The alleviative effects of taurine supplementation on growth, antioxidant enzyme activities, hepatopancreas morphology and mRNA expression of heat shock proteins in freshwater prawn Macrobrachium nipponense (De Haan) exposed to dietary lead stress
Funding information
This research was supported by Zhejiang Province Public Welfare Technology Application Research Project (CN; No. LGN21C190003, LGN21C190004), the Natural Science Foundation of Huzhou (No. 2019YZ04), and the Major Scientific, Technological Special Project of Zhejiang, China (No. 2014C02011).
Abstract
To investigate the potential alleviative effect of taurine supplementation on growth and oxidant stress in freshwater prawn Macrobrachium nipponense (De Haan) exposed to dietary lead, pelleted feeds were prepared with two concentrations of lead (0 and 10 mg/kg diet) and two levels of taurine (0 and 15 g/kg diet). Healthy prawns were randomly distributed between the four treatment groups, with three tanks per group and 50 prawns per tank. Dietary lead decreased weight gain and the activities of superoxide dismutase, catalase and glutathione peroxidase; increased malondialdehyde content; altered hepatopancreas morphology; and decreased the expression of heat shock protein 90 (HSP90; p < .05). A significant interactive effect was found between dietary lead and taurine on the malondialdehyde level, and taurine supplementation significantly decreased the malondialdehyde level under lead stress (p < .05). No significant effect on growth, hepatopancreas morphology and HSP90 expression was observed with taurine supplementation under lead stress. Overall, dietary lead affected growth, induced oxidative stress and impaired hepatopancreas morphology of the prawns, and dietary taurine significantly alleviated oxidative stress through a decreased malondialdehyde level, whereas the capacity of taurine to alleviate the decreased growth rate, hepatopancreas damage and low HSP90 expression under lead stress was limited.
1 INTRODUCTION
With rapid development and modern industrialization, large quantities of various metals are introduced into the environment, including aquatic ecosystems (Lapanje et al., 2010). Metal ions are not biodegradable, and they can infiltrate into the food chain resulting in biomagnification of these toxic substances (Hu et al., 2014). Lead is one of the most dangerous metals to humans and other life forms owing to its persistent accumulation and high toxicity (Rubio & Hardisson, 1999). In mammals, lead causes adverse effects on the central and peripheral nervous systems, hematopoietic system, cardiovascular system, kidneys, reproductive organs and the antioxidant defence system (Pal et al., 2015).
In aquatic ecosystems, exposure to lead in the water or dietary lead can disrupt the physiology and biochemistry of aquatic organisms. Studies of the physiological responses to lead exposure in such organisms have reported lowered antioxidant capacity in the sea cucumber Apostichopus japonicus (Wang et al., 2015), altered haematological parameters in juvenile rockfish Sebastes schlegelii (Kim & Kang, 2015) and the generation of reactive oxygen species (ROS) and induced oxidative stress in fish (Sevcikova et al., 2011). Moreover, besides antioxidant defence system scavenging ROS, chaperone molecules are also involved in this process. Heat shock proteins (HSPs), as molecular chaperones, play a crucial role in maintaining protein homeostasis under sublethal stress conditions (Hofmann et al., 2002) and can be sensitive molecular biomarkers for aquatic monitoring of heavy metal pollution (Kim et al., 2014).
Interestingly, many studies have proved that proper nutritional supplementation can alleviate multiple stressors imposed by the environment, including heavy metals pollution (Hashish and Elgaml, 2015; Mezzomo et al., 2019; Ramos et al., 2018; Yu et al., 2016;). Taurine, as found in most animal tissues, is a sulphur-containing β-amino acid (Ripps & Shen, 2012). Taurine plays a role in regulating various physiological functions, including bile acid conjugation, cellular osmoregulation, anti-inflammation, anti-apoptosis, neuromodulation and immunity, as well as in antioxidative defence (Mezzomo et al., 2020; Salze & Davis, 2015). Research with higher animals demonstrated that the administration of taurine could reduce inflammation and oxidative stress in the lungs of adult mice, as induced by short-term exposure to cigarette smoke (Ramos et al., 2018); that dietary taurine had a protective effect in piglets with diquat-induced oxidative stress (Wen et al., 2019); and that taurine supplementation alleviated the negative effects of heat stress in laying quails (Orhan et al., 2020). In aquatic animals, exogenous taurine could mitigate the adverse effect of ammonia poisoning as manifested in physiological disorders in juveniles of yellow catfish Tachysurus fulvidraco (Zhang et al., 2018), and pretreatment with taurine could prevent stress-induced behavioural and biochemical changes in zebrafish (Mezzomo et al., 2019).
The freshwater prawn Macrobrachium nipponense (De Haan, 1849) is an economically and nutritionally important aquaculture species widely distributed in China, Japan and Southeast Asian countries (Yang et al., 2004). Our previous study showed that chronic exposure to lead can impair growth, bioaccumulate in muscle, damage intestinal morphology and induce oxidant stress or neurotoxicity-related effects in this species (Ding et al., 2019). Based on the alleviating effects of taurine to various stressors, we hypothesized that dietary taurine can protect against lead exposure in M. nipponense. Therefore, this study aimed to evaluate the alleviative effects of taurine supplementation on growth, antioxidant enzyme activities, hepatopancreas morphology and mRNA expression of HSPs in M. nipponense exposed to dietary lead stress.
2 MATERIALS AND METHODS
2.1 Diet preparation
Two concentrations (0 and 10 mg/kg diet) of lead (Pb) in the form of lead acetate (CAS 6080–56–4, 99.5% purity) and two taurine levels (0 and 15 g/kg diet) were added to the experimental basal diet. Four experimental diets were formulated (Pb0Tau0, Pb0Tau15, Pb10Tau0 and Pb10Tau15), and the proximate analysis of the diets is listed in Table 1. The hygienic standard for feeds in China (GB 13078–2017, 2017) stipulates that the lead limit in artificial feeds is 5 mg/kg, and therefore, we selected a level double this concentration as representative of lead stress in freshwater prawns. Our choice of taurine inclusion level referred to research on taurine supplementation in juveniles of Chinese mitten crab Eriocheir sinensis (Chen et al., 2018).
| Ingredients | Pb0 | Pb0Tau15 | Pb10 | Pb10Tau15 |
|---|---|---|---|---|
| Casein (82.57% crude protein) | 380 | 380 | 380 | 380 |
| Fish meal (70% crude protein) | 100 | 100 | 100 | 100 |
| Corn starch | 250 | 250 | 250 | 250 |
| Fish oil | 40 | 40 | 40 | 40 |
| Soybean oil | 20 | 20 | 20 | 20 |
| Lead acetate (mg/kg diet) | 0 | 0 | 18.3 | 18.3 |
| Taurine | 0 | 15 | 0 | 15 |
| Vitamin mixa | 20 | 20 | 20 | 20 |
| Mineral mixb | 30 | 30 | 30 | 30 |
| Lecithin | 5 | 5 | 5 | 5 |
| Cholesterol | 5 | 5 | 5 | 5 |
| Choline chloride | 5 | 5 | 5 | 5 |
| Cellulose | 125 | 110 | 125 | 110 |
| Sodium carboxymethylcellulose | 20 | 20 | 20 | 20 |
| Proximate composition (g/kg) | ||||
| Crude protein | 381.1 | 387.2 | 380.7 | 388.3 |
| Crude lipid | 80.5 | 81.2 | 79.3 | 82.2 |
- a Vitamin mixture (100 g−1 mixture): vitamin A, 420000 IU; vitamin C, 6000 mg; α-tocopherol acetate, 2000 mg; vitamin D3, 120000 IU; vitamin K, 1000 mg; vitamin B1, 1000 mg; vitamin B2, 1000 mg; vitamin B6, 1600 mg; vitamin B12, 2 mg; niacin, 5000 mg; folic acid, 400 mg; inositol, 6000 mg; biotin, 10 mg; calcium pantothenic, 3500 mg.
- b Mineral mixture (mg g−1 mixture): KCL, 28; MgSO4·7H2O, 100; NaH2PO4, 215; KH2PO4 100; Ca(H2PO4)2·H2O, 265; CaCO3, 105; C6H10CaO6·5H2O, 165; FeC6H5O7·5H2O, 12; ZnSO4·7H2O, 4.76; MnSO4·H2O, 1.07; AlCL3·6H2O, 0.15; CuCl2·2H2O, 0.24; CoCl2·6H2O, 1.4; KI,0.23; α-cellulose, 2.15.
All dry ingredients were accurately weighed and fully blended, and then thoroughly mixed to homogeneity with distilled water. The wet mixture was extruded into pellets (1.5-mm diameter) using a pelleting machine. The pellets were dried in a forced-air oven at 40°C to approximately 10% moisture and then stored at −20°C before being used in the feeding trial.
2.2 Experimental animals and feeding trials
Juvenile prawns were supplied by a local farmer. Prior to the feeding trial, the prawns were fed a commercial diet (38% protein) for 1 week to acclimate to the culture conditions. Thereafter, healthy prawns with an average initial weight of 0.104 g were randomly stocked in 12 tanks (300 L), with 50 prawns per tank (three replicates per dietary group). Prawns were fed twice a day (07:30 and 17:00 h) at 8% of body weight for 8 weeks. During the feeding trial, the water conditions were maintained at 25–28°C, dissolved oxygen >6.5 mg L−1 and ammonia and nitrate <0.1 mg L−1, under a natural photoperiod. Water was continuously aerated using air stones, with a daily water exchange rate of 1/3 of the tank volume. Some nylon fishing nets were placed in each tank as an artificial shelter to minimize disturbance to the animals.
2.3 Sample collection
Feeding was stopped 24 h prior to termination of the experiment. The hepatopancreas of six prawns per dietary group was dissected and fixed separately in Bouin's solution for 24 h before histological analysis, and the other hepatopancreas samples were stored at −80°C for subsequent analyses.
2.4 Growth parameters and biochemical analysis
Growth performance was measured using the following parameters: weight gain (%) = (final body weight−initial body weight)/(initial body weight) × 100; specific growth rate (%) = (ln final weight−ln initial weight)/(days of the experiment) × 100; and survival rate (%) = (final prawn number)/(initial prawn number) × 100.
The crude protein, crude lipid and moisture in the prepared diets were determined conducted using standard procedures (AOAC, 2005).
2.5 Lipid-peroxidation levels and antioxidant enzyme activities
The hepatopancreas of 10 prawns (intermolt) per tank were obtained for assaying enzyme activities and then combined as one sample. The hepatopancreas samples were homogenized with ice-cold 0.86% (w/v) NaCl solution to get a crude extract. Lipid-peroxidation levels (malondialdehyde, MDA) and antioxidant enzymes were determined using commercially available kits (Nanjing Jiancheng Bioengineering Institute). MDA levels were determined following the thiobarbituric acid method (Buege & Aust, 1978). Superoxide dismutase (SOD) activity was determined based on the method of Beauchamp and Fridovich (1971). Catalase (CAT) activity was determined by measuring the decrease in H2O2 concentration (Aebi, 1984). Glutathione peroxidase (GPx) activity was evaluated from the decrease in absorption at 340 nm due to the oxidation of NADPH to NADP+ (Balic et al., 2012).
2.6 Histological assay
The fixed hepatopancreas were dehydrated in an ascending alcohol concentration. Dehydrated tissues were embedded in paraffin and cut as continuous section blocks into 5-μm sections. The sections were stained with haematoxylin and eosin to reveal changes in the hepatopancreas and then examined with CaseViewer software (3DHISTECH Ltd).
2.7 qRT-PCR analysis of heat shock proteins
Total RNA was extracted from the hepatopancreas of five prawns (intermolt) per tank, using a commercial assay kit (Aidlab Biotechnologies Co) in accordance with the manufacturer's protocols. cDNA for qRT-PCR was synthesized via reverse transcription using a PrimeScript™ RT-PCR Kit (Takara). Reaction conditions were those recommended by the manufacturer.
Gene-specific primers were designed using online Primer3 software based on the cDNA sequences in GenBank. The upstream primer of HSC70 (DQ660140.1) is GCGTCTTATTGGTGATGC, and the downstream primer is TAAAGGGCCAATGTTTCA. The upstream primer of HSP90 (GU319963.1) is GAAGGAAAGGGACAAGGA, and the downstream primer is GGTCCATAAAGGCTTGGT. The upstream primer of β-actin (FL589653.1) is GTGCCCATCTACGAGGGTTA, and the downstream primer is CGTCAGGGAGCTCGTAAGAC. qRT-PCR analysis was performed using a SYBR® Premix Ex Taq™ Kit (Takara) in the CFX96™ Real-Time PCR Detection System (Bio-Rad). Amplification was performed in a total volume of 20 μl containing 10 μl 2 × SYBR Green Premix Ex Taq, 0.2 μl of each primer (10 μM), 2 μl of cDNA and 7.6 μl of PCR-grade water. The cycling conditions were as follows: 95°C for 30 s followed by 40 cycles at 94°C for 15 s, 58°C for 20 s and 72°C for 20 s, with an 0.5°C/5 s incremental increase from 60 to 95°C. During the detection, each sample was run in triplicate. The amplification efficiencies of all genes were approximately equal. The relative mRNA expression of genes was calculated with the 2−ΔΔCt comparative CT method (Livak & Schmittgen, 2001).
2.8 Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows ver. 17.0 (SPSS Inc.). Data are mean ± standard deviation (SD). Data were analysed by two-way analysis of variance (ANOVA) to determine whether there was any interaction between dietary lead level and taurine level. At the same lead level, an independent-samples t-test was used to determine significant differences between 0 and 15 g/kg taurine concentration. At the same taurine level, an independent-samples t-test was used to determine significant differences between 0 and 10 mg/kg dietary lead concentration. A p-value of <.05 was considered statistically significant, and a value of <0.01 was considered extremely significant.
3 RESULTS
3.1 Growth performance
As shown in TABLE 2, there was a significant main effect of dietary lead on weight gain or specific growth rate (p < .05) in M. nipponense. Prawns fed the diet with 10 mg/kg lead displayed significantly less weight gain and a lower specific growth rate compared with prawns fed 0 mg/kg lead regardless of the dietary taurine level (p < .05). The addition of dietary taurine did not improve the growth performance of prawns. The survival rate was not significantly affected by the dietary levels of lead or taurine (p > .05). There were no significant interactive effects between dietary lead and dietary taurine on weight gain, specific growth rate and survival rate (p > .05).
| Groups | Final body weight/g | Weight gain/% | Specific growth rate (%/d) | Survival rate/% |
|---|---|---|---|---|
| Pb0 | 0.47 ± 0.03B | 355.54 ± 33.62B | 2.39 ± 0.13B | 59.67 ± 4.16 |
| Pb0Tau15 | 0.49 ± 0.05B | 371.93 ± 52.36B | 2.45 ± 0.20B | 61.33 ± 3.51 |
| Pb10 | 0.33 ± 0.05A | 224.44 ± 53.39A | 1.77 ± 0.31A | 57.67 ± 2.51 |
| Pb10Tau15 | 0.37 ± 0.04A | 256.27 ± 43.09A | 1.95 ± 0.22A | 59.00 ± 4.35 |
| Lead effect | 0.002 | 0.002 | 0.003 | 0.341 |
| Taurine effect | 0.373 | 0.394 | 0.394 | 0.503 |
| Lead * Taurine | 0.815 | 0.780 | 0.667 | 0.940 |
Note
- Capital letters denote significant differences between 0 and 10 mg/kg dietary lead concentration at the same taurine supplementation level.
3.2 Malondialdehyde content and activities of antioxidant enzymes
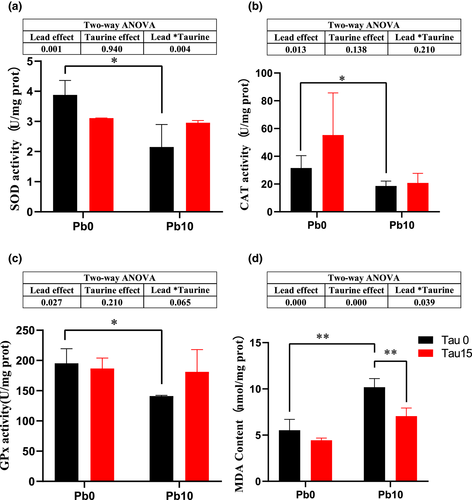
Figure 1 shows the effects of dietary lead and taurine on MDA content and the antioxidant enzyme activities of the prawns. There were significant main effects of dietary lead on SOD, CAT, GPx and MDA indexes (p < .05), but no significant main effect of dietary taurine on the activities of SOD, CAT and GPx. Dietary lead significantly decreased SOD, CAT and GPx activities and increased MDA content in hepatopancreas when compared with prawns given feed with no lead (p < .05). There was a significant main effect of dietary taurine on MDA (p < .05), and lead-contaminated diets with taurine supplementation significantly decreased MDA content in hepatopancreas (p < .01). Significant interactive effects were obtained between dietary lead and dietary taurine on SOD and MDA (p < .05).
3.3 Hepatopancreas morphology
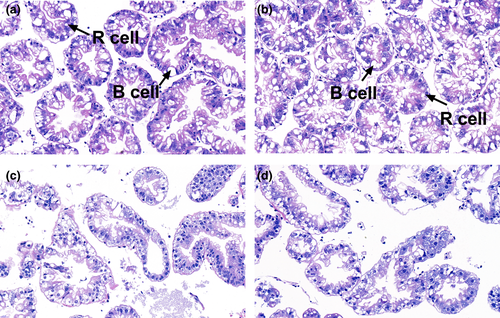
As shown in Figure 2, histology of the hepatopancreas in prawns of the Pb0Tau0 and Pb0Tau15 groups was normal, with tightly connected cells of the pseudostratified epithelium arranged along the hepatopancreatic tubule. The hepatopancreas of prawns fed Pb0Tau15 had more blister cells (B cells) than those fed Pb0Tau0. No difference was observed in the numbers of resorptive cells (R cells) between groups Pb0Tau0 and Pb0Tau15. Histopathological alterations in hepatopancreas were observed in group Pb10Tau0, which exhibited enlarged or disintegrated lumen. The taurine addition to the lead-contaminated diet (Pb10Tau15) resulted in no obvious improvement to the histological morphology of the hepatopancreas. There was no clear change in the proportions of R cells and B cells between groups Pb10Tau0 and Pb10Tau15.
3.4 mRNA expression of HSC70 and HSP90
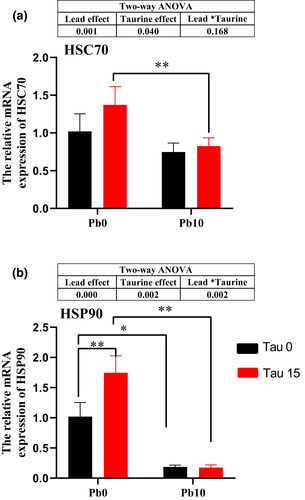
The mRNA expressions of HSC70 and HSP90 in the hepatopancreas are shown in Figure 3. There was a significant main effect of dietary lead or dietary taurine on HSC70 mRNA expression, but no significant interactive effect was obtained between dietary lead and taurine supplementation. No difference in HSC70 mRNA expression was observed at the same level of lead (p > .05). Dietary lead significantly decreased the expression of HSC70 in prawns given 15 g/kg dietary taurine but not in prawns given no taurine supplementation. There was a significant main effect of dietary lead or dietary taurine on HSP90 mRNA expression, and a significant interactive effect was obtained between dietary lead and dietary taurine (p < .05). Dietary taurine significantly increased HSP90 mRNA expression under no dietary lead contamination (p < .01). Dietary lead significantly decreased HSP90 mRNA expression regardless of the dietary taurine level (p < .05). There was no significant difference in HSP90 mRNA expression in prawns fed a lead-contaminated diet with or without taurine addition (p > .05).
4 DISCUSSION
The feeding experiment investigated whether dietary taurine could be effective in countering the effects of lead toxicity in M. nipponense. The hygienic standard for feeds in China stipulates that the lead content of a formulated feed should not exceed 5 mg/kg. In the present study, 10 mg/kg lead added to the diet decreased growth of the prawns. This result agrees with our previous study, which found that chronic lead exposure (26.26 μg/L) could adversely affect the growth of M. nipponense (Ding et al., 2019). Similarly, the growth performance of juvenile rockfish Sebastes schlegelii was significantly decreased by dietary lead exposure (Kim & Kang, 2015). The decreased growth might be attributable to poorer food assimilation caused by stress (Hansen et al., 2002) and by reallocation of energy from growth and development to detoxification (Kim & Kang, 2015). The survival rate of the prawns was not affected by dietary lead, which is consistent with the results of lead exposure in the rearing water (Ding et al., 2019). Thus, the survival rate of M. nipponense may not be a sufficiently sensitive indicator of chronic lead exposure through either the water or diet. Previous studies have suggested that an appropriate addition of taurine can improve growth performance in some animals (Han et al., 2020; Lin and Lu, 2020). However, our data did not suggest a growth-promoting effect of taurine under dietary lead stress, which might be related to the amount of taurine supplementation and/or to the different metabolic systems of the experimental animals. Further study is needed to determine the optimal concentration of taurine supplementation in a diet for M. nipponense.
It has been shown that lead exposure can produce ROS and then trigger oxidative stress (El-Fara et al., 2017; Sudjaroen & Suwannahong, 2017). The antioxidant response can be a reliable and sensitive indicator for evaluating oxidative stress following exposure to metals (Lee et al., 2019). SOD catalyses dismutation of superoxide radicals to hydrogen peroxide, which can, in turn, be removed by CAT and GPx (Livingstone, 2003). In the present study, there were main effects of dietary lead on antioxidant enzyme activities, as the activities of SOD, CAT and GPx were inhibited in the prawns given lead-contaminated feed, which might indicate its reduced capacity to scavenge H2O2 and lipid hydroperoxides produced in organ tissues because of oxidative stress (Ballesteros et al., 2009). Although no significant increase was observed in the antioxidant enzyme activities of prawns given taurine supplementation under lead stress compared with prawns not given the supplementation, a significant decrease in lipid peroxidation in terms of MDA was observed in the hepatopancreas. This indicates that both the level of dietary lead and dietary taurine can affect lipid peroxidation in this freshwater prawn. The more dramatic change in the MDA level than in the enzyme activities will be because oxidative stress originating from heavy metals directly affects the MDA level (Ates et al., 2008). Antioxidant activity is one of the most important biological functions of taurine (Cassol et al., 2010; Das et al., 2012). Previous research suggested that the interaction of taurine with the plasma membrane results in a modulation of the membrane lipid ratio, thereby leading to a decrease in membrane fluidity and increased resistance to oxidative damage (Hamaguchi et al., 1991). The significant interaction of dietary taurine with dietary lead indicates that, to some extent, taurine has a protective role in terms of peroxidative damage caused by dietary lead. Although literature is lacking on the direct effects of taurine on lead stress, the known modulatory effects of taurine to various stresses indicate its capacity to alleviate oxidative damage (Mezzomo et al., 2019; Orhan et al., 2020; Ramos et al., 2018; Wen et al., 2019; Zhang et al., 2018).
The hepatopancreas, as an important organ for the digestion, absorption, storage and metabolism of nutrients, plays a very important role in the normal physiology of crustaceans (Vogt, 2019). Additionally, the organ is important in immune defences and the detoxification of pollutants (Müller et al., 2020; Roszer, 2014). Consequently, when the histological structure of the hepatopancreas is abnormal, the normal physiological functioning of crustaceans is impacted. In the present study, dietary lead induced structural damage of the hepatopancreas, which aligns with the results reported for silver sailfin molly Poecilia latipinna (Mobarak & Sharaf, 2011) and three terrestrial isopod crustacean species (Mazzei et al., 2014) exposed to lead. Similarly, hepatopancreas structural change was also observed after exposure to other heavy metals (Boudet et al., 2015; Mu et al., 2017). Histopathologies are related to environmental quality, demonstrating the high sensitivity of the hepatopancreas to the change in environmental quality (Boudet et al., 2015). R cells and B cells are important epithelial cells in the hepatopancreas (Liu et al., 2019); B cells have a secretory function, and R cells are involved in nutrient storage and detoxification processes (Chen et al., 2017; Sousa et al., 2005; Yeganeh et al., 2020). The relatively high proportion of B cells in the hepatopancreas of prawns fed taurine without lead stress may indicate that energy and nutrient utilization increased. However, the mitigation of damage to hepatopancreas morphology by dietary taurine under lead stress appeared to be limited in this study. The present results suggest that the hepatopancreas cell damage caused by dietary lead is unlikely to be achieved through taurine addition alone.
After an organism is subjected to stress, HSPs can maintain the homeostasis of the organism and resist sequential stresses by maintaining protein conformation, anti-apoptosis, cell protection and other functions (Basu et al., 2002). As a constituent heat stress protein, HSC70 can degrade and remove damaged and denatured proteins and play a role in protecting and repairing cells (Boorstein et al., 1994; Kiang & Tsokos, 1998). Data on HSC70 in relation to metal stress are limited, but some studies have shown that HSC70 can be induced (Oksala et al., 2014; Tawk et al., 2000). However, in our study, HSC70 transcript was not induced by a higher lead level in the diet. This result concurs with a study showing lack of change in HSC70 expression with heat shock and heavy metal exposure in rainbow trout hepatocytes (Boone and Vijayan, 2002). Interestingly, in our study, dietary lead significantly decreased the expression of HSC70 at 15 g/kg taurine concentration but not at 0 g/kg taurine concentration, and no interactive effects were observed on HSC70 expression between dietary lead and taurine. This may suggest HSC70 is not a sensitive index of dietary lead contamination in this prawn. HSP90 is highly expressed in the cytoplasm of eukaryotic cells; it not only plays the function of molecular chaperone but also an important role in intracellular transport, protein degradation, cell signal transduction and other processes (Xie et al., 2015). Choi et al. (2008) reported that cadmium (Cd) treatment increased HSP90 gene expression in the digestive gland and gill tissue of Pacific oyster Crassostrea gigas. However, Ivanina et al. (2008) reported that Cd exposure did not affect the expression of HSP90. Compared with other heavy metals, a relatively low modulation of the HSP90 gene was observed in the experiment with dietary lead regardless of the dietary taurine level in the M. nipponense. Organisms cannot achieve appropriate levels of metabolic function under strong oxidative stress (Zhang et al., 2004), so the decreased expression of HSP90 might be due to a decrease in the metabolic capacity of the organism caused by the strong toxicity of dietary lead. This damage can be confirmed from the abnormal structure of the hepatopancreas in M. nipponense. In a study with poultry, HSP90 gene expression was increased in the liver by acute heat stress but limited after chronic heat stress (Wang et al., 2013), so it seems stress duration may affect the HSP90 expression. The present experiment lasted for 2 months; therefore, different results might be observed whether the stress duration was changed. Although dietary taurine improved HSP90 expression in this prawn under no lead stress, its modulation of HSP90 expression under lead stress is indeterminate. Similarly, taurine supplementation in broilers (poultry) under heat stress resulted in lower expressions of HSP60 and HSP70 but not HSP90 (Belal et al., 2018).
5 CONCLUSIONS
Overall, the present study demonstrated that the main effects of dietary lead (10 mg/kg of feed) in M. nipponense included decreased growth, oxidative stress and histopathological changes in the hepatopancreas. Dietary taurine had an interactive effect with lead on the MDA level in the hepatopancreas, which alleviated the oxidative stress caused by lead consumption. The potential of dietary taurine to alleviate impaired growth, hepatopancreas damage and low HSP90 expression under dietary lead stress is limited.
ACKNOWLEDGEMENTS
This research was supported by Zhejiang Province Public Welfare Technology Application Research Project (No. LGN21C190003, LGN21C190004), the Natural Science Foundation of Huzhou (No. 2019YZ04), and the Major Scientific, Technological Special Project of Zhejiang, China (No. 2014C02011).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




