Impacts of dietary zinc on growth performance, haematological indicators, transaminase activity and tissue trace mineral contents of soft-shelled turtle (Pelodiscus sinensis)
Abstract
A 12-week growth trial was conducted to investigate the impacts of dietary zinc on growth performance, whole-body composition, haematological parameters, liver aspartate aminotransferase and alanine transaminase activity and tissue trace mineral contents of soft-shelled turtle (Pelodiscus sinensis). Approximately 4 g of turtles were fed fishmeal-based diets with 35.43, 46.23, 55.38, 66.74, 75.06 and 85.24 mg Zn/kg. Respiratory burst, red blood cells and intestine somatic index enhanced with escalating dietary Zn concentrations up to 55.38 mg/kg and then kept constant beyond this level. Escalating dietary Zn inclusion up to 66.74 mg/kg improved weight gain, survival rate, feed intake, albumin, haemoglobin, liver aspartate aminotransferase and alanine transaminase activity, carapace ratio and crude protein of turtles, beyond which they decreased. Osmolality, hepatosomatic index and crude lipid of turtles firstly declined with elevating dietary zinc levels from 35.43 to 55.38 mg/kg diet and then increased at a higher zinc level. Liver and muscle Zn concentrations enhanced with escalating dietary zinc inclusion, whereas Cu and Fe concentrations reduced. In summary, the optimal dietary zinc requirements for turtles were 61.27, 60.46 and 60.03 mg/kg diet, based on quadratic regression analysis derived from weight gain, aspartate aminotransferase and alanine transaminase activity, respectively.
1 INTRODUCTION
Soft-shelled turtle (Pelodiscus sinensis) is cultured as a commercial species in Asian countries due to its high nutritional values, elegant taste and economic profit. Soft-shelled turtle is also used in Chinese medicine, because it contains many minerals including selenium, zinc and iron. Soft-shelled turtle has been the most popular and crucial freshwater species in China. It has been recognized as a highly valuable food source and its culture has received more attention (Kou et al., 2018).
Zinc is an important trace element demanded for aquaculture and has various biological functions. It is a necessary component of a cofactor of several enzymes (Tan & Mai, 2001). Zinc is linked to many metabolic pathways and participates in various metabolisms (Kumar et al., 2017), such as affecting the formation of related enzymes (Li & Huang, 2016) and regulating the body's immunity (Muralisankar et al., 2015). Zinc is considered to have a major influence on the immune system (Rink & Kirchner, 2000). Zinc can scavenge free radicals in the body, block the peroxidation of lipids, participate in anti-oxidation in the body (Powell, 2000) and maintain the integrity of cell membranes (Cai et al., 2015). Zinc is also related to more complex physiological processes, such as immune response, nerve transmission and signal transmission (Beyersmann, 2002; Murakami & Hirano, 2008). In addition, Zinc can play a role in anti-inflammatory and anti-diarrhoea and maintain the integrity of the epithelial barrier (Patel et al., 2010; Roselli et al., 2003). Similar to IGF-I, Zn can improve the protein content of bone and have an effect on bone growth together with IGF-I (Ma and Yamaguchi, 2001a; Ma and Yamaguchi, 2001b).
Water includes Zn and other trace minerals, but they are not assimilated enough amounts to satisfy the demand of aquaculture; thus, addition in the diets is recognized as a more important source of trace minerals (National Research Council (NRC), 1993). Although these sources contain many intrinsic zinc contents, practical aquaculture diets are generally replenished with inorganic zinc to meet the nutrition demand for the species (Do Carmo et al., 2004). Excess supplementation of Zn and other micronutrients not only improve the cost of feeds, but also enhance the input of minerals to the cultured environment and pollute the culture environment (Maage & Julshamn, 1993). In addition, excessive Zn can interfere with absorption and transport systems and thus influence the nutrition condition of other metals, such as Fe, Ca and Cu. It is expected to prevent extra high supplementation of Zn, which may restrict the absorbing of minerals in the cultured environment (Buentello et al., 2009). Nutritional imbalances, especially for the larval and juvenile aquatic animal, play a crucial role in growth, disease resistance and survival (Viswanath, 2012). Thus, Dietary addition of Zn was a balance between satisfying the Zn demand and preventing excess and deficiency of Zn (Luo et al., 2011).
Up to now, mineral requirements for soft-shelled turtle, including phosphorus and calcium (Huang et al., 2003), magnesium (Chen et al., 2014; Chen & Huang, 2015), iron (Chu, Chen, & Huang, 2007, 2009), zinc (Huang et al., 2010) and copper (Wu & Huang, 2008) have been reported.
In previous study, the impacts of Zn inclusions in the diets on growth, tissue trace mineral inclusions, Zn in the serum and the haematology of soft-shelled turtles were reported. The protein inclusion of the basal diets in the previous work was 44.3%, and the protein source was casein (Huang et al., 2010). The protein content in the basal diets of soft-shelled turtles is high, which ranged from 42% to 47%. Recently, it has been found that the protein content of soft-shelled turtle's feed can be reduced to 32.8% (Wang et al., 2014). So in our study, fish meal (protein source) is applied in the basic diet with a protein content of 32.45%, which can maintain the requirement of the Chinese soft-shelled turtles (Kou et al., 2021).
The aim of the paper was to estimate the impacts of dietary zinc on survival rate (SR), weight gain(WG), feed intake (FI), somatic index (hepatosomatic index [HIS], intestine somatic index [ISI], carapace ratio), whole-body composition, haematological parameters (respiratory burst [RB], red blood cells [RBCs], albumin [Alb], haemoglobin [Hb], osmolality), aspartate aminotransferase(AST) activity and alanine transaminase (ALT) activity in the liver and tissue trace element contents of juvenile soft-shelled turtle fed diets with 32.45% protein.
2 MATERIAL AND METHODS
2.1 Animals
Juvenile healthy (2 weeks old) soft-shelled (P. sinensis) turtles with a body weight of around 4 g were bought from a commercial farm located in Guangzhou, China. The obtained turtles were acclimatized and fed twice a day with basal diet (Table 1), 2 weeks prior to the experiments.
| Ingredients | Percentage (%) |
|---|---|
| White fish meal | 38 |
| Soybean meal | 9 |
| α-starch | 23 |
| Wheat meal flour | 16 |
| Yeast | 4 |
| Ca(H2PO4)2 | 1.7 |
| 50% Choline | 0.2 |
| Cellulose | 3 |
| Vitamin premixa | 0.3 |
| Mineral premixb | 0.8 |
| Corn oil | 4 |
| Proximate composition | |
| Crude protein (%) | 32.45 |
- a One kilogram of the vitamin premix contains 200,000 IU vitamin A, 1000 IU vitamin D3, 5 g Vitamin E, 3 g vitamin K3, 6 g vitamin B1, 6 g vitamin B2, 5 g vitamin B6, 50 mg vitamin B12, 20 g niacin, 20 mg biotin, 100 g inositol, 8 g calcium pantothenate and 5 g folic acid.
- b One kilogram of the mineral premix contains: MgSO4·7H2O, 137 g; KH2PO4, 239.8 g; NaCl, 3 g; CuSO4·5H2O, 50 mg; FeSO4·7H2O, 33.7 g; MnSO4·H2O, 0.8 g; KI, 0.15 g; CoCl2·6H2O, 1 g; AlCl3·6H2O, 0.15 g; NaSeO3·5H2O, 0.04 mg.
2.2 Experiment diets
The basal diet was formulated to include 38% of white fish meal and 32.45% of crude protein without Zn supplementation (Table 1), and all other ingredients were dried, measured carefully and grounded into powder before sieving through 200 µm mesh, and this diet was recognized as the control basal diet. Diets were formulated using the control feed supplied with ZnSO4·7H2O (Sigma Chemical) at the inclusions of 0, 10, 20, 30, 40 and 50 mg of Zn/kg and the final zinc content in the diet was 35.43, 46.23, 55.38, 66.74, 75.06 and 85.24 mg/kg, respectively (Table 2). The diets were dried well at 50°C, sealed in plastic bags and kept at −20°C.
| Added Zn (mg/kg) | Dietary Zn (mg/kg) |
|---|---|
| 0 | 35.43 |
| 10 | 46.23 |
| 20 | 55.38 |
| 30 | 66.74 |
| 40 | 75.06 |
| 50 | 85.24 |
2.3 Feeding trial
360 turtles were randomly distributed to 360 individual plastic cylindrical containers (30 cm D × 18 cm H) to prevent injury from fighting and divided into six different groups (60 nos ×6 groups). The diet dough was freshly prepared during the time of feeding with distilled water at 1:1.2 ratios. All the turtles were fed (about 3% of body weight, fed at 08:30 and 16:30 h) to apparent satiation twice a day for 12 weeks. Rearing water temperature, pH and Zn concentrations were monitored daily and kept at 27 ± 2°C, 7.9 and 10 µg L−1, respectively. Animal handling and experimental methods were conducted following research protocols supported by the institutional Animal Care and Use Committee of South China Normal University.
2.4 Analysis and measurement
2.4.1 Calculations
Survival rate (SR), weight gain (WG), feed intake (FI) and somatic index evaluated by hepatosomatic index (HSI), intestine somatic index (ISI) and carapace ratio were calculated as follows:
SR (%) = 100(Initial–Dead turtle number)/Initial turtle number
WG (%) = 100(Final weight−Initial weight; g)/Initial weight (g).
FI (%/day) = dry diet fed (g)/{(initial body weight [g] + final body weight [g]) × days of the experiment/2}
HSI (%) = 100* liver weight (g)/body weight (g).
ISI (%) = 100*intestine weight (g)/body weight (g).
Carapace ratio (%) = 100* carapace weight (g)/body weight (g).
2.4.2 Sample collection
All turtles were fasted for 1 day after 12-week feeding experiment and estimated for growth performance and other nutritional parameters. Five turtles selected from each treatment were randomly taken, anaesthetized by 500 mg/L tricaine methane sulfonate (MS-222; Hubei Xinyuanshun Pharmaceutical Chemical Co. Ltd.) and decapitated. Blood samples were collected, and a half of the blood placed into Eppendorf tubes, which contained 68 USP units of sodium heparin. The rest of the blood was centrifuged for 10 mins (1075 × g, 4°C) to obtain serum. The entire liver tissues, intestine and carapace were removed aseptically from five turtles of each treatment, washed using ice-cold phosphate-buffered saline and kept at −80°C after immediately frozen by immersion in liquid nitrogen. Muscles of five turtles from each treatment were sampled, blended and kept at −80°C until use.
2.4.3 Measurement of phytic acid contents in the diets
Phytic acid contents were measured following the method of Latta and Eskin's (1980). After extracted by hydrogen chloride (HCl) and then extracted by chloroform, the samples were centrifuged and the supernatant was subsequently eluded by an anion exchange column. The effluent was then blended with the agent (0.03% FeCl3, 0.3% sulfosalicylic acid) and tested by a spectrophotometer. The content of dietary phytic acid was 1.03 g/kg.
2.4.4 Measurement of diets and whole-body composite of turtles
The proximate composition of the diet and whole body of turtles were sampled and determined for inclusions of crude protein, crude lipid, ash and moisture following our previous study (Kou et al., 2021).
2.4.5 Measurement of haematological parameters
Respiratory burst activity was assessed following previously published methods (Cheng et al., 2007).
Red blood cells (RBCs) were determined under a light microscope following Abdel-Tawwab's method (Abdel-Tawwab et al., 2010).
The amounts of Hb and Alb in the blood were measured according to the relevant assay kit instructions (Jiancheng Institute of Biotechnology).
The osmolality of the blood samples taken from the neck and stored in Eppendorf tubes with 68USP units of sodium heparin was tested using an osmometer (Beijing XianglongHuanyu Biotechnology Co., Ltd).
2.4.6 Measurement of Alanine transaminase activity and Aspartate aminotransferase activity
Liver and serum aspartate aminotransferase (AST) activity and alanine transaminase (ALT) activity were tested according to our previous work (Kou et al., 2021).
2.4.7 Measurement of trace element inclusions in muscle and liver of turtles
The tissue samples were digested by a microwave sample preparation system (Anton Parr) before measurement of trace mineral element. Zn, Cu and Fe were tested by atomic absorption spectrometer (AA-240Z, Varian).
2.5 Statistical methods
All data were statistically dealt with using SPSS 19.0 software for windows. One-way ANOVA and Duncan's new multiple range comparison were applied to estimate changes among treatment means. The requirement of dietary Zn in the turtle was evaluated by quadratic regression analysis using weight gain, AST and ALT as the response criteria.
3 RESULTS
3.1 Growth performance
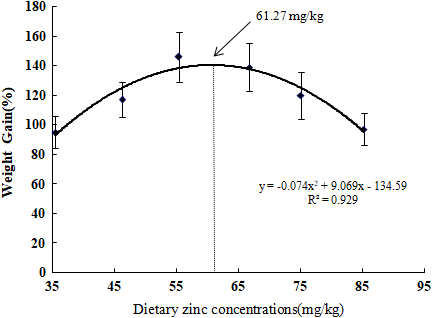
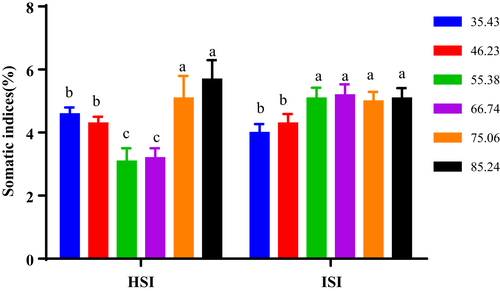
The survival rate (SR), weight gain (WG), feed intake (FI), hepatosomatic index (HSI), intestine somatic index (ISI) and carapace ratio of soft-shelled turtles were presented in Figures 1 and 2 and Table 3.
| Dietary zinc contents (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| 35.43 | 46.23 | 55.38 | 66.74 | 75.06 | 85.24 | |
| Initial weight | 4.09 ± 0.22 | 4.13 ± 0.36 | 4.07 ± 0.23 | 4.15 ± 0.30 | 4.07 ± 0.22 | 4.12 ± 0.31 |
| Final weight | 8.01 ± 0.58c | 8.97 ± 0.59b | 10.17 ± 0.63a | 9.89 ± 0.61a | 9.05 ± 0.54b | 8.13 ± 0.56c |
| Weight gain (%) | 95.14 ± 11.05c | 117.13 ± 12.16b | 146.26 ± 17.03a | 139.52 ± 16.14ab | 120.39 ± 16.21b | 97.41 ± 11.28c |
| Survival rate (%) | 78.33 ± 0.92c | 81.67 ± 1.04bc | 98.33 ± 2.15a | 95.00 ± 3.02a | 88.33 ± 1.63b | 85.00 ± 1.46b |
| Feed intake (%/d) | 1.43 ± 0.08a | 1.42 ± 0.07a | 1.21 ± 0.05b | 1.17 ± 0.04b | 1.42 ± 0.06a | 1.38 ± 0.08a |
| Carapace ratio (%) | 14.52 ± 0.61c | 16.92 ± 0.97b | 18.14 ± 1.07a | 17.89 ± 1.02a | 16.95 ± 0.86b | 15.02 ± 0.62c |
Note
- Values in the same column with different superscripts are significantly different (p < .05).
Survival rate of the turtles significantly improved in the 55.38 mg/kg group reaching 98.33%. The lowest survival rate was observed in the 35.43 mg/kg group with a survival rate of 78.33%. As seen in Table 3, WG escalated with elevating dietary Zn inclusions up to 146.26% in the 55.38 mg Zn/kg feed group. Groups fed diets with 66.74 and 75.06 mg Zn/kg showed the next highest WG levels. Increasing Zn in the diet beyond 55.38 mg Zn/kg did not escalate the growth, but rather decreased the WG to 97.41% at 85.24 mg/kg. This was nearly in parallel with the lowest Zn concentration of 35.43 at 95.14%. Turtles fed 55.38 and 66.74 mg Zn/kg showed the best WG without evident variations contrasted with the rest of the groups (p > 0.05). The suitable inclusion of dietary Zn by WG was analysed by quadratic regression analysis. This resulted in a value of 61.27 mg/kg, and the quadratic regression was y = −0.074x2 + 9.069x−134.59, R² = 0.929 (Figure 1).
Turtles fed with 55.38 and 66.74 mg Zn/kg showed the highest feed intake, while no evident difference was found among other groups (Table 3).
In this study, turtles fed with 55.38 and 66.74 mg Zn/kg had the lowest HSI, and the highest HSI was found in turtles fed diets with 75.06 and 85.24 mg Zn/kg (Figure 2).
As shown in Figure 2, turtles fed with 35.43 and 46.23 mg Zn/kg group had lower ISI than other groups. The ISI of turtles levelled off when the turtles were fed diets with more than 46.23 mg Zn/kg.
Turtles fed with 55.38 and 66.74 mg Zn/kg had the highest carapace ratio, followed by 46.23 mg Zn/kg group and 75.06 mg Zn/kg group and the least in turtles fed diets with 35.43 and 85.24 mg Zn/kg (Table 3).
3.2 Whole-body composition of turtles
The whole-body composition of turtles fed diets with various zinc inclusions was shown in Table 4. Turtles fed diets with 55.38 and 66.74 mg Zn/kg had higher body protein than other groups, and least in turtles fed with 35.43 mg Zn/kg. Body crude lipid was smallest in turtles fed with 55.38 and 66.74 mg Zn/kg, followed by 35.43 and 46.23 mg Zn/kg and the highest in turtles fed with 85.24 mg Zn/kg. Body crude ash enhanced significantly with the escalating dietary zinc levels, and turtles fed diets with 75.06 and 85.24 mg Zn/kg had the highest body crude ash content among all the treatments.
| Dietary zinc contents (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| 35.43 | 46.23 | 55.38 | 66.74 | 75.06 | 85.24 | |
| Moisture (%) | 80.26 ± 1.18 | 79.03 ± 0.97 | 78.69 ± 1.19 | 78.47 ± 1.25 | 78.03 ± 1.20 | 78.47 ± 1.14 |
| Crude protein (%) | 12.07 ± 0.10d | 14.27 ± 0.14c | 17.89 ± 0.15a | 17.39 ± 0.14a | 16.09 ± 0.12b | 15.82 ± 0.18b |
| Crude lipid (%) | 1.43 ± 0.03ab | 1.37 ± 0.05b | 1.28 ± 0.01c | 1.27 ± 0.02c | 1.38 ± 0.04b | 1.48 ± 0.05a |
| Ash (%) | 4.78 ± 0.10c | 5.03 ± 0.21c | 5.16 ± 0.11bc | 5.48 ± 0.14b | 6.08 ± 0.13a | 6.05 ± 0.15a |
Note
- Values in the same row with different superscripts are significantly different (p < .05).
3.3 Haematological parameters
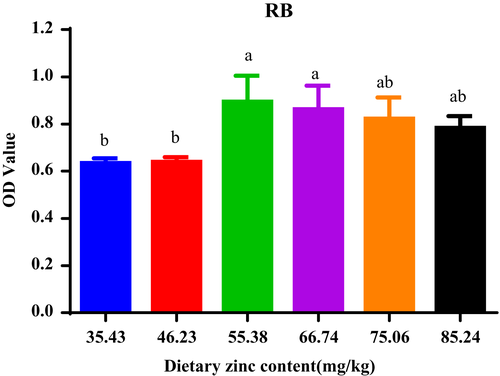
Haematological parameters, involving Haemoglobin (Hb), red blood cells (RBCs) and Albumin (Alb), respiratory burst (RB) and osmolality, were presented in Figures 3-5.
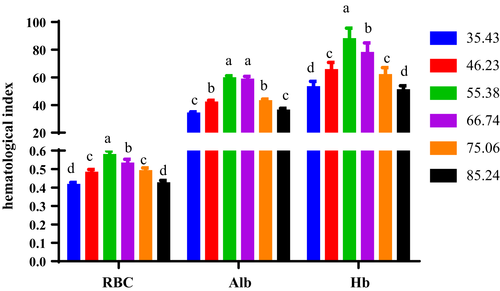
As the increase in dietary zinc concentration, the RBCs show a tendency to rise first and then decrease (Figure 3). When the dietary zinc content was 55.38 mg/kg, the RBC of the turtle is significantly higher than other experimental groups. When the dietary zinc levels were 35.43 mg/kg and 85.24 g/kg, there was no evident change in the total number of RBCs in turtle between the two groups, which was greatly smaller than other experimental groups.
When dietary zinc contents were 35.43 mg/kg and 85.24 mg/kg, the haemoglobin (Hb) content of the soft-shelled turtles was significantly smaller than that of other groups, with a minimum value of 51.12−53.27 g/L(Figure 3).
With the elevation of dietary zinc inclusion, the content of albumin (Alb) in turtle showed a trend of first improving and then declining. When dietary zinc content was 55.38 and 66.74 mg/kg, there was no evident variation in the content of Alb in the turtle, but significantly higher than other groups. When zinc content in feed was 46.23 and 75.06 mg/kg, no significant change was recognized in the content of Alb in the blood of turtle between the two groups, but it was evidently smaller than that in the 55.38 mg/kg group (Figure 3).
Respiratory burst (RB) enhanced with escalating dietary Zn inclusions up to 55.38 mg/kg and then kept plateau beyond this level (Figure 4).
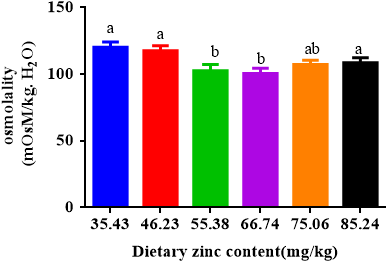
Osmolality firstly decreased with escalating dietary Zn inclusions from 35.43 to 53.38 mg/kg and then increased at a higher Zn level. When dietary zinc levels were 55.38 and 66.74 mg/kg, there was no evident change in the osmolality of soft-shelled turtles between the two groups, but the values were significantly smaller than that of other groups (Figure 5).
3.4 Alanine transaminase activity and Aspartate aminotransferase activity
The transaminase enzyme activities of soft-shelled turtles fed graded zinc levels were shown in Figures 6-8.
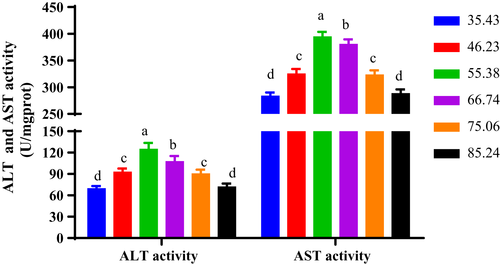
Aspartate aminotransferase (AST) activity firstly increased with escalating dietary Zn provision from 35.43 to 55.38 mg/kg diet and then reduced at higher Zn inclusions (Figure 6). The aspartate aminotransferase (AST) activity of the turtles fed with 55.38 mg Zn/kg showed the largest value among the groups.
Alanine transaminase (ALT) activity showed a similar trend with the aspartate aminotransferase (AST) activity. Turtles fed diets with 55.38 mg Zn/kg had the highest ALT value, followed by 66.74 mg Zn/kg group, 46.23 mg Zn/kg group and 75.06 mg Zn/kg group and the least in turtles fed with 35.43 mg Zn/kg and 85.24 mg Zn/kg (Figure 6).
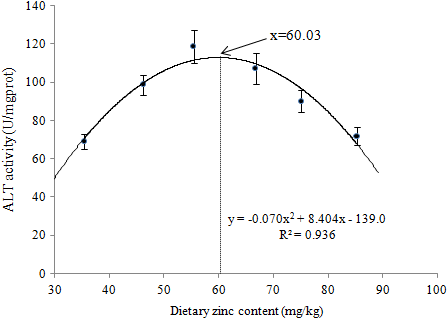
The relation between ALT activity and dietary Zn concentrations was presented by a quadratic regression analysis (Figure 7), which revealed that the required dietary Zn inclusion for ALT activity of soft-shelled turtles was 60.03 mg Zn/kg in diet.
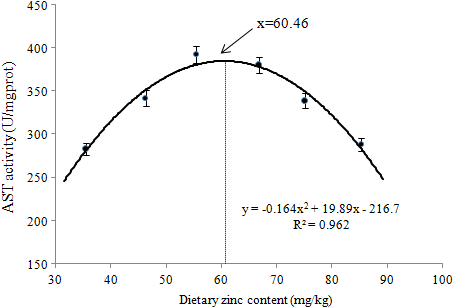
A quadratic regression model was applied to evaluate the correlation between AST activity and dietary Zn levels. The result suggested that the estimated dietary Zn concentration for AST activity of juvenile soft-shelled turtles was 60.46 mg Zn/kg in diet (Figure 8).
3.5 Impacts of dietary zinc content on trace elements in muscle and liver of soft-shelled turtle
The zinc content, iron content and copper inclusion in the muscle of soft-shelled turtle were reported in Figure 9.
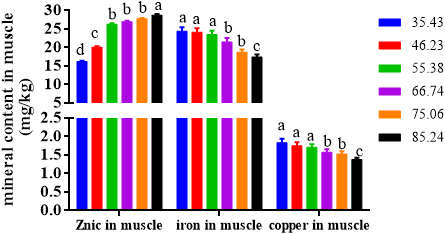
The zinc content in the muscle of turtle enhanced gradually with the elevation of dietary zinc content. The lowest zinc content was presented in turtles fed diet with 35.43 mg Zn/kg. Turtles fed with 85.24 mg Zn/kg had the highest zinc content.
With the increase in dietary zinc inclusion, the iron content in the muscle of turtle decreased gradually. When the dietary zinc content was 35.43, 46.23 and 55.38 mg/kg, there was no evident change in the iron content in the muscles of turtles among each group. When dietary zinc content was 66.74 and 75.06 mg/kg, the iron inclusion in the muscle of the turtle was significantly lower than those in the 35.43, 46.23 and 55.38 mg/kg groups. When the dietary zinc content was 85.24 mg/kg, the iron concentration in the muscles of turtles was the minimum.
The copper content in the muscle of turtle paralleled well with the iron content of the turtles. Turtles fed diets with 35.43, 46.23 and 55.38 mg/kg had the highest copper content, followed by 66.74 and 75.06 mg/kg group, and 85.24 mg Zn/kg group had the lowest copper content.
The zinc content, iron content and copper content in the liver of soft-shelled turtle were presented in Figure 10.
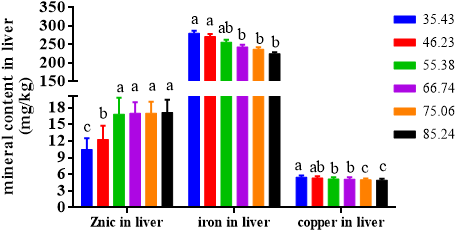
The zinc inclusion in the liver of turtle improved when dietary Zn escalated up to 66.74 mg /kg. When beyond this dietary inclusion, the zinc content levelled off (Figure 10).
The iron inclusion in the liver of turtle decreased gradually with an increase in dietary Zn level up to 66.74 mg Zn/kg and then levelled off. When the dietary zinc contents were 35.43, 46.23 and 55.38 mg/kg, the iron content in the liver of the turtle was the highest, and there were no significant variations among the groups. When dietary zinc contents were 55.38 and 66.74 mg/kg, no evident difference was shown in the iron content in the liver of turtles.
The liver copper content of soft-shelled turtle declined gradually with the elevation of dietary zinc content. Turtles fed feeds with 75.06 and 85.24 mg Zn/kg group had the lowest copper content.
4 DISCUSSION
After 12 weeks of feeding turtles with varying doses of Zn, the effects on growth performance were assessed. Inclusion of supplemental Zn caused a significant WG up to 66.74 mg/kg compared with controls. Turtles in the 55.38 mg/kg group showed the highest survival rate and WG. Similar patterns of results were found in carp (Ogino and Yang, 1979), channel catfish Ictalurus punctatus (Gatlin & Wilson, 1984) and Nile tilapia Oreochromis niloticus (Huang et al., 2015; Do Carmo et al., 2004; Eid & Ghonim, 1994). However, some reports indicated that growth performance was not influenced by Zn concentrations in blue tilapia Oreochromis aureus(McClain & Gatlin, 1988), Atlantic salmon Salmo salar (Maage & Julshamn, 1993), European sea bass Dicentrarchus labrax (Fountoulaki et al., 2010), yellow catfish Pelteobagrus fulvidraco (Luo et al., 2011) or Nile tilapia Oreochromis niloticus (Do Carmo et al., 2004). These findings indicated that the impact of dietary Zn on growth in various fish differs between species. However, discrepancies in nutritional requirements found among members of the same species might be attributed to different feed ingredients (Do Carmo et al., 2004; Lin et al., 2008), differences in body size and variability in culture conditions (Mjoun et al., 2010).
In our study, a high inclusion of dietary Zn inversely impacted the growth of soft-shelled turtles. This may be because high inclusions of dietary Zn may negatively influence the uptake of several minerals such as iron, calcium and magnesium (Clearwater et al., 2002; Eid & Ghonim, 1994; Huang et al., 2010; Park & Shimizu, 1989). Huang et al. (2010) observed no evident discrepancies in WG of soft-shelled turtle when turtles were given casein-based diets, including seven different concentrations of Zn over a 10 week period. Two possible explanations for the inconsistency in WG observed in the present study are that there might have been different ingredients in the feed used, as well as variations in the culture periods.
Feed intake is a major parameter for determining the growth of the aquatic animals, because the amount of an aquatic animal eats is a valuable estimation for how much it grows (Truong et al., 2021). The content of dietary zinc reduced the feed intake up to 66. 74 mg/kg and then elevated when the higher dietary zinc increased. Dietary high zinc may prevent the use and deposition of other minerals in soft-shelled turtles. The turtles fed with non-optimal zinc may eat more feed to acquire more energy to fight with malnutrition stress and maintain its normal growth and physical activity. Our results indicated that high dietary zinc may negatively influence the growth of the aquatic animals. This agreed with previous study on freshwater prawn Macrobrachium rosenbergii (Muralisankar et al., 2015) and Nile tilapia (Oreochromis niloticus) (Huang et al., 2015). In the findings of turbot (Scophthalmus maximus), they found that the feed intake increased up to optimal dietary zinc and then level off at higher zinc levels (Ma et al., 2014), while the feed intake of yellow catfish Pelteobagrus fulvidraco, rainbow trout, Oncorhynchus mykiss was not affected by different dietary zinc levels (Kucukbay et al., 2006; Luo et al., 2011). The difference may result from different species and different diets.
With the elevation of zinc inclusion in the diet, the carapace ratio of soft-shelled turtle showed a tendency of first increasing and then decreasing. This is similar to the fact that zinc inclusion in the vertebrae of aquaculture gradually enhanced with the elevation of dietary zinc content. The concentration of minerals in the carapace of soft-shelled turtles is large and gradually deposits in the carapace as the dietary mineral elements increase. However, over-optimal zinc content will reduce the carapace ratio, and the turtles fed with excess zinc content were smaller in size and not easy to grow up. When dietary Zn concentration exceeded the required amount, Zn toxicity would occur.
High HSI is commonly considered as to be linked to aquaculture's bad growth and health (Li et al., 2014; Ren et al., 2011). In our paper, dietary 75.06 and 85.24 mg Zn/kg showed significant elevations in HSI, which resulted in suppression of the growth performance and antioxidant activities enzymes. Opposite to our result, dietary Zn inclusions had no evident impact on hepatosomatic index (HSI) or viscerosomatic index (VSI) of grass carp (Ctenopharyngodon idella) (Liang et al., 2012), common carp (Cyprinus carpio var. Jian) (Liang et al., 2020) and Jian carp (Cyprinus carpio var. Jian) (Tan et al., 2011). This difference may possibly result from different species. In our present study, turtles fed with inadequate concentration of Zn diets showed poor ISI. The author thinks that dietary addition of Zn can promote digestive enzymes secretion, so it can improve the weight and length of intestine. As we all know, the dietary supplemented Zn can improve these digestive enzymes secretion of aquatic animals, such as hybrid tilapia Oreochromis niloticus (L.) × Oreochromis aureus (Steindachner), Jian carp (Cyprinus carpio var. Jian), giant fresh water prawn Macrobrachium rosenbergii and rats (Jing et al., 2009; Li et al., 2007; Muralisankar et al., 2015; Tan et al., 2011).
With the elevation of dietary zinc inclusion, the crude protein concentration of turtle in whole body has a tendency to rise first and then decline, and the highest level of dietary zinc content is 55.38 mg/kg. The crude fat content of the turtle has a minimum value when the dietary zinc content is 55.38 and 66.74 mg/kg. Crude ash of the turtles increased with elevated dietary zinc levels. The author thinks that when dietary zinc content exceeds a certain dose, excess zinc has a toxic effect on the turtles and causes the reduction in the protein deposition. The reason for this is that aquaculture needs much energy to prevent the Zn toxicity impact, which was acquired by breaking down body protein. Normally, variations in protein and lipid concentrations in aquaculture could be related to alterations in their synthesis and/or accumulation in aquaculture body. The effect of Zn inclusion in diet on body proximate composition of aquaculture differs by species. Dietary Zn deficiency greatly escalated moisture concentration, declined crude protein and ash concentration of the aquaculture body (Ogino and Yang, 1979; Satoh et al., 1987). However, contrary to this result, moisture and total ash improved greatly; meanwhile, the concentrations of crude lipids and protein reduced significantly with Zn exposure (Abdel-Tawwab et al., 2016). The crude lipid increased with elevated dietary zinc levels in rainbow trout, Oncorhynchus mykiss, while the crude protein and ash reduced with elevated dietary zinc levels (Welker et al., 2016). The crude protein and crud lipid declined with increased dietary zinc inclusions in hybrid tilapia (Oreochromis niloticus × O. aureus) (Li & Huang, 2016). The concentrations of crude lipid, crude protein and ash of juvenile abalone, Haliotis discushannai Ino (Tan & Mai, 2001) and Jian carp (Cyprinus carpio var. Jian) (Tan et al., 2011) were not changed by dietary Zn. Our results were contradicted with previous study on soft-shelled turtles (Huang et al., 2010). In his study, muscles of turtles fed with 32 mg/kg showed the lowest moisture and the largest ash value. The muscular crude protein and lipid inclusion in the turtles were not affected by dietary zinc content, but in our paper, we analysed the whole-body proximate content of soft-shelled turtle. The difference may also result from different duration of the trials, as the soft-shelled turtles in our study were cultured for 12 weeks not 10 weeks.
Zinc exposure elevated greatly total ash and Zn inclusions in the whole body of aquatic animal. Similar results were found in Channel fish (Murugan Senthil et al., 2008), hybrid tilapia (Oreochromis niloticus × O. aureus) (Li & Huang, 2016), Nile tilapia (Abdel-Tawwab et al., 2012, 2016), tilapia, Oreochromis niloticus (Kishawy et al., 2020) and common carp (Abdel-Tawwab et al., 2013). Shell mineralization of soft-shelled turtles at its developmental stage may influence determined ash content (Zhou et al., 2013). Zinc is important for immunity and bone formation (Huber and Gersho, 1970). Optimal dietary zinc content was useful for bone formation, which caused high carapace ratio. Excess dietary Zinc prevents the deposition of other minerals, so reduces carapace ratio.
Normal cellular damage, malnutrition and disease of aquatic animals are generally determined by measuring activities of enzymes such as AST and ALT (Cowey, 1994). ALT and AST activities are considered as parameters of better liver function, high AST and ALT activities in the serum and low liver AST and ALT activities normally represent damage or a weakening of normal function for liver (Kim & Lee, 2009). The essential role of dietary Zn for the immunity of soft-shelled turtle was clearly stated in our study. In our study, liver AST and ALT activities in turtles fed with deficiency or excess dietary zinc content were greatly smaller than others. Considering that the largest HSI was found in groups with deficiency or excess dietary zinc content, the results indicated that turtles fed with deficit or excess dietary zinc content had abnormal liver functions. It may cause liver injury as a result of inadequate or excess dietary Zn and thus cause high HSI and low liver ALT and AST activities. These results coincide with reports for Nile tilapia (Abdel-Tawwab et al., 2016; Huang et al., 2015). ALT and AST activities in serum of Nile tilapia in the un-added group were evidently larger than those in the replenished groups, which suggest probable liver damage of aquatic animal fed the feed without Zn addition (Abdel-Tawwab et al., 2016).
Blood chemistry is considered to be a crucial physiological, pathological and toxicological biomarker and is normally applied in the estimation of nutrition condition, health condition and the ability of adaptation to the external environment in various animals involving aquaculture (Khoshbavar-Rostami et al., 2004; Kopp et al., 2009). It may be that the high zinc-containing feed inhibits the absorption of iron by the soft-shelled turtle, which also reduces the haemoglobin content. Optimal dietary zinc improved the count of Hb and albumin levels. Similar phenomena were found in tilapia, Oreochromis niloticus (Kishawy et al., 2020).
Red blood cells count is an important parameter for aquaculture blood health. In agreement with our result, diet supplemented with 30 mg/kg Zn greatly elevated RBCs count of grass carp whereas at higher supplementation (60 mg/kg) declined RBCs values (Faiz et al., 2015). However opposed to this, dietary zinc inclusion has no impact on RBCs count of hybrid tilapia, Oreochromis niloticus × O. aureus (Li & Huang, 2016) and tilapia, Oreochromis niloticus (Kishawy et al., 2020). This discrepancy may result from different species, zinc levels or diet composition.
Cells are associated with a large number of reactive oxygen species (ROS) during phagocytosis. The resulting process is called respiratory eruption. These reactive oxygen species are cytotoxic oxidants, including superoxide anions(O2−), hydrogen peroxide(H2O2) and hydroxyl radicals [OH] and so on, and it has a vital impact on the immune defence of killing and digesting pathogenic micro-organisms.
Respiratory burst is a fast release of reactive oxygen species thought to protect against bacterial pathogens (Immanuel et al., 2009). A diet supplemented with ZnO significantly increased respiratory burst of rohu, Labeo rohita (Hamilton) (Swain et al., 2019). Similarly, our result indicated that respiratory burst activity improved with increasing zinc level up to a certain range. Contrary to our results, elevated zinc presented an inhibitory effect on the respiratory burst activity of Atlantic salmon (S. salar) (Lygren & WaagbØ, 1998, 1999) and European sea bass (Dicentrarchus labrax) (Fountoulaki et al., 2010). The difference may result from different species.
Zn is vital for Zn-containing enzymes. It is served as a cofactor in several enzyme systems. Lack of Zn will not only decline growth but also damage physiological parameters in aquaculture. In our work, deficiency or excess dietary zinc content decreased the content of Hb in the soft-shelled turtles. As we all know, Fe is an essential element for Hb. Deficiency or excess dietary zinc content decreased the content of Fe in muscle and liver of turtles. Therefore, reduction in Fe in the tissue would decrease the content of Hb in the soft-shelled turtles.
Plasma osmolality is an indicator for evaluating the healthy status of renal function. High plasma viscosity and high blood glucose are two major reasons for the increase in plasma osmolality. With the increase in dietary zinc levels, plasma osmolality decreased firstly and then increased. It may be that inadequate or excess zinc concentrations affect enzymes related to sugar metabolism, resulting in elevated blood glucose and increased osmolality. In accordance with our result, zinc deficiency increased the osmolality of Nile tilapia (Oreochromis niloticus) (Do Carmo et al., 2004).
The nutritional value of mineral elements in the diet is related not only to their inclusion in the diet but also to the bioavailability of the mineral for animal (Paripatananont & Lovell, 1995). It has been demonstrated that Zn concentrations in tissues, involving the scales, bone, muscle and liver, are vital parameters of the Zn nutritional conditions of animals and are positively related to Zn inclusions in the diets (Liang et al., 2012; Luo et al., 2011). As the distribution of Zn in different body tissues is unequal (Jeng & Sun, 1981), excess Zn greatly leads to Zn deposition in the muscle and liver, whereas Zn deficiency declined Zn inclusion in the muscle and liver. Iron and zinc have similar positions and chemical characteristics in the periodic table. Both iron and zinc are divalent ions, occupying part of the common transport channel. The absorption of the two mineral exists a competitive relationship. Some metal transporting proteins that play a role in the intestinal absorption, involving the divalent cation transporter 1, present a high affinity for both iron and zinc. Zn and Fe may compete for binding with the divalent cations transporter 1 (Cousins & McMahon, 2000). Dietary excess zinc will reduce the absorption of iron. Dietary zinc inclusions were also observed to inversely influence the concentrations of the tissues Fe and Cu of the aquaculture, such as channel catfish (Gatlin & Phillips, 1989), carp (Ogino and Yang, 1979), eel (Park & Shimizu, 1989), Nile tilapia (Eid & Ghonim, 1994), carapace of soft-shelled turtle, Pelodiscus sinensis (Huang et al., 2010), grass carp, Ctenopharyngodon idella (Liang et al., 2012), rainbow trout, Oncorhynchus mykiss (Welker et al., 2016), and hybrid tilapia, Oreochromis niloticus × O. aureus (Li & Huang, 2016). C. auratus fed with Zn-added diets showed evident improvements in Cu and Fe inclusion up to 60 mg/kg and reduced at elevated zinc inclusion in diets (Rani et al., 2012). There also exist some contradicted results. No significant effect was obtained on the liver and muscle Fe of soft-shelled turtle (Huang et al., 2010) by supplementation of dietary zinc. In addition to different ingredients in the diet, duration of the trials could also be a different parameter, as the turtles in this paper were cultured for 12 weeks.
With the elevation of dietary copper content, the content of iron and zinc in muscles and liver gradually increased when copper was supplemented to the feed of soft-shelled turtle (Wu & Huang, 2008). However, the findings of this study demonstrated that the inclusions of copper and iron in muscle and liver declined with the elevation of zinc content in feed. The author speculates that the relationship between copper and iron and zinc is not equivalent, and copper cannot inhibit the absorption of iron and zinc, but adding zinc to the feedstuff can inhibit the absorption of copper and iron in turtles. These discrepancies have possibly resulted from different dietary ingredients, different culture time, different protein levels and different cultural environments.
To our knowledge, phytic acid used as a strong negative divalent cations chelator is rich in vegetable-based diets and will limit the absorption of Zn in aquatic animals (Erdman, 1979; Ma et al., 2014). It may form in soluble complexes with minerals in the digestive tract and prevent zinc transport across the intestine mucosa. Aquatic animals fed diets are probably to have evidently higher dietary zinc demand because of the existence of tricalcium phosphate and phytic acid obtained from feed ingredients, which can obstruct zinc in the feed and influence the bioavailability of zinc for aquaculture (Denstadli et al., 2006; Laining et al., 2010; Liang et al., 2020). Compared with previous study, the zinc requirement (60.03–61.27 mg Zn/kg) for soft-shelled turtle was higher than that in previous work (42–46 mg Zn/kg) (Huang et al., 2010). The high optimal supplemented inclusion of zinc observed in our study may probably result from phytic acid existed in soybean meal, which declines the bioavailability of Zn. The different evaluation parameter is also another reason for the discrepancy.
5 CONCLUSIONS
Findings of our study demonstrated that an optimal inclusion of dietary Zn was necessary for growth, whole-body composition, haematological indicators and tissue trace element contents of juvenile soft-shelled turtles. Deficit and excess Zn not only caused poor growth, but also had a negative effect on haematological parameters, AST activity and ALT activity in the liver.
In conclusion, findings of the work have revealed that the suitable dietary Zn demand for soft-shelled turtles is 61.27, 60.46 and 60.03 mg/kg diet, using weight gain, AST and ALT activity as indicators, respectively.
ACKNOWLEDGEMENTS
This finding was financially supported by Research start-up funds for annual salary staff of Zhongkai University of Agriculture and Engineering (KA210319242) and Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2019KJ141).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




