase (SOD) by its capacity to suppress the superoxide radical-dependent reactions (McCord & Fridovich, 1969). Catalase (CAT) activity was measured through Aebi (1984) technique according to the rate constant of decomposing hydrogen peroxide (H2O2) by the CAT enzyme.
2.6 Measurement of immunological parameters
Serum total protein (TP) and albumin (ALB) were determined using biuret and bromocresol green dye binding approach (Doumas et al., 1971). The lysozyme (LYZ) activity was estimated by turbidimetric assay, and hen egg white lysozyme was used as standard (Ellis, 1990). An amount of 175 µl of a suspension of Micrococcus lysodeikticus at 0.2 mg ml−1 concentration in 0.5 M phosphate buffer with pH 6.2 was added to 25 µl of serum in each well of a 96-well plate. The reduction in OD was documented at 530 nm after 1 and 5 min at 22°C. One unit of LYZ activity was determined as a decrease in absorbance of 0.001 per min. The phenoloxidase (PO) activity was measured photometrically through the red pigment DOPA-chrome formation, after oxidation of the enzyme substrate L-dihydroxy-phenylalanine (Perazzolo & Barracco, 1997). PO was declared as the variation in 490 nm absorbance/min/mg of the total protein in the samples.
2.7 Statistical analyses
Excel software was used to process the experimental data which were expressed as means ± standard error (SE). Kolmogorov–Smirnov and Levene's tests were applied to evaluate the normality and homogeneity of the data, respectively. Data were analysed using SPSS 26.0 (IBM). One-way ANOVA was used to assess the differences amongst the TUR, CUR and NMC treatments when the data had homogeneous variance. To specify the differences amongst the treatments, Duncan's multiple range test at 95% confidence was applied.
3 RESULTS
3.1 Growth performance and body composition
The results related to the growth performance of P. vannamei juveniles fed the control and experimental diets are presented in Table 2. Overall, growth performance was influenced by 9 weeks of feeding with experimental diets, and important effects were registered amongst the control and experimental treatments. TUR, CUR and NMC treatments caused the improvement of growth parameters including final weight (FW), WG %, SGR and FCR compared with the control. CUR 0.075 and NMC 0.150 induced the best growth performance. Significantly lower mortality was noticed in shrimps fed NMC treatments during the entire trial period. NMC treatments, especially NMC 0.150, improved the SR and the resultant BG in comparison with the control and other experimental treatments (p < .05). TUR, CUR and NMC treatments caused no significant increase in ECR compared with the control meaning, and no increase in production cost was induced by these supplements. CUR 0.075 showed the lowest and NMC 0.300 and control showed the highest ECR.
| Growth parameter | Control | TUR 2.5 | TUR 5 | TUR 10 | CUR 0.075 | CUR 0.150 | CUR 0.300 | NMC 0.075 | NMC 0.150 | NMC 0.300 |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial weight (g) | 2.91 ± 0.06 | 2.93 ± 0.01 | 2.94 ± 0.03 | 2.94 ± 0.06 | 2.95 ± 0.01 | 2.96 ± 0.01 | 2.94 ± 0.03 | 2.97 ± 0.01 | 2.98 ± 0.02 | 2.96 ± 0.01 |
| Final weight (g) | 14.91 ± 0.07f | 15.86 ± 0.27abc | 15.46 ± 0.03e | 15.43 ± 0.07e | 16.20 ± 0.01a | 15.51 ± 0.06de | 15.83 ± 0.08bcd | 15.73 ± 0.11cde | 16.11 ± 0.11ab | 15.86 ± 0.04abc |
| WG (%) | 412.93 ± 10.99c | 441.43 ± 10.28ab | 425.68 ± 4.71bc | 425.20 ± 9.86bc | 449.85 ± 2.00a | 423.10 ± 3.66bc | 437.88 ± 8.10ab | 429.10 ± 5.09abc | 440.94 ± 6.84ab | 436.73 ± 1.84ab |
| SGR (% day−1) | 2.59 ± 0.03c | 2.68 ± 0.03ab | 2.63 ± 0.01bc | 2.63 ± 0.03bc | 2.71 ± 0.01a | 2.63 ± 0.01bc | 2.67 ± 0.02ab | 2.64 ± 0.02abc | 2.68 ± 0.02ab | 2.67 ± 0.01ab |
| FCR | 1.68 ± 0.02a | 1.55 ± 0.02b | 1.58 ± 0.01ab | 1.57 ± 0.06b | 1.49 ± 0.03b | 1.56 ± 0.03b | 1.52 ± 0.03b | 1.54 ± 0.02b | 1.52 ± 0.00b | 1.54 ± 0.05b |
| BG (g) | 358.71 ± 18.14c | 363.99 ± 5.58c | 353.04 ± 3.52c | 299.54 ± 5.22d | 416.82 ± 9.08b | 362.12 ± 10.13c | 388.57 ± 14.93bc | 422.40 ± 9.74b | 485.21 ± 1.09a | 420.23 ± 18.64b |
| SR (%) | 75.00 ± 2.50d | 75.00 ± 1.44d | 75.00 ± 0.00d | 65.83 ± 0.83e | 82.50 ± 1.44c | 77.50 ± 1.44cd | 80.83 ± 2.20c | 87.50 ± 1.44b | 97.50 ± 0.00a | 90.00 ± 2.89b |
| ECR ($/kg) | 1.45 ± 0.03ab | 1.35 ± 0.02cd | 1.38 ± 0.02bcd | 1.39 ± 0.10bcd | 1.32 ± 0.04d | 1.41 ± 0.05bcd | 1.43 ± 0.04abc | 1.37 ± 0.03bcd | 1.39 ± 0.01bcd | 1.50 ± 0.09a |
Note
- Values are presented as mean ± SE of three replicates. Mean values with different superscripts are significantly different within row (p < .05).
- Abbreviations: BG, biomass gain; ECR, economical conversion ratio; FCR, feed conversion ratio; SGR, specific growth rate; SR, survival rate; WG (%), weight gain percentage.
Body proximate composition of the shrimps fed the control diet and different levels of TUR, CUR and NMC are shown in Table 3. The CUR in its different forms affected the whole-body composition and the amount of crude protein and crude lipid was the highest in the shrimps fed CUR 0.075 and NMC 0.150 (p < .05).
| Parameter | Control | TUR 2.5 | TUR 5 | TUR 10 | CUR 0.075 | CUR 0.150 | CUR 0.300 | NMC 0.075 | NMC 0.150 | NMC 0.300 |
|---|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 25.02 ± 0.11ed | 26.14 ± 0.15a | 25.51 ± 0.27bcd | 25.89 ± 0.05ab | 26.37 ± 0.37a | 25.25 ± 0.22cd | 25.80 ± 0.12abc | 25.02 ± 0.10de | 25.03 ± 0.11de | 24.46 ± 0.15e |
| Crude protein | 17.94 ± 0.05e | 18.73 ± 0.06abc | 18.22 ± 0.17de | 18.46 ± 0.02bcd | 18.90 ± 0.13a | 18.41 ± 0.10cd | 18.62 ± 0.01abc | 18.46 ± 0.18bcd | 18.79 ± 0.12ab | 18.69 ± 0.02abc |
| Crude lipid | 1.81 ± 0.03d | 1.84 ± 0.03cd | 1.83 ± 0.07cd | 1.86 ± 0.09cd | 1.97 ± 0.03a | 1.87 ± 0.07bcd | 1.91 ± 0.03abc | 1.95 ± 0.04ab | 1.95 ± 0.03ab | 1.91 ± 0.04abc |
| Ash | 2.60 ± 0.01d | 2.73 ± 0.05c | 2.62 ± 0.01d | 2.62 ± 0.03d | 2.68 ± 0.04bcd | 2.67 ± 0.02cd | 2.72 ± 0.03bc | 2.77 ± 0.03ab | 2.84 ± 0.02a | 2.75 ± 0.04abc |
Note
- Values are presented as mean ± SE of three replicates. Mean values with different superscripts are significantly different within row (p < .05).
3.2 Haematological findings
The effects of control and experimental treatments on serum biochemical parameters of juvenile P. vannamei have been shown in Figure 2. ALP was not different between the control and different experimental treatments (p > .05). The levels of ALT and AST decreased in shrimps fed with different dosages of TUR, CUR and NMC additives compared with the control (p < .05). The highest and lowest levels of ALT and AST were detected in the control and NMC 0.150, respectively (p < .05). About the ACP, the values were elevated in all NMC treatments compared with the control. NMC 0.150 had the highest level of ACP compared with the control and all experimental treatments (p < .05).

3.3 Antioxidant findings
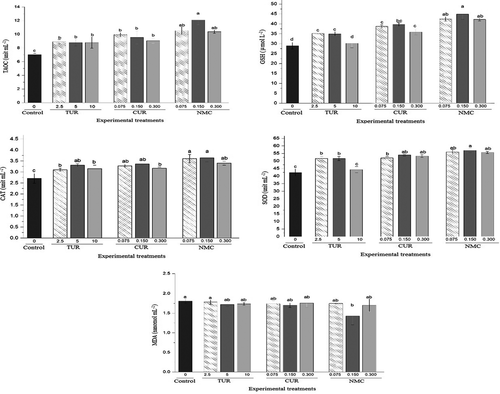
In Figure 3, the impacts of control and experimental treatments on the antioxidant parameters of juvenile P. vannamei have been presented. TUR, CUR and NMC additives increased the levels of TAOC in shrimps fed with different dosages of them compared with the control (p < .05). NMC treatments displayed higher levels of TAOC than TUR and CUR treatments. The lowest and highest levels of TAOC was detected in the control and NMC 0.150, respectively (p < .05). For GSH, an incremental trend was observed from the control to NMC treatments, and NMC 0.150 showed the highest level of GSH (p < .05). The same incremental trend was also detected for CAT and SOD, and the lowest and highest levels of these parameters were detected in the control and NMC 0.150, respectively. MDA was significantly lower in NMC 0.150 compared with the control but not significantly different between the control and other experimental treatments (p > .05).
3.4 Immunological findings
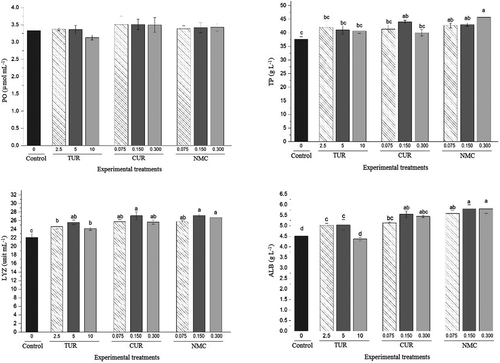
The effects of control and experimental treatments on immunological parameters of juvenile P. vannamei have been displayed in Figure 4. PO level was not different between the control and different experimental treatments (p > .05). Both TUR and CUR treatments increased the level of TP compared with the control, although the difference was not significant (p > .05). The significant increase of TP compared with the control was observed in NMC treatments. NMC 0.300 showed the highest level of TP compared with the other treatments. The level of LYZ was the lowest in control, however, the TUR, CUR and NMC additives increased the LYZ significantly compared with the control (p < .05). About the ALB, the lowest level was detected in the control and TUR 10 treatment. TUR, CUR and NMC additives increased the levels of ALB in shrimps fed with different dosages of them compared with the control (p < .05). CUR and NMC treatments, especially NMC 0.150 and NMC 0.300, displayed higher levels of ALB than TUR treatments.
4 DISCUSSION
This study compared the nutritional and immunological impacts of dietary TUR, CUR and NMC for the first time in a shrimp species. The consequences of supplementing diet with different dosages of TUR and its bioactive compound CUR on the Pacific white shrimp performance were investigated and compared with the effects of CUR incorporation into a nanocarrier. The findings revealed that the growth and immunological parameters of P. vannamei were influenced by the CUR form in the juvenile shrimp diet.
The improvement of growth and survival affects the productivity and profitability of aquaculture activities (Minabi et al., 2020). Results of this study indicated that TUR and its natural products in the forms of CUR and synthesized NMC did not negatively affect the shrimp growth and even, offered a better nutritional status. According to the present results, CUR 0.075 and NMC 0.150 treatments showed a relatively better growth performance than the other experimental treatments and improved the FW, WG, SGR and FCR values. The SR of shrimps was clearly influenced by the experimental diets and the NMC treatments improved the SR compared with the control and other experimental treatments. NMC 0.150 caused the highest survival rate and this led to the maximal level of BG for this treatment.
Yonar et al. (2019) reported that the WG and SGR increased whilst the FCR decreased in O. mykiss fed with different levels of CUR. The same growth improving impacts were previously reported by dietary supplementation of CUR in C. auratus (Jiang et al., 2016), grass carp Ctenopharyngodon idella (Ming et al., 2020), C. carpio (Abdel-Tawwab & Abbass, 2017; Giri et al., 2019), O. niloticus (Mahmoud et al., 2017) and Mozambique tilapia Oreochromis mossambicus (Sruthi et al., 2018). In P. vannamei, CUR supplementation at 0.2 g kg−1 diet enhanced the growth and feed intake when the diet was supplemented with aflatoxin (García-Pérez et al., 2020). In one of the other scarce CUR studies on P. vannamei, aflatoxin B1 decreased the growth performance in juvenile shrimps whilst 100 mg kg−1 Zn(II)-CUR ameliorated the toxic effects of aflatoxin on the growth (Yu et al., 2018). On the other hand, no significant enhancement in SR, growth parameters and feed utilization were detected in O. mykiss fed CUR-supplemented diets compared with the control (Kohshahi et al., 2019). The same finding was reported for this species fed with CUR at 200 mg kg−1 diet and the FCR did not differ compared with the control (Akdemir et al., 2017). Diet supplementation with TUR powder at 1, 2 and 5 g kg−1 caused no significant change in FCR of C. carpio too (Abdel-Tawwab & Abbass, 2017). Dietary CUR also induced no significant effect on the FW, WG and SGR of C. auratus compared with the control (Yang et al., 2020).
According to Abdel-Tawwab and Abbass (2017), the incorporation of TUR in C. carpio diet also increased diet digestion and nutrient digestibility, and improved feed utilization and fish growth. Based on the present results, the overall improved growth performance and the enhanced survival rate and BG in shrimps fed CUR and NMC diets may also be attributed to a better health status caused by improved antioxidant capacity and immune parameters in the shrimps, which is more described later in the discussion. Another criteria to evaluate the growth rate in fish and shellfish is to determine the ECR that indicates the economic aspect of the feed conversion ratio. When the ECR increases, it means that the production cost has increased (Öz et al., 2021). The values of ECR in experimental treatments except NMC 0.300 were lower than the control group despite not statistically significant in some treatments. However, the ECR value of NMC 0.300 was not statistically more than the control group. CUR 0.075 showed the best ECR but there was no statistical difference between CUR 0.075 and NMC 0.150, which had a better SR and BG. These data reflects that in P. vannamei culture, supplementing the diet with TUR, CUR and NMC has economic benefits.
According to the proximate composition analysis, CUR supplementation affected the shrimp body composition and the amount of crude protein and crude lipid was the highest in the shrimps fed CUR 0.075 and NMC 0.150 treatments (p < .05). Similar results were reported by Mahmoud et al. (2017) and the content of protein and lipid of the O. niloticus body was increased in fish fed CUR-supplemented diets. Hwang et al. (2013) declared that TUR powder incorporation in the diet of black rockfish Sebastes schlegeli decreased crude lipid and increased crude protein and ash contents of the body. There are also some studies indicating that body composition has not been affected in C. caprio (Abdel-Tawwab & Abbass, 2017) by TUR or in O. mykiss (Kohshahi et al., 2019) and C. auratus (Jiang et al., 2016) by CUR supplementation in the diet. The increase of body protein and lipid following the feeding with TUR or CUR could be attributed to the regulation of intestinal microbiota and the subsequently improved nutrient utilization (Mahmoud et al., 2017) or the modulatory effect of CUR on the activities of digestive enzymes (Jiang et al., 2016). However, the alterations in body protein and lipid content could be linked with the growth performance of the animal, as was observed in the present study (Abdel-Tawwab & Abbass, 2017).
Haematological parameters are generally applied to determine the physiological and immunological condition and evaluate the health status of animals. AST and ALT as sensitive indicators of invertebrate hepatopancreas play important roles in the body's metabolism. ACP is a major lysosomal enzyme playing a key role in immunity and is used as a marker of macrophage activation in animal models (Li et al., 2019). In the present study, serum biochemical parameters including ALP, ALT, AST and ACP were investigated under the influence of different dosages of dietary TUR, CUR and NMC. ALP was not different between the treatments, but the experimental additives reduced the ALT and AST and increased the ACP compared with the control. The lowest values of ALT and AST and the highest values of ACP were detected in NMC 0.150, which indicates the improved innate immune response of the shrimps in this treatment. From the viewpoint of animal nutritional biotechnology, CUR is more efficiently utilized using different nanocarriers and this may have led to the improved values of serum biochemical parameters with NMC treatments. In fact, more bioavailability of CUR is provided by NMC approach and thus, more effective components are absorbed by the body affecting the serum biochemical parameters. The shrimps fed the TUR, CUR and NMC treatments displayed a decreasing trend in the activities of ALT and AST compared with the control indicating the beneficial roles of CUR in exerting a protective effect against the hepatopancreas damage and improving the hepatopancreas health status. Toxic and stressful conditions or damages to the liver or hepatopancreas made by cellular destruction enhance the activities of AST, ALP and ALT (Afshari et al., 2021; Kaya et al., 2015). However, the present values of these enzymes reveal no stressful or toxic condition following the applied supplementation procedure.
In C. carpio, TUR administration at 10 g kg−1 diet diminished the blood ALT and AST compared with the dietary copper exposed fish which was attributed to the hepatoprotective effects of TUR (Rajabiesterabadi et al., 2020). The same finding and conclusion were reported by Ajani et al. (2020) in O. niloticus and the serum AST decreased when the fish fed diets containing dietary TUR at 0.25%, 0.5%, 0.75% and 1.0% diet. In P. vannamei, CUR supplementation to the diet at 0.15, 0.2 and 0.3 g kg−1 decreased ALP activity when the diet was supplemented with aflatoxin (García-Pérez et al., 2020). In another study on P. vannamei, a diet supplemented with aflatoxin B1 + 100 or 200 mg kg−1 Zn(II)-CUR decreased the ALT level compared with the diet only supplemented with the aflatoxin B1. However, the ALT level in these CUR-treated groups was not different compared with the control. AST was not significantly different amongst all the treatments (Yu et al., 2018). The hepatoprotective effects of CUR have been attributed to the enhancement of hepatocyte antioxidative ability and the restriction of the NF-kB signalling pathway and associated cytokines, involving TNF- α, IL-1β and IL-12 (Abd El-Hakim et al., 2020; Cao et al., 2015; Giri et al., 2019).
The antioxidant system is greatly related to health and immunity, and several studies have described a positive relation between the antioxidant activity and immune response of fish and shellfish. SOD and CAT, as main endogenous enzymes of this system, preserve the cells from oxidative stress and catalyse the reactive oxygen species into less reactive forms (Ibrahim et al., 2019; Kazemi et al., 2020). SOD enzyme stimulates the oxidation and reduces the superoxide anion to hydrogen peroxide, and then, other antioxidant enzymes such as CAT uses it as a substrate for decomposing hydroperoxide radicals. Therefore, oxidative damage can happen when the antioxidants are deficient (Afshari et al., 2021; Yonar et al., 2019). MDA is the product of lipid peroxidation and indicates the oxidative damages to the lipids. The findings of this study indicate that the supplementation of TUR, CUR and NMC to the P. vannamei diet affected the antioxidant parameters. The experimental additives inserted an incremental trend from the control to NMC treatments and enhanced TAOC, GSH, CAT and SOD, reaching the highest in NMC 0.150. A reverse trend was observed for MDA, reaching the lowest in NMC 0.150. These results reveal the improved antioxidant defence system of the P. vannamei after dietary incorporation of TUR, CUR and NMC.
In C. carpio, TUR administration at 10 g kg−1 diet ameliorated the harmful effect of copper and enhanced the antioxidant and immunological parameters including SOD, CAT, LYZ and GSH-Px (Rajabiesterabadi et al., 2020). Giri et al. (2019) reported the enhanced levels of serum SOD, CAT and LYZ and the decreased level of MDA following CUR supplementation in the fish diet. The MDA decrease indicated the fortified antioxidant defence system. Moreover, 0.5% and/or 1.0% dietary CUR elevated SOD, TAOC and GSH but inhibited MDA formation in the livers of CCl4-treated carp (Cao et al., 2015). In O. mykiss, the dietary CUR prompted the activities of SOD, CAT and GSH in the spleen, head kidney and liver compared with the control, but a reverse trend was reported in MDA (Yonar et al., 2019). In O. niloticus, the dietary CUR elevated both non-enzymatic (GSH) and enzymatic (CAT) antioxidants with a decrease in MDA level, chiefly at a concentration of 50 mg kg−1 diet (Mahmoud et al., 2017). In C. auratus, the dietary CUR enhanced the contents of antioxidant parameters including CAT, SOD and GSH in the intestine. Moreover, CUR dietary incorporation enhanced the relative mRNA expression of antioxidant enzymes in this species (Jiang et al., 2016). In P. vannamei, a diet supplemented with aflatoxin B1 + 100 or 200 mg kg−1 Zn(II)-CUR decreased the GSH and MDA level compared with the diet only supplemented with the aflatoxin B1. However, the GSH and MDA levels in these CUR-treated groups were not different compared with the control. Moreover, SOD and CAT were not significantly different amongst all the treatments (Yu et al., 2018). CUR exerts its protective impacts by scavenging free radicals and stimulating antioxidant parameters (Manju et al., 2012; Xu et al., 2018). The enhancement in antioxidant parameters observed in the present study may be described with probable augmentation of antioxidant capacity. CUR, rather than its direct antioxidant activity, may also act indirectly as an antioxidant by increasing the synthesis of glutathione, which is GSH-Px substrate (Biswas et al., 2005; Mahmoud et al., 2017). Another mechanism for antioxidant properties of CUR is the transcription induction of antioxidant enzyme by activating the nuclear factor erythroid 2 (Nrf2) signalling pathway, which is involved in the free radical scavenging (Kwak et al., 2004). To date, there has been no research conducted on NMC in shellfish nutrition assessing the haemato-immunological and antioxidant parameters. In infertile men, the daily administration of 80 mg NMC for 10 weeks resulted in the increase of plasma TAOC and the decrease of plasma MDA. This was assigned to the role of CUR in diminishing the probability of free radical production due to the presence of sulphydryl groups in CUR structure or improving the activities of antioxidant enzymes (Alizadeh et al., 2018).
Phenoloxidase, TP and LYZ are amongst the main components of the shrimp's innate immune system (Ayiku et al., 2020; Chen et al., 2018). PO induces antimicrobial substances into the serum to increase the phagocytosis in the shrimp haemocytes. Serum TP, which is made up of ALB and globulin, is often measured as an indicator associated with the health and immune status in aquatic animals and its increase displays a more robust innate immune status. LYZ is considered as a first-line barrier of the defence system that diminishes the disease by the inhibition of bacterial infections through hydrolyzing bacterial cell wall peptidoglycans. Immunological parameters of the P. vannamei were influenced by the addition of TUR, CUR and NMC to the diet. PO was not different between the treatments, but the experimental additives increased TP, ALB and LYZ compared with the control. NMC treatments showed a better immunological performance than the other treatments.
In shrimps, a diet supplemented with aflatoxin B1 + 100 mg kg−1 Zn(II)-CUR (AFB1/100 Zn-CUR) increased the PO activity in P. vannamei juveniles compared with the diet only supplemented with aflatoxin B1. However, the PO activity in the shrimps fed AFB1/100 Zn-CUR was not significantly different from the control shrimps (Yu et al., 2018).
Yonar et al. (2019) announced that dietary CUR noticeably increased the TP level in O. mykiss by inducing humoral immunity. The LYZ was also elevated in this fish, fed diets containing CUR because of the stimulation of the immune system. Mahmoud et al. (2017) used four concentrations of CUR including 50, 100, 150 and 200 mg kg−1 in the diet of O. niloticus and reported that the LYZ activity significantly enhanced in the fish fed 50 and 100 mg kg−1 diet but was not different in the other treatments compared with the control. In rohu Labeo rohita, the TP level and LYZ activity in the serum of the TUR-fed fish were decreased compared with the control (Sahu et al., 2008). The rise of serum LYZ activity following the use of immunostimulants is either as a result of an increment in the number of phagocytes secreting LYZ or an enhancement in the extent of LYZ synthesized per cell (Edahiro et al., 1990). Although the studies on the enhancement of curcumin bioavailability through nanotechnology approaches and nanocarrier systems such as nanomicelles have not been undertaken in fish and shellfish yet, but based on the studies in other animals, micelles can promote the gastrointestinal absorption, thus giving higher plasma levels and lower kinetic elimination that result in the improvement of natural drugs bioavailability. Micelles as aggregates of surfactant molecules with hydrophilic and hydrophobic tails can dissolve CUR (Kumar et al., 2016). In fact, loading the CUR in nanomicelles induces its dissolution and dispersion in water and gives CUR the ability to be more absorbed by the body (Rahimi et al., 2016). This may be the main reason for the improved haemato-immunological responses and antioxidant status of the shrimps in NMC treatments especially in NMC 0.150, which based on the economic evaluation, was also recommendable. Nanomicelles as novel delivery strategies offer significant promise and are worthy of further exploration in fish and shellfish nutrition and health.
5 CONCLUSION
The findings of this study confirmed that the dietary administration of TUR, CUR and NMC positively affected the growth and immunity of the Pacific white shrimp. Amongst the experimental treatments, the least CUR concentration of 0.075 and NMC concentration of 0.150 induced a better growth performance. NMC treatments improved the survival more than other treatments with the highest value in NMC 0.150, which led to the maximal level of BG for this treatment. TUR, CUR and NMC reduced the ALT and AST compared with the control reaching the lowest level in NMC 0.150. The highest levels of ACP, TAOC, GSH, CAT and SOD and the lowest level of MDA were detected in NMC 0.150. The highest value of TP was observed in NMC 0.300. LYZ and ALB reached the highest values in NMC 0.150 and NMC 0.300. Therefore, CUR alone or encapsulated in nanocarriers such as nanomicelles can be safely used in shrimp feeds, representing a promising dietary supplement to improve the quantity and quality of the production. The economic evaluation also approved this finding. However, further studies on the mode of action and the definite performance mechanisms should be conducted to comprehensively realize the effects of different forms of dietary CUR in shrimp culture.
ACKNOWLEDGEMENTS
We wish to express our thanks to the manager and experts of Kolahi breeding center, Hormozgan province, for providing the facilities and to the Exir Nano Sina company, Tehran, Iran for donating the curcumin nanomicelles (SinaCurcumin®).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data will be available on request from the authors.




