Dietary lysine requirement of greater amberjack juvenile (Seriola dumerili, Risso, 1810)
Abstract
An 8-week feeding trial was conducted to determine the dietary lysine requirement of greater amberjack, Seriola dumerili. Six experimental diets resulting from a practical basal formulation were produced to containing mainly plant ingredients (25% fish meal) were supplied with graded levels of crystalline L-lysine-HCl and the analysed lysine concentration in each diet was found to be 1.93 (basal diet; CL1.93), 2.01 (CL2.01), 2.11 (CL2.11), 2.15 (CL2.15), 2.20 (CL2.20) and 2.29 (CL2.29) g/100 g diet respectively. Seriola dumerili of 32.8 g ± 3.0 (mean ± SD) were randomly assigned to 18 experimental small cages, and each was stocked with 25 fish per cage in triplicates. The fish were hand-fed with the experimental diets twice daily (09:00 h and 15:00 h) to apparent satiation, 6 days a week for 56 days. No significant differences were observed in weight gain (WG), daily growth index (DGI), specific growth rate (SGR) and protein efficiency ratio (PER) among the dietary groups. However, the pairwise linear regression of WG showed that the dietary lysine requirement of greater amberjack juvenile is 2.11% of the diet. The activity of catalase enzyme among the dietary treatments differed significantly (p < .05) in both the liver and intestine. Similar results were found for the heat shock proteins (HSP70 and HSP90) with a tissue-specific response. Based on the results obtained, the dietary lysine requirements that can support maximum WG and PER of greater amberjack juvenile were found to be between 2.03% and 2.11% of the diet (4.55%–4.73% of dietary protein).
1 INTRODUCTION
As the demand for fish food increases globally, there is a need for the aquaculture industry to expand its farmed fish species base in order to increase productivity and sustainability. To unlock a species potential for aquaculture, the nutrient requirement of the fish in terms of protein, amino acids, lipids, minerals and vitamins must be well understood to ensure efficient nutrient utilization and rapid growth. The new development in aquafeed production has witnessed commercial fish feed industries incorporating large quantities of plant-based ingredient in fish feed formulation (Lee et al., 2020). However, the main constraint when alternative proteins of plant origin are used in the fish diets is the limiting content in some essential amino acids (EAA), mainly lysine and methionine. Lysine is the most common limiting EAA in aquafeeds, especially where plant protein forms the main dietary ingredient (Lee et al., 2020; Wilson, 2003). The assessment of lysine requirement has been a subject of particular attention in aquatic animal research (Adesola et al., 2018; Campelo et al., 2018; Jirsa et al., 2014; Deng et al., 2010; Hauler & Carter, 2001). The optimal use of such lysine-limiting protein source ingredients in aquafeeds will depend on the precise estimation of lysine requirements of the fish as this will determine the efficiency of protein utilization and normal physiological function of the animal. For instance, lysine deficiency or excess dietary lysine has been reported to cause growth reduction, lipid accumulation and/or poor feed utilization in different fish species (Ebeneezar et al., 2019; Li et al., 2014; Deng et al., 2010; Zhou et al., 2007; Ahmed & Khan, 2004). Thus, species-specific lysine nutritional requirements must be defined for the accurate supplementation of lysine in plant protein-based aquafeed to be nutritionally balanced.
The greater amberjack (Seriola dumerili, Risso 1810) is one of the emerging fish species that is common to Mediterranean aquaculture and is much valued among the consumers (Porrello et al., 1993; Takakuwa et al., 2006, 2020). This fish species has a high potential for aquaculture due to its adaptability to tank culture conditions, fast growth rate, excellent meat quality and consumer acceptability (Mazzola et al., 2000; Monge-Ortiz et al., 2018; Sicuro & Luzzana, 2016). As the culture of greater amberjack is gaining attention in different countries, little is known about its nutritional requirements (Sarih et al., 2019; Skaramuca et al., 2001; Takakuwa et al., 2006, 2020; Vidal et al., 2008) and this presents a major bottleneck towards the development of a cost-effective feed.
The available literature indicates that the dietary protein requirement of S. dumerili range between 40 and 54% (Sarih et al., 2019; FAO, 2016; Sicuro & Luzzana, 2016; Takakuwa et al., 2006), but information on the EAA requirements for this species is lacking. Furthermore, as the need for increased incorporation of plant-based ingredients in aquafeed intensifies, information on EAA requirements is of the utmost importance to optimize a nutritional balance diet for greater amberjack. Very few studies have published data on the dietary lysine requirements of Mediterranean fish species such as gilthead sea bream, Sparus aurata (Marcouli et al., 2006), European sea bass, Dicentrarhus labrax (Tibaldi & Lanari, 1991) and meagre, Argyrosomus regius (Kotzamanis et al., 2018). On the other hand, several studies have investigated the dietary lysine needs of other farmed finfish species, for instance, Atlantic salmon Salmon salar, (Espe et al., 2007; Anderson et al., 1993), rainbow trout, Oncorhynchus mykiss (Lee et al., 2020; Yun et al., 2016; Rodehutscord et al., 1997; Walton et al., 1986), common carp, Cyprinus carpio (Nose, 1979), channel catfish, Ictalurus punctatus (Robinson et al., 1980), dusky kob, Argyrosomus japonicus (Adesola et al., 2018) and Silver pompano, Trachinotus blochii (Ebeneezar et al., 2019), significantly contributing to the development and production of cost-effective diets for the aquaculture industry. As regards the fish of Seriola genus, lysine requirements have been estimated only for Japanese amberjack, Seriola quinqueradiata (Ruchimat et al., 1997) and California yellowtail, Seriola lalandi (Jirsa et al., 2014).
The increased utilization of alternative protein sources in commercial aquafeed production, especially plant-origin proteins, has predicated the need for the supplementation of limiting EAA for optimum protein utilization and growth. Studies conducted on the effects of the inclusion of alternative ingredients in the diets of greater amberjack (Seriola dumerili) are still scarce (Takakuwa et al., 2020; Dawood et al., 2015; Tomás et al., 2005).
Recently, Yang et al. (2020) found that dietary lysine is related to the activity of the fish immune system and can modify immune response in largemouth bass (Micropterus salmoides). In grass carp (Ctenopharyngodon idellus) fry, Huang, Liang, et al. (2021) found that appropriate dietary lysine levels improve the growth and diminish the lipid content in parallel with the promotion of glycolysis and lipolysis. Furthermore, in another study of Huang, Maulu, et al. (2021), similar lysine levels in the same fish species were found to improve the immune and the antioxidant capacities of the fish. This is in agreement with the effect of dietary lysine on the antioxidant status of sub-adult grass carp (Ctenopharyngodon idella) including the enzymatic activity of catalase (Li et al., 2014). In addition, lysine supplementation relieves the antioxidant decrease, including that of catalase, of soybean meal inclusion in the diet of yellow catfish (Pelteobagrus fulvidraco) according to Jiang et al. (2018).
Dietary nutrients including amino acids, proteins and bioactive peptides have been described to adjust the heat shock response individually or in an additive pattern with other potential stressors in several vertebrates (e.g., Moura et al., 2018). In European sea bass (Dicentrarchus labrax), soy-based diets enriched with different taurine concentrations affect both the enzymatic activity of catalase and the expression and activation of heat shock proteins (HSPs) (Feidantsis et al., 2014). Limited are the studies on the effects of dietary lysine on HSPs in teleosts, although in zebrafish (Danio rerio), it appears to affect the myotomal muscle proteome profile (de Vareilles et al., 2012).
To our understanding, there is dearth of knowledge with regards the amino acids requirements of this important cultured fish species. Hence, the aim of the present study was to define the nutritional requirements of lysine in a dose-response trial evaluating the effects which different dietary levels of lysine may have on growth, nutrient utilization, whole-body composition, antioxidant status and expression of heat shock proteins in juvenile greater amberjack, Seriola dumerili, fed low fish meal-based diets.
2 MATERIALS AND METHODS
2.1 Ethics statement
All animal handling and sampling procedures were conducted in compliance with Greek (PD 56/2013) and EU (Directive 63/2010) laws and regulations regarding the protection of animals used for scientific purposes. Furthermore, the aqua-laboratories of the Hellenic Centre for Marine Research are certified by the Greek Veterinary authorities for the breeding and use of animals for scientific purposes (ΕL-25- BIO-037). The use of experimental fish (juvenile greater amberjack, Seriola dumerili) was carried out according to the scientific research protocols of the Greek Veterinary authorities according to all relevant local and/or international animal welfare laws, guidelines and policies.
2.2 Experimental diets and feeding trial
Six isonitrogenous (446 g Kg−1 CP ± 1.2) and isoenergetic (22 MJ kg−1 GE ± 1.5) experimental diets (173 g Kg−1 ± 2.3 lipid content) resulting from a basal formulation were produced to containing mainly plant ingredients (25% fish meal) and supplemented with graded levels of crystalline L-lysine-HCl at the expense of wheat meal (0, 0.10, 0.21, 0.31, 0.41 and 0.52) (Table 1). Dietary ingredients were weighed according to their proportion in the formulation and thoroughly mixed, and extruded feeds (2.5 mm pellets) were produced at the Skretting Aquaculture Research Centre (SARC), Norway. Then, the feeds were shipped to the experimental facilities of HCMR in Ag. Kosmas, Athens, Greece, where they were stored in a temperature-controlled room (4℃) until use. Before the feeding trial, feed samples were taken for amino acid analysis determination and the lysine concentration in each diet was found to be 1.93 g/100 g diet (control diet; CL1.93), 2.01 g/100 g diet (CL2.01), 2.11 g/100 g diet (CL2.11), 2.15 g/100 g diet (CL2.15), 2.20 g/100 g diet (CL2.20) and 2.29 g/100 g diet (CL2.29) (Table 2).
| Diets | ||||||
|---|---|---|---|---|---|---|
| Ingredients (g/kg) | CL1.93 | CL2.01 | CL2.11 | CL2.15 | CL2.20 | CL2.29 |
| Fish meal (71%)a | 250 | 250 | 250 | 250 | 250 | 250 |
| Wheat mealb | 286.0 | 285.0 | 283.9 | 282.9 | 281.9 | 280.8 |
| Corn glutenc | 100 | 100 | 100 | 100 | 100 | 100 |
| Wheat glutend | 219.5 | 219.5 | 219.5 | 219.5 | 219.5 | 219.5 |
| Soya concentratee | 10.1 | 10.1 | 10.1 | 10.1 | 10.1 | 10.1 |
| Fish oilf | 123.3 | 123.3 | 123.3 | 123.3 | 123.3 | 123.3 |
| Lysine HClg | 0.0 | 1.0 | 2.1 | 3.1 | 4.1 | 5.2 |
| Monoammonium phosphateh | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 | 6.1 |
| Mineral & Vitamin premixi | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Analysed chemical composition of diets (g/kg or specified) | ||||||
| Crude Protein | 445.8 | 448.3 | 446.3 | 445.2 | 445.3 | 446.8 |
| Crude Fat | 176.5 | 174.7 | 172.4 | 171.9 | 170.1 | 173.8 |
| Ash | 51.4 | 53.4 | 53.1 | 52.3 | 51.6 | 51.5 |
| Moisture | 78.7 | 86.6 | 84.1 | 86.5 | 85.2 | 81.3 |
| Carbohydrate* | 247.6 | 237.0 | 242.1 | 244.1 | 247.8 | 246.6 |
| Gross energy (MJ kg−1) | 21.90 | 21.63 | 21.55 | 21.58 | 21.52 | 21.78 |
Note
- Nordsildmel, Norway.a,f, Statkorn, Norway.b, Cargill, USAc, Cerestar Scandinavia AS, Denmark.d, ADM, Holland.e, Eurolysine, France. g, Minerarira Saciliese, Italy. hVitamin and mineral supplementation to meet or exceed requirements of fish (NRC, 1993). Proprietary of Skretting Aquaculture Research Center.i
- *Calculated by difference: 100-(%protein + %fat + %ash + %moisture) (i.e., N-free extract + crude fibre).
| Diets | ||||||
|---|---|---|---|---|---|---|
| Amino acids | CL1.93 | CL2.01 | CL2.11 | CL2.15 | CL2.20 | CL2.29 |
| Essential amino acids (EAA) | ||||||
| HyPro | 0.19 | 0.19 | 0.19 | 0.17 | 0.17 | 0.18 |
| His | 0.87 | 0.87 | 0.88 | 0.88 | 0.86 | 0.88 |
| Ile | 1.63 | 1.67 | 1.64 | 1.62 | 1.63 | 1.67 |
| Leu | 3.66 | 3.69 | 3.63 | 3.64 | 3.61 | 3.72 |
| Arg | 1.93 | 1.98 | 1.96 | 1.94 | 1.92 | 1.97 |
| Met | 0.95 | 0.83 | 0.93 | 0.94 | 0.93 | 0.96 |
| Thr | 1.50 | 1.53 | 1.52 | 1.51 | 1.50 | 1.54 |
| Lys | 1.93 | 2.01 | 2.11 | 2.15 | 2.20 | 2.29 |
| Phe | 2.02 | 2.06 | 2.01 | 2.00 | 2.00 | 2.06 |
| Val | 1.82 | 1.86 | 1.83 | 1.81 | 1.81 | 1.86 |
| Non-essential amino acids (NEAA) | ||||||
| Ala | 2.12 | 2.14 | 2.16 | 2.13 | 2.11 | 2.16 |
| Pro | 3.60 | 3.60 | 3.52 | 3.53 | 3.55 | 3.68 |
| Cys | 0.33 | 0.33 | 0.32 | 0.32 | 0.32 | 0.33 |
| Tyr | 1.23 | 1.27 | 1.22 | 1.22 | 1.22 | 1.26 |
| Gly | 1.98 | 2.03 | 1.99 | 1.97 | 1.97 | 2.02 |
| Asp + Asn | 2.93 | 2.96 | 3.05 | 2.96 | 2.93 | 3.00 |
| Glu + Gln | 10.93 | 10.84 | 10.91 | 10.78 | 10.84 | 11.23 |
| Tau | 0.14 | 0.14 | 0.14 | 0.14 | 0.13 | 0.14 |
| Ser | 2.08 | 2.10 | 2.09 | 2.08 | 2.06 | 2.13 |
Juveniles of greater amberjack were obtained from a broodstock which was reproduced in captivity at the Institute of Marine Biology, Biotechnology and Aquaculture, HCMR, Crete, Greece. Once fish were transferred to the HCMR’s facility in Agios Kosmas, Athens, they acclimated to the experimental rearing condition for a period of 2 weeks. Then, the individuals (32.8 g ± 3.0) were randomly allocated to 18 trial cages (1.0 m × 1.5 m × 1.5 m), and each was stocked with 25 fish (3 replicate cages per dietary treatment). The rearing experimental conditions were according to Kotzamanis et al. (2018) with slight modifications. Briefly, the cages were placed in two large rectangular concrete tanks (36-m3 water capacity), suspended around 20 cm from tank's bottom and were continuously supplied with filtered seawater (salinity 35 ppt). Moreover, a piece of tarp was placed inside and perimetrically in each cage, ten centimetres above and below the sea water level to avoid pellets escape from one cage to the other. Seawater was channelled into each 36-m3 tank from 10 different pipes at 400 L/h and aerated using stone diffusers to maintain oxygen saturation above 80%. Water temperature throughout the experimental period was in average of 19.8℃ ± 1.7. The photoperiod was in line with the natural cycle of the season, the oxygen level was kept close to saturation (>7.5 mg L−1), the pH of the seawater ranged between 6.7 and 7, and the levels of total ammonia (<0.3 ppm), nitrites (<0.2 ppm) and nitrates (<30 ppm) levels were photometrically recorded twice a week. The fish were hand-fed with the experimental diets twice daily (09:00 h and 15:00 h) to apparent satiation, 6 days a week for 56 days. The remaining feed was collected by siphoning and weighed to monitor daily feed consumption. Five fish were randomly collected from the initial fish population, sacrificed using an overdose of anaesthesia (2-phenoxyethanol; Pharmaqua, Athens, Greece), minced and freeze dried, and the initial whole-body composition determined.
2.3 Proximate composition and amino acid analysis
Diets and fish samples from each dietary group at the end of the trial, as well as the initial fish samples, were analysed for the proximate composition according to the AOAC (1995) protocol. Ten fish were randomly collected from each tank and pooled (30 fish per treatment) for whole-body analysis. The amino acid composition of the diets was carried out according to Kotzamanis et al. (2018). Briefly, feeds after acid hydrolysis (6N, 110℃, 24 h), were derivatized by AccQ-Tag™ Ultra according to the amino acid analysis application solution (Waters Corporation) (Table 2). DL-Norvaline (Sigma) 2.5 mM was used as an internal standard. Ultra-performance liquid chromatography (UPLC) was performed on an Acquity system (Waters Corporation) and equipped with a photodiode array (PDA) detector, and the detection wavelength was set at 260 nm. The column used was the Ethylene-bridged hybrid (BEH) C18 column (100mm × 2.1 mm i.d., 1.7 μm) from Waters Corporation, Milford, MA, USA The flow rate was 0.7 ml/min, and the column temperature was kept at 55ºC. Peak identification and integration were performed by the software Empower v.2.0 (Waters Corporation) using an amino acid hydrolysate standard (Waters, USA) as an external standard. All analyses were in duplicate. When the standard deviation of the mean values between replicates was >5%, then a new duplicate analysis was performed. Tryptophan was not quantified as a result of its susceptibility to acid hydrolysis, while cysteine reacts with cysteine, forming a disulphide bridge to produce cystine. Additionally, during the acid hydrolysis procedure, asparagine is converted to aspartate and glutamine to glutamate, so the presented values for these amino acids (Asx and Glx) constitute the sum of both amino acids.
2.4 Sample collection and analysis
Five fish per tank (15 fish per treatment) were slightly anesthetized with 2-phenoxyethanol (250 mg/L), and the blood sample was collected from the caudal vein and transferred to a centrifuge tube. Following centrifugation at 3,000 × g for 10min at 4℃, the serum samples were collected into a plain tube for biochemical analyses using an automated analyser (Flexor E; Vital Scientific, Holland). The parameters analysed were aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose and total protein, using commercial kits (Sentinel Diagnostics), and the protocol was as described by the manufacturer. The liver and mid-intestine of the sampled fish were removed to determine the catalase (CAT) enzyme activity and the expression of heat shock proteins (HSP 70 and HSP 90).
The catalase (CAT, EC 1.11.1.6) activity was assayed following the methods of (Cohen et al., 1970) as described in Antonopoulou et al. (2020). Briefly, the assay mixture contained 50 mmol l−1 phosphate buffer of 7.4 pH and tissue extract. The reaction was initiated by adding 300 μl of 30 mmol L–1 H2O2, while activity was established following changes in H2O2 absorbance at 240 nm (extinction coefficient ε = 0.0394 mM−1 cm−1) and expressed as μ moles of substrate/min/mg protein.
2.5 Growth performance and survival
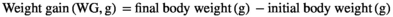
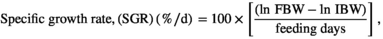
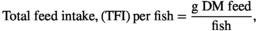
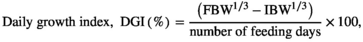
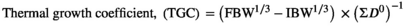
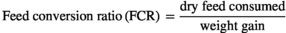
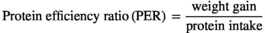
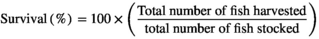
2.6 SDS-PAGE and Immunoblot analysis
Frozen tissue samples (liver and mid-intestine) were homogenized in 3 ml/g of cold lysis buffer (20 mM β-glycerophosphate, 50 mM NaF, 2 mM EDTA, 20 mM Hepes, 0.2 mM Na3VO4, 10 mM benzamidine, pH 7, 200 μM leupeptin, 10 μΜ trans-epoxy succinyl-L-leucylamido-(4-guanidino) butane, 5 mM dithiotheitol, 300 μΜ phenyl methyl sulfonyl fluoride (PMSF), 50 μg/ml pepstatin, 1% v/v Triton X-100) as described in Antonopoulou et al. (2014). Thereafter, the homogenized samples were extracted on ice for 30 min and centrifuged at 10,000 x g for 10 min at 4℃. The supernatants were boiled with 0.33 volumes of SDS/PAGE sample buffer (330 mM Tris-HCl, 13% v/v glycerol, 133 mM DTT, 10% w/v SDS, 0.2% w/v bromophenol blue). Protein concentrations were determined using the BioRad protein assay. Equal amounts of proteins (50 μg) were separated in 10% (w/v) acrylamide and 0.275% (w/v) bis-acrylamide slab gels and electrophoretically transferred to nitrocellulose membranes (0.45 μm, Schleicher & Schuell, Keene H. 03431, USA). Non-specific binding sites on the membranes were blocked with 5% (w/v) non-fat milk in Tris-Buffered Saline Tween 20 (TBST) (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 0.1% (v/v) Tween 20) for 30 min at room temperature. All nitrocellulose membranes were dyed with Ponceau S stain to ensure both good transfer quality and equal protein loading. Subsequently, the membranes were incubated overnight with the appropriate primary antibodies. The antibodies used were as follows: monoclonal mouse anti-heat shock protein, 70 kDa (Cat. No. H5147, Sigma, Darmstadt, Germany), monoclonal mouse anti-heat shock protein, 90 kDa (Cat. No. H1775, Sigma, Darmstadt, Germany) and rabbit β-actin protein, 45 kDa (Cat. No. 4967; Cell Signaling, Beverly, MA, USA). After washing with TBST (3 periods, 5 min each time), the blots were incubated with horseradish peroxidase-linked secondary antibodies and washed again with TBST (3 periods, 5 min each time), and the bands were detected using enhanced chemiluminescence (Chemicon) with exposure to Fuji Medical X-ray films. Blots were quantified by Image Studio Lite, LI-COR Biosciences.
2.7 Statistical analyses
Data were tested for normality and homogeneity of variance before been subjected to one-way ANOVA using Kolmogorov–Smirnov and Levene tests respectively. Significant differences between means were determined using Tukey's test. The level of significance was set at p < .05. All statistical tests were performed using the general linear model (STATISTICA version 12.0, StatSoft). The breakpoint for lysine (Lys) concentration was estimated by using the broken-line regression method of Robbins et al. (1979). The data from WG and Lys concentrations were modelled using the piecewise linear regression with breakpoint (STATISTICA version 12.0, StatSoft). The model was estimated using least squares: y = (b01 + b11 *x1 +...+bm1 *xm) *(y ≤ bn) + (b02 +b12 *x1 +...+bm2 *xm)*(y > bn). In order to estimate changes in the expression of HSP70 and HSP90 in the liver and in the intestine between the different dietary groups, Kruskal–Wallis was performed for significance at the 5% level and post hoc comparisons were performed using the Dunns’ post hoc test.
3 RESULTS
3.1 Growth performance and survival
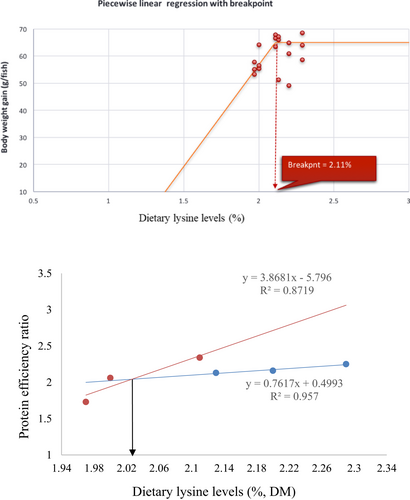
The growth response indices of greater amberjack fed different levels of lysine are shown in Table 3. The results showed that the fish fed the CL2.11 diet had a numerically higher weight gain, DGI, SGR TGC and PER, but those values were not statistically different from other dietary groups (p > .05) (Table 3). The results of the pairwise linear regression of WG against lysine levels showed that the dietary lysine requirements corresponding to 86% for maximum response of weight gain of greater amberjack juvenile were 2.11% of the diet (Figure 1). However, the broken-line regression analysis of PER showed a breakpoint at 2.03% of dietary lysine (Figure 2). All diets were well accepted by fish and the total feed intake, PER and FCR values among the dietary treatments were found to be unaffected (p > .05). The survival of fish in all dietary treatments ranged from 88% to 98%, with the CL2.15 diet group showed the highest mortality (Table 3).
| Diets | ||||||
|---|---|---|---|---|---|---|
| CL1.93 | CL2.01 | CL2.11 | CL2.15 | CL2.20 | CL2.29 | |
| Initial body weight (g) | 32.77 ± 0.55 | 32.90 ± 0.40 | 33.00 ± 0.61 | 32.57 ± 0.40 | 32.77 ± 0.55 | 32.67 ± 0.23 |
| Final Body weight (g) | 88.11 ± 1.86 | 91.61 ± 5.18 | 98.97 ± 2.91 | 93.98 ± 3.25 | 91.07 ± 3.62 | 96.35 ± 3.65 |
| WG | 55.35 ± 2.31 | 58.71 ± 4.79 | 65.97 ± 2.32 | 61.41 ± 4.78 | 58.31 ± 4.28 | 63.68 ± 4.96 |
| DGI % | 2.32 ± 0.08 | 2.41 ±0.13 | 2.63 ± 0.05 | 2.50 ± 0.26 | 2.40 ± 0.26 | 2.57 ± 0.16 |
| SGR | 1.83 ± 0.07 | 1.90 ± 0.08 | 2.03 ± 0.02 | 1.96 ± 0.17 | 1.89 ± 0.18 | 2.00 ±0.10 |
| TGC × 1,000 | 1.16 ± 0.04 | 1.22 ± 0.07 | 1.33 ± 0.02 | 1.26 ± 0.13 | 1.21 ± 0.13 | 1.30 ± 0.08 |
| TFI | 72.8 ± 2.59 | 71.0 ± 3.41 | 79.3 ± 7.41 | 82.3 ± 7.45 | 75.9 ± 4.29 | 79.5 ± 10.14 |
| FCR | 1.25 ± 0.05 | 1.21 ±0.06 | 1.19 ± 0.05 | 1.22 ± 0.10 | 1.27 ± 0.11 | 1.22 ± 0.04 |
| PER | 1.73 ± 0.05 | 2.06 ± 0.09 | 2.34 ± 0.14 | 2.13 ± 0.46 | 2.16 ± 0.28 | 2.25 ± 0.11 |
| Survival | 93.47 ±6.6 | 98.20 ± 0.10 | 97.44 ± 4.44 | 88.62 ± 15.15 | 95.56 ±7.70 | 97.78 ± 3.85 |
Note
- Data are presented as means ± SD (n = 3). Row means that have no superscript in common are significantly different from each other (Tukey's HSD, p < .05).
- Abbreviations: DFI, daily growth index (%);DGI, daily growth index; FCR, feed conversion ratio; PER, protein efficiency ratio; SGR, specific growth rate; TFI, total feed intake (g) per fish; TGC, thermal growth coefficient; WG, weight gain (g/fish).
3.2 Whole-body composition
The result of the whole-body composition of the fish fed the experimental diets is shown in (Table 4). Body protein, moisture and ash content were not significantly affected by the dietary treatments (p > .05). However, fish fed the CL1.93 and CL2.11 diets showed a body lipid content similar to CL2.01, CL2.20 and CL2.29, but significantly higher (p < .05) compared with the CL2.15 diet group (p < .05; Table 4).
| Diets | ||||||
|---|---|---|---|---|---|---|
| CL1.93 | CL2.01 | CL2.11 | CL2.15 | CL2.20 | CL2.29 | |
| Moisture | 70.9 ± 1.2 | 72.8 ± 0.9 | 70.8 ± 0.9 | 71.6 ± 0.5 | 72.3 ± 1.0 | 72.0 ± 1.3 |
| Crude Protein | 15.4 ± 0.3 | 14.7 ± 0.4 | 15.5 ± 0.5 | 15.5 ± 0.1 | 15.1 ± 0.6 | 15.2 ± 0.9 |
| Crude Lipid | 8.8 ± 1.0 a | 7.7 ± 0.5 ab | 8.9 ± 0.1 a | 7.4 ± 0.3 b | 7.6 ± 0.4 ab | 8.1 ± 0.3 ab |
| Ash | 3.4 ± 0.0 | 3.2 ± 0.1 | 3.2 ± 0.3 | 3.3 ± 0.1 | 3.2 ± 0.0 | 3.1 ± 0.1 |
| Gross energy (MJ kg−1) | 7.38 | 6.80 | 7.46 | 6.97 | 6.89 | 7.08 |
Note
- Initial whole-body composition: moisture (75.9%), crude protein (15.5%), crude lipid (3.6%), ash (4.1%). Data are presented as means ± SD. Different superscripts in row means indicate statistically significant differences (Tukey's HSD, p < .05).
3.3 Blood chemistry parameters
The results in Table 5 showed that there were no significant differences (p > .05) observed in the total protein, glucose, ALT and AST activities in fish fed different level of lysine. Although no significant differences were observed between the groups, fish fed the CL1.93, CL2.01 and CL2.11 diets recorded the lowest serum glucose concentration.
| Diets | ||||||
|---|---|---|---|---|---|---|
| CL1.93 | CL2.01 | CL2.11 | CL2.15 | CL2.20 | CL2.29 | |
| Metabolites | ||||||
| Total protein (g/dl) | 3.1 ± 0.1 | 2.9 ± 0.3 | 3.1 ± 0.1 | 3.0 ± 0.2 | 2.9 ± 0.2 | 2.9 ± 0.3 |
| Glucose (mg/dl) | 179.7 ± 10.0 | 173.6 ± 14.3 | 175.4 ± 18.1 | 191.8 ± 21.3 | 195.9 ± 17.7 | 185.7 ± 20.4 |
| Enzymes | ||||||
| ALT (U/l) | 212.4 ± 24.1 | 189.8 ± 29.6 | 190.5 ± 23.3 | 181.9 ± 18.7 | 189.0 ± 19.5 | 181.8 ± 22.3 |
| AST (U/l) | 162.9 ± 33.2 | 200 ± 28.2 | 164.3 ± 28.0 | 172.8 ± 29.2 | 205.3 ± 35.3 | 173.5 ± 31.7 |
Note
- Data are presented as means ± SD. Different superscripts in row means indicate statistically significant differences (p < .05).
- Abbreviations: ALT, alanine aminotransferase (GPT); AST, aspartate aminotransferase (GOT).
3.4 Catalase activity and HSPs expression
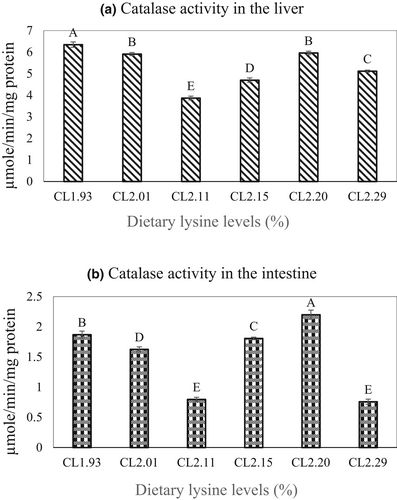
The specific activity of CAT in the greater amberjack was significantly (p < .05) lower in the liver of fish fed the CL2.11 diet compared with the higher value recorded for the other dietary treatments (Figure 2a), whereas CAT activity in the intestine of groups fed CL2.20 was found to be higher compared with the lowest value recorded for fish fed CL2.11 and CL2.29 (p < .05) (Figure 2b).
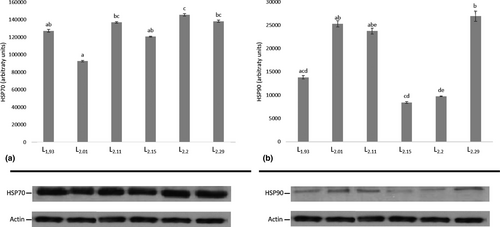
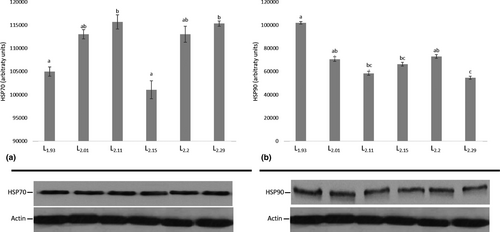
Regarding HSP induction, the results for HSP70 and HSP90 showed a differential expression profile in both the liver and the intestine between the different dietary groups (Figure 3 and Figure 4). The highest HSP70 levels in the liver were found in CL2.20 group, whereas the lowest in the CL2.01 group and whereas the other dietary groups remained similar (Figure 3a). The highest hepatic HSP90 levels were found at CL2.01, CL2.11 and CL2.29 compared with CL1.93, CL2.15 and CL2.20 (Figure 3b). In the intestine, HSP70 lowest levels were found in CL1.93 and CL2.15 (Figure 4a), whereas the highest expression levels of HSP90 were recorded in CL1.93 dietary group (Figure 4b).
4 DISCUSSION
Lysine is the first limiting essential amino acid in many plant protein sources used in fish feeds, so synthetic lysine is commonly added to a practical fish feed that is based mainly on plant protein ingredients to meet the fish requirements (NRC, 2011). To the best of our knowledge, this is the first study to determine the lysine requirement of juvenile greater amberjack (Seriola dumerili). In this investigation, lysine dietary supplementation did not significantly improve growth performance; however, a numerically higher weight gain was observed in the CL2.11 dietary group. Based on the piecewise linear regression of WG and broken-line regression of PER, the dietary lysine requirement of juvenile greater amberjack was found to be between 2.03% and 2.11% of the diet, corresponding to 4.55% and4.73% of dietary protein and falls within the range reported in several other species (NRC, 2011). The values recorded in the current study (4.55%–4.73% of dietary protein) are similar to those reported for European sea bass fingerlings—4.82% (Tibaldi & Lanari, 1991), grass carp—4.73% (Li et al., 2016) but higher than those of rainbow trout—3.71% (Kim et al., 1992), sub-adult grass carp—3.89% (Li et al., 2014), Atlantic salmon—3.98% (Anderson et al., 1993), although lower than those reported for gilthead sea bream—5.04% (Marcouli et al., 2006), meagre—5.94% (Kotzamanis et al., 2018), juvenile silver pompano—5.71%–5.83% (Ebeneezar et al., 2019), juvenile dusky kob—7.35% (Adesola et al., 2018) and African catfish—5.7% (Fagbenro et al., 1998). The results of the current study showed that dietary lysine requirement of the great amberjack (Seriola dumerili) was higher than those previously reported for Japanese amberjack (Seriola quinqueradiata) (4.13% of the dietary protein) (Ruchimat et al., 1997), another member of Seriola family. The variance observed among the different fish species could be related to species-specific differences, age, protein source and content of the diet, domestication, genetics, etc. Hua (2013) deduced through a multilevel analysis that the dietary protein content of a diet has a significant effect on the lysine requirement of fish, while Kotzamanis et al. (2018) underlined the noticeable role of the dietary level of fishmeal in determining the optimal dietary lysine needs of fish. In addition, Lee et al. (2020) found that the lysine requirement of rainbow trout significantly differs by strain.
Lysine supplementation has been reported to enhance whole-body protein and reduces lipid content in different fish species (Ebeneezar et al., 2019; Lee et al., 2020; Ruchimat et al., 1997). However, in the current study, no significant difference was found in the whole-body protein content similar to the observation of Deng et al. (2011). Deficiency of lysine has been observed to cause an increase in body lipid deposition in several fish species such as rainbow trout (Lee et al., 2020), adult Lambari (Campelo et al., 2018), silver pompano (Ebeneezar et al., 2019), juvenile Cobia (Zhou et al., 2007) and Japanese amberjack (Ruchimat et al., 1997). However, in the current study, both fish fed the low and high dietary lysine levels had an increased lipid deposition. Furthermore, it was observed that at a higher dietary level above the optimum requirements (2.11% of dry diet), the whole-body lipid deposition was significantly reduced. Similarly, Ahmed and Khan (2004) recorded a higher body lipid deposition at optimum dietary lysine level for Cirrhinus mrigala.
Blood parameters are important tools often used for the evaluation of physiological stress response, general health conditions and welfare of fish towards nutritional and environmental changes (Congleton & Wagner, 2006; Fawole et al., 2020; Maita et al., 2002; Peres et al., 2013). In the present study, dietary lysine supplementation did not significantly affect serum total protein and glucose concentration, regardless of the level fed. However, Zhou et al. (2007) reported that cobia fed increasing levels of lysine tended to have higher total protein and glucose than those subjected to low lysine diets, which is consistent with our findings for glucose. Transaminases (ALT, AST) are liver-specific enzymes that catalyse the exchange of amino groups and characterize liver function. Elevated levels of blood transaminases are noted in the case of liver diseases or during the feeding of fish related to increased processing of energy substrates by the liver, leading to increased transmembrane transport of ions and water, the elevation of hepatic enzymes activities and increased outflow of the enzymes into the blood (Congleton & Wagner, 2006). In this study, dietary lysine levels did not affect significantly the activity of AST and ALT enzymes, which indicate that the organ integrity was not hampered as a result of feeding incremental levels of lysine. In contrast, Campelo et al. (2018) observed an increase in serum levels of AST and ALT in Astyanax altiparanae fed the highest levels of lysine (1.5%–2.3%), and assumed that this was due to the increased deamination of excess lysine for energy production (Stone et al., 2003).
The antioxidant capacity was examined by determining the liver and intestine catalase (CAT) enzyme activity of the fish. CAT is an important enzyme in the protection of cell from oxidative damage by reactive oxygen species (ROS), detoxifies excess of hydrogen peroxide, which is a harmful by-product of many normal cellular metabolic processes, into the less-reactive oxygen and water molecules, to prevent from damaging the cells and tissues being protected from oxidative stress. According to Li et al. (2016), increased antioxidant activity may be correlated with the protective ability of dietary lysine against oxidative damage, and they reported that lysine supplementation increased the activity of the CAT enzyme in the intestine of grass carp. Li et al. (2014) reported a similar increase in CAT activity when lysine-based diets were fed to sub-adult grass carp. However, in the work of Li et al. (2014), it was observed that a higher level of dietary lysine beyond 10.8 g/kg resulted in decreased intestinal CAT activity. They believed that dietary lysine could increase antioxidant capacity in fish. In contrast, in the current work, we recorded a significantly reduced CAT level in the liver and the intestine of greater amberjack with an increase in the level of lysine to 2.11% and this corresponds to the optimal level for growth, thus pointing to a likely protection mechanism of lysine in this dose. However, this effect was not found in fish fed other diets with higher concentrations of lysine supplementation. Furthermore, in the intestine, a significant decrease in CAT was found in both the medium (2.11%) and higher level (2.29%) of lysine supplementation. The reason for this variation in comparison with other studies remains unclear, and this needs to be further investigated, as little information is available on this subject in fish.
Molecular responses are evaluated by examining the protein expression of heat shock proteins (HSP70 and HSP90) in the liver and intestine. HSPs proteins are molecular chaperones with roles to vital cell functions. Concerning HSPs, the results on HSP70 and HSP90 showed a differential expression profile within the same tissue (liver or intestine) between the different dietary groups. The lowest levels of HSP90 were found in the 2.15 and 2.20 dietary groups in the liver and the lowest levels of HSP70 in the intestine of the 2.15 group. Therefore, a tissue-specific response is apparent. Although much research has been done on the expression of HSPs, their role in nutrition and metabolism is not fully understood. In fish, HSPs are expressed in different amounts in various tissues and cells and are often used as indicators of cellular stress, tolerance and health status (Iwama et al., 1999), and can be induced by various stress factors, both abiotic such as temperature, or biotic such as starvation and refeeding (Antonopoulou et al., 2013) or a combination of them (Antonopoulou et al., 2020), or different dietary supplementations (Antonopoulou et al., 2014; Feidantsis et al., 2014). The insignificant effects noticed in the expression of HSPs in the current study could be due to the influx of free amino acids from the diets for protein synthesis as reported by (Hendrick & Hartl, 1993; Ronnestad et al., 1999), who they proposed that more HSPs will be required for a different aspect of protein metabolism including repair or translocation of newly synthesized protein caused by the availability of free amino acids from food. Thus, it seems that the supplemental lysine might have increased protein synthesis, thereby resulting in an increased level of HSPs in the greater amberjack. This is similar to what was reported also in European sea bass fed a taurine-enriched diet (Feidantsis et al., 2014).
5 CONCLUSION
In conclusion, the results of this study showed that the dietary lysine requirements, based on the broken-line model, which can support maximum weight gain and protein utilization in amberjack juveniles fed a diet that contained mainly a blend of plant ingredients and 250 g/kg of fish meal were between 2.03% and 2.11% of the diet (4.55%–4.73% of dietary protein). Lysine supplementation was found to affect the specific activity of catalase enzyme in both the liver and intestine of greater amberjack fed the diet containing 2.11% dietary lysine. The data presented in the current study could be useful in developing balanced commercial diets for intensive culture of greater amberjack (Seriola dumerili), particularly when fishmeal is replaced by plant protein blends. Evaluation of other EAA requirements of this fish species should also be conducted.
ACKNOWLEDGEMENT
This work was supported by the European Union's Seventh Framework Programme for Research, Technological Development, and Demonstration project DIVERSIFY [7FP-KBBE, grant number 603121].
CONFLICT OF INTEREST
The authors declare that the research was conducted with no commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




