Effects of bile acids on the growth performance, lipid metabolism, non-specific immunity and intestinal microbiota of Pacific white shrimp (Litopenaeus vannamei)
Abstract
Effects of dietary bile acids on the growth performance, lipid metabolism, non-specific immunity and intestinal microbiota of Pacific white shrimp (Litopenaeus vannamei) were studied. Five diets were formulated to contain 0, 0.1, 0.2, 0.3 and 0.5 g kg−1 (control, BA1, BA2, BA3 and BA4) of bile acids, respectively. Shrimp (1.09 ± 0.04 g) were assigned randomly to 15 tanks (400 L, three tanks in each group, 60 shrimp in each tank) for 60 days. Results indicated that BA2 group had the highest final weight and weight gain. The contents of triglyceride decreased significantly with increasing bile acids level (p < .05). The contents of high-density lipoprotein and glutathione, total antioxidant capacity as well as protease, lipase and superoxide dismutase activities in BA2-4 groups increased significantly (p < .05). Additionally, bile acids enhance the immune response of shrimp through NF-κB-mediated signalling pathway and 0.5g kg−1 bile acids could cause oxidative damage to shrimp. High-throughput sequencing analysis revealed that the intestinal microbiota diversity and richness were significantly altered by bile acids, and functional genes associated with metabolism and bile acids biotransformation were strongly activated in BA2 and BA4 groups. Together, we recommend the shrimp diet supplemented with bile acids at the level of 0.2 g kg−1.
1 INTRODUCTION
Currently, the yield of shrimp, especially pacific white shrimp (Litopenaeus vannamei), has been steadily increased worldwide (FAO, 2020). In order to meet the yield demand, practices based on the increased stocking density and excessive overfeeding have become the most commonly poor management practices (Limsuwan, 2010), while these practices could result in the imbalance of nutrition and the degradation of culture environment, which not only increase the burden of hepatopancreas in shrimp but also increase the susceptibility of shrimp to diseases. Recently, adding functional supplements into feeds has been gradually developed into a general method to promote the health status of shrimp (Hoseinifar et al., 2016; Zhou et al., 2020). Specifically, owing to the simple biodegradation and relative safety, natural bioactive substances extracted from animals or plants are considered as significant functional supplements, which have a great application potential in aquaculture practice (Bu et al., 2020).
Bile acids as endogenous molecules synthesized from cholesterol in vertebrates (Reschly et al., 2008) are regarded as pleiotropic mediators that regulate multiple physiological processes and mediate the utilization for different nutrients. From an evolutionary perspective, the presence of bile acids in vertebrates and their absence in invertebrates are deemed as a major innovation from invertebrates to vertebrates (Nes, 2012). By virtue of their amphipathic structure, bile acids are capable of solubilizing lipids by forming micelles, thus enhancing the digestion of fats, cholesterol and lipid-soluble vitamins (Alrefai & Gill, 2007). Besides, bile acids could also function as hormones or signalling molecules, performing pleiotropic activities by activating nuclear hormone receptors. It has been clarified that bile acids could take part in the regulation of the antioxidant defence (Ljubuncic et al., 2000; Mitsuyoshi et al., 1999) and immune response (D'Aldebert et al., 2009) of mammals. Previous research also proved that dietary supplementation of bile acids could exert obviously beneficial effects on fish, including the better growth performance, promoted feed conversation ratio, enhanced liver function, higher digestive enzyme activity and alleviated stress response (Alam et al., 2001; Ding et al., 2020; Guo et al., 2020; Jiang et al., 2018; Liao et al., 2020; Maita et al., 1996; Zhou et al., 2018). Whereas, effects of dietary bile acids on invertebrates including shrimp without the ability to synthesize bile acids de novo have not been reported (Teshima, 1971), and whether bile acids could regulate the detoxification system, antioxidant defence and immune response of shrimp is totally unknown.
Intestinal microbiota considered as the metabolic ‘organ’ could participate in the digestion or absorption of food to improve the growth performance of host and produce numbers of metabolites or signalling molecules to regulate the physiological function of host (Wahlström et al., 2016). Bile acids have been perceived as the main regulators of intestinal microbiota by means of preventing the metabolism of bile acids-sensitive microorganisms and stimulating the metabolism of bile acids-metabolizing microorganisms (Ridlon et al., 2014). In return, intestinal microbiota could alter the physicochemical properties and increase their diversity through a complex biological transformation process. The resulting secondary bile acids could bind to and activate more receptors to a greater extent than primary bile acids (Kawamata et al., 2003). Recently, the relationship between bile acids and intestinal microbiota of mammals or fish has been widely investigated (Islam et al., 2011; Zheng et al., 2017). Nevertheless, information regarding how dietary bile acids affect intestinal microbiota of invertebrates is scarce. Besides, given that vertebrates could synthesize bile acids, researches on bile acids in vertebrates either used bile sequestrants or ligated bile ducts, or took the effects of their own bile acids into account. Consequently, an obvious advantage of conducting experiments conducted in invertebrates to figure out the effects of bile acids on intestinal microbiota is that there is no need to consider the ‘interference’ of the body's own bile acids synthesis. In this case, studies in invertebrates may help us cognize the effects of bile acids on the intestinal microbiota more accurately and more comprehensively.
Based on the above background, the experiment was carried out to investigate whether dietary supplementation of bile acids could promote growth performance, lipid metabolism and non-specific immunity of shrimp for the first time and discuss the optimal supplement of bile acids in shrimp diets. Besides, the effects of bile acids on the intestinal microbiota of shrimp were revealed. Moreover, biotransformation process of bile acids through intestinal microbiota was preliminarily analysed as well. The findings will help us to further understand the metabolic mechanism of bile acids in invertebrates, which could shed lights on the development of feed supplements of shrimp.
2 MATERIALS AND METHODS
2.1 Preparation of experimental diets
Diet formulations were shown in Table 1. The bile acids were supplemented at the levels of 0, 0.1, 0.2, 0.3 and 0.5 g kg−1, and corresponding diets were prepared as groups of control, BA1, BA2, BA3 and BA4. In brief, all the dry components were smashed, weighed and then mixed; after that, fish oil and bile acids were added and fully mixed with the above dry components for about 20 min. Subsequently, after the addition of the proper amount of distilled water, all the above ingredients were mixed for about 15 min to form soft dough. A dough with even consistency was extruded as pellets (1.2 mm). All diets were air-dried at 50℃. Diets from each group were stored at −20℃ before usage.
| Ingredient | Diets | ||||
|---|---|---|---|---|---|
| Control | BA1 | BA2 | BA3 | BA4 | |
| Fish meal | 270 | 270 | 270 | 270 | 270 |
| Soybean meal | 210 | 210 | 210 | 210 | 210 |
| Peanut meal | 190 | 190 | 190 | 190 | 190 |
| Wheat flour | 263 | 262.9 | 262.8 | 262.7 | 262.5 |
| Fish oil | 25 | 25 | 25 | 25 | 25 |
| Vitamin C ester (35%) | 1 | 1 | 1 | 1 | 1 |
| Ca(H2PO4)2 | 10 | 10 | 10 | 10 | 10 |
| Choline chloride (50%) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Soybean phospholipid | 20 | 20 | 20 | 20 | 20 |
| Cholesterol | 1 | 1 | 1 | 1 | 1 |
| Vitamin premixa | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Mineral premixb | 5 | 5 | 5 | 5 | 5 |
| Bile acidsc | 0 | 0.1 | 0.2 | 0.3 | 0.5 |
| Proximate analysis | |||||
| Crude protein | 386.75 | 385.83 | 382.27 | 385.63 | 389.36 |
| Crude lipid | 73.93 | 73.46 | 75.92 | 77.22 | 78.81 |
- a Vitamin premix contains the following g kg−1 premix: VA 8,000,000 IU, VB1 5g, VB2 15 g, VB6 8g, VB12 40 g, VD 2,000,000 IU, VE 30 g, VK 10 g, calcium pantothenate 25 g, folic acid 2.5 g, biotin 0.08 g, nicotinic acid 40 g, inositol 100 g.
- b Mineral premix contains the following g kg−1 premix: MgSO4·H2O 12 g, KCl 90 g, Met-Cu 3 g, Met-Co 0.16 g, FeSO4·H2O 1 g, ZnSO4·H2O 10 g, Ca(IO3)2 0.06 g, NaSeO3 0.0035 g.
- c Bile acids: hyodesoxycholic acids 67.52%, chenodeoxycholic acids 19.81%, hyocholic acids 8.60%; Longchang Animal Health Products Co., Ltd. Jinan, China.
2.2 Animal culturing
Shrimps were purchased from a shrimp farm, located in Qingdao, China. During the acclimation stage for 14 days, shrimps were fed with diets without bile acids. Then, shrimps (1.09 ± 0.04 g) were randomly assigned to the tank (400 L, three tanks in each group, 60 shrimp in each tank), respectively. All groups were fed at a rate of 5–7% of the biomass for three times every day (8:00, 16:00 and 24:00) till obvious satiation, and one third of water in tanks was exchanged each day. During the experiment period, the water temperature, pH, salinity and dissolved oxygen were maintained at 26.8–27.3℃, 7.8–8.1, 31‰ and 6.2–6.6 mg L−1, respectively.
2.3 Sample collection
Shrimp of every tank were randomly selected for sampling according to Su et al., 2020. Briefly, haemolymph (0.25 ml) of each shrimp was drawn from central sinus with the sterilized syringe containing the anticoagulant of 0.25 ml and then centrifuged at 800 × g for 10 min. The supernatants were kept at −80℃ for the determination of parameters related to immune response and serum biochemical indexes. The whole intestine and hepatopancreas of every shrimp were sampled, placed into liquid nitrogen and kept at −80℃ before usage. The intestines were divided into two parts; specifically, intestines of ten shrimps were used for intestinal microbiota analysis, and intestines of the other shrimp were used for the RNA extraction. After ground, hepatopancreas was homogenized in phosphate buffer on ice bath, which was subsequently centrifuged at 3000 g for 10 min and then 13,000 g for 30 min at 4℃, and the supernatants (plasma) were kept at −80℃ for the determination of activities of enzymes.
2.4 Survival and growth
The weight of shrimp was measured on day 0 and day 60. Survival rate (%), weight gain (%), specific growth rate (SGR) and feed conversion rate (FCR) were calculated according to the following formulas:
Survival rate (%) = (Number of total shrimps -Number of dead shrimps)/ Number of total shrimps × 100.
Weight gain (WG) (%) = (Weight of day 60 - Weight of day 0) / Weight of day 0 × 100.
Specific growth rate (SGR) (%/d) = 100 × (Ln weight of day 60 - Ln weight of day 0) / Duration of experiment days.
Feed conversion rate (FCR) = feed consumed / weight gain.
2.5 Assessment of immune response
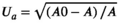
2.6 Assessment of enzyme activity
The activities of amylase, lipase and protease were presented as specific activity (U/mg protein), and one unit (U) was defined as 1 μg of maltose, fatty and acid tyrosine released per min, respectively. The activity of amylase was measured with the soluble starch as substrate based on the 3,5-dinitrosalicylic acid colorimetric method (Qing & Xing, 1997). The activity of lipase was assayed with the olive oil as substrate in line with the method of JIANG et al., 2007. The activity of protease was determined with the casein as substrate in terms of the method reported by Qing & Xing, 1997. The concentration of soluble protein was determined using the method of Lowry et al., 1951.
The activity of superoxide dismutase (SOD) was determined on the basis of the capability of SOD to inhibit the auto-oxidation of pyrogallol, which was measured at the absorbance of 325 nm (Marklund & Marklund, 1974). The content of glutathione (GSH) was spectrophotometrically assayed by means of the method elucidated by (Monostori et al., 2009). Total antioxidant capacity (T-AOC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were measured following commercial kits’ instructions (Nanjing Jiancheng Bioengineering Institute, China).
2.7 Assessment of gene expression
RNAiso Plus reagent (TaKaRa, Dalian, China) was used to extract RNA from intestines of shrimp, and the genomic DNA was removed by RNase-free DNase (TaKaRa, Dalian, China). Afterwards, electrophoresis with 1% agarose gel and A260/A280 ratio were used to evaluate the integrity, quantity and purity of the extracted RNA. Subsequently, RNA samples were reversely transcribed by Prime Script RT-PCR Kit (TaKaRa, China). Expressions of the four immune-related genes, including Dorsal, Relish, penaeidins (Pen) and crustins (Cru) were analysed by means of real-time quantitative PCR (qPCR); besides, β-actin gene was selected as the housekeeping gene due to its lowest variation in the present study. Primers of genes of β-actin, Dorsal, Relish, Pen and Cru were presented in Table 2. The qPCR mixture was performed on a 96-well rotor, and the amplification was carried out in 10 systems consisted of 3.6 μl deionized water, 0.2 μl of each primer, 5.0 μl SYBR Green master Mix (2×) and 1.0 μl diluted cDNA. The cycling conditions were as follows: denaturation programme (95℃ for 3 min) and then forty cycles of 95℃ for 10 s, 57℃ for 20 s and 72℃ for 30 s, followed by the temperature from 60℃ to 95℃ to get data acquisition, and 20℃ for 10 s as the last step. The expression levels of genes were analysed based on the relative Ct method (Livak & Schmittgen, 2001).
| Primer name | Primer sequences (5′ to 3′) | GenBank accession number | Product size (bp) |
|---|---|---|---|
| 515F | GTGCCAGCMGCCGCGGTAA | ||
| 907R | CCGTCAATTCCTTTGAGTTT | ||
| Dorsal-F | TTGCGACCACCAGACAAGAG | SRP132193 | 142 bp |
| Dorsal-R | GCAAGGTAACGACTAATCTTCTCTG | SRP132193 | |
| Relish-F | CTACATTCTGCCCTTGACTCTGG | FJ592176 | 152 bp |
| Relish-R | GGCTGGCAAGTCGTTCTCG | FJ592176 | |
| Pen-F | CACCCTTCGTGAGACCTTTG | Y14926 | 141 bp |
| Pen-R | AATATCCCTTTCCCACGTGAC | Y14926 | |
| Cru-F | GTTCCAACGACTACAAGTGTGC | AY488496 | 185 bp |
| Cru-R | CCAAAACATCGGTCGTTCTTCAG | AY488496 | |
| β-actin-F | CCACGAGACCACCTACAAC | AF300705 | 142 bp |
| β-actin-R | AGCGAGGGCAGTGATTTC | AF300705 |
2.8 Assessment of intestinal microbiota
Total genomic DNA of intestinal microbiota of shrimps in BA2 (the group with the best growth performance) and BA4 (the group with the highest bile acids level) groups was extracted and verified according to the method described by Zhang et al., 2019. Then, the samples of control, BA2 and BA4 groups were analysed by Novogene Biological Information Technology Co. (Tianjin, China). PCR reactions were carried out by Phusion® High-Fidelity PCR Master Mix, and 515F and 907R (Table 2) were used as universal primers to amplify the V4-V5 regions of the 16S rRNA gene. Sequencing libraries were constructed using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA), and index code was respectively added. Lastly, libraries were sequenced on the IlluminaHiSeq2500 platform. The obtained sequences were available in the GenBank database with the accession number of PRJNA629040.
Sequence with ≥97% similarity was designated as the same OTUs by UPARSE (Edgar, 2013). OTUs with the single read were discarded. Taxonomic classifications of OTU-representative sequence were achieved by MOTHUR program via SILVA database with the confidence threshold of 80% (Quast et al., 2012). The abundance of OTUs was then normalized according to Su et al., 2020 to calculate the alpha diversity using QIIME (version 1.7.0). Venn diagram and Principal co-ordinates (PCoA) analyses were conducted. The functional predictions of OTUs were performed using Tax4Fun (v1.0) (Aßhauer et al., 2015).
2.9 Statistical analysis
Data were shown as means ± standard deviation (SD) and statistically analysed by SPSS 24.0 software. Statistical significances among control, BA1, BA2, BA3 and BA4 groups were determined using one-way ANOVA and post hoc Duncan multiple range tests. p < .05 was used to reveal the statistical significance.
3 RESULTS
3.1 Growth performance and survival
Table 3 elucidated the significant effects of dietary bile acids on the growth performance of shrimp after sixty days of feeding (p < .05). As compared to control group, the final weight, weight gain and specific growth rate in BA2 and BA3 groups were significantly increased (p < .05), and the feed conversion rate was significantly decreased in BA2 and BA3 groups (p < .05). Meanwhile, there were no significant differences in survival rate (%) among all groups (p > .05).
| Index | Treatment | One-way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Control | BA1 | BA2 | BA3 | BA4 | F value | p value | |
| SR% | 95.56 ± 2.55 | 93.33 ± 1.67 | 90.56 ± 6.94 | 89.44 ± 0.96 | 92.78 ± 4.19 | 1.139 | .392 |
| Initial weight/g | 1.09 ± 0.04 | 1.09 ± 0.04 | 1.09 ± 0.04 | 1.09 ± 0.04 | 1.09 ± 0.04 | 0.000 | 1.000 |
| Final weight/g | 8.67 ± 0.21a | 8.92 ± 0.30ab | 9.41 ± 0.51b | 9.33 ± 0.36b | 9.29 ± 0.24ab | 4.356 | .011 |
| WG% | 697.55 ± 19.16a | 720.86 ± 27.79ab | 765.64 ± 46.61b | 758.9 ± 33.24b | 754.91 ± 21.70ab | 4.356 | .011 |
| SGR/(%/d) | 3.46 ± 0.04a | 3.51 ± 0.06ab | 3.60 ± 0.09b | 3.58 ± 0.06b | 3.58 ± 0.04b | 4.338 | .011 |
| FCR | 1.77 ± 0.11a | 1.79 ± 0.12a | 1.63 ± 0.06b | 1.60 ± 0.08b | 1.69 ± 0.10ab | 3.365 | .024 |
- Data are presented as mean ± SD. Data with different letters are significantly different (p < .05) among groups.
3.2 Serum biochemical
As shown in Figure 1, TG was significantly decreased and HDL was significantly increased in BA2-4 groups compared with control group (p < .05). And no significant difference in LDL content was observed among BA1-4 and control groups (p > .05). Meanwhile, compared with the control group, there were no significant differences in ALT and AST in BA1-3 groups (p > .05), and ALT and AST in BA4 group were significantly increased (p < .05).
3.3 Digestive enzyme activity
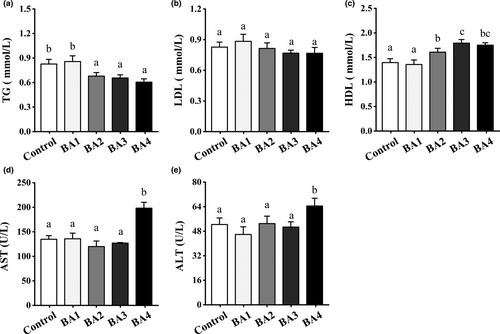
Figure 2 clarified dietary bile acids could significantly affect the activities of digestive enzymes of shrimp (a-c). As compared to control group, the activities of lipase and protease of BA2-4 groups were significantly higher (p < .05), while the activities of protease and lipase among BA2-4 groups were not significantly different (p > .05). The activity of amylase of BA2-4 groups was decreased slightly without significant differences from control group (p > .05). Effects of dietary bile acids on antioxidant system of shrimp were clarified in Figure 2d-f. The BA2-4 diets significantly increased the T-AOC, SOD and GSH by comparison with the control group throughout the experiment (p < .05). BA3 group had the highest antioxidant capacity in comparison with other groups (control, BA1, BA3 and BA4).
3.4 Immune parameters and gene expression
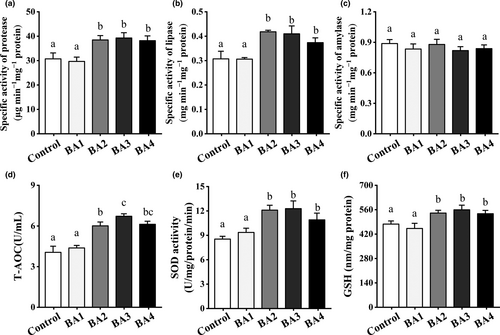
Figure 3a-c showed that phagocytic activity and antibacterial activity of BA2 and BA3 groups were significantly increased than those of control group (p < .05). Meanwhile, Figure 3d-g exhibited the effects of dietary bile acids on expression levels of immune-related genes in the intestine of shrimp. As compared to control group, the expression level of Dorsal of BA2-4 groups was significantly increased (p < .05) and BA4 diet induced the highest expression level of Relish (p < .05). As for antimicrobial peptides (AMPs), both BA3 and BA4 groups significantly stimulated the expressions of Pen and Cru in the intestine of shrimp (p < .05).
3.5 Intestinal microbiota
To examine effects of dietary bile acids on intestinal microbiota of shrimp, samples of control, BA2 (with the best growth performance) and BA4 (with the highest bile acids level) groups were analysed. Table 4 presented the Shannon and Simpson indexes of control, BA2 and BA4 groups. Shannon and Simpson indexes were increased with increasing dietary bile acids level, and BA4 group had the highest Shannon and Simpson indexes in comparison with other groups (p < .05). In addition, Chao and ACE values were calculated to show the intestinal bacterial richness of control, BA2 and BA4 groups. Results showed that Chao value was decreased in BA4 group and increased in BA2 group with significant differences from control group (p < .05), and ACE value was significantly increased in BA2 group (p < .05).
| Index | Treatment | One-way ANOVA | |||
|---|---|---|---|---|---|
| Control | BA2 | BA4 | F value | p value | |
| Shannon | 5.06 ± 0.12a | 5.72 ± 0.29b | 5.61 ± 0.10b | 5.506 | .044 |
| Simpson | 0.92 ± 0.01a | 0.95 ± 0.02ab | 0.97 ± 0.02b | 6.759 | .029 |
| Chao | 337.90 ± 30.91ab | 391.96 ± 15.85b | 302.61 ± 13.96a | 8.190 | .019 |
| ACE | 339.51 ± 15.12a | 388.31 ± 25.93b | 298.35 ± 9.57a | 12.566 | .007 |
| Good's coverage | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.00 ± 0.00a | 1.323 | .334 |
- Data are presented as mean ± SD. Data with different letters are significantly different (p < .05) among groups.
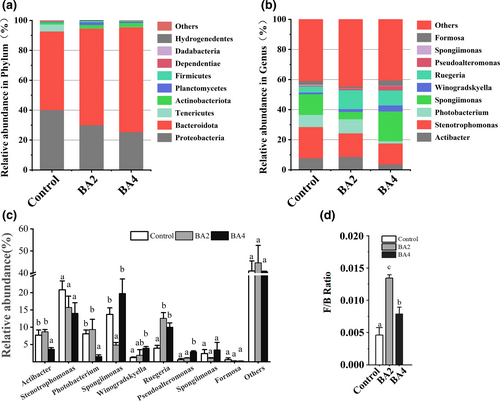
As shown in Figure 4, Proteobacteria and Bacteroidetes followed by Tenericutes, Actinobacteria and Planctomycetes were the most dominated phyla of all groups (control, BA2 and BA4). By comparison with control group, the abundance of Proteobacteria was decreased and the abundances of Bacteroidetes and Actinobacteria were increased in BA2 and BA4 groups. At the genus level, Actibacter, Stenotrophomonas and Photobacterium were the most dominant genera of all groups (control, BA2 and BA4). The abundance of Actibacter and Photobacterium was significantly decreased in BA2 and BA4 groups (p < .05), and the abundance of Ruegeria was significantly increased in BA2 and BA4 groups (p < .05). The Firmicutes to Bacteroidetes (F/B) ratio were significantly increased in BA2 and BA4 groups (p < .05). As shown in Figure 5, 312 OTUs were shared by all samples. The PCoA analysis elucidated that the intestinal microbiota of bile acids groups, which could be divided into two categories, was separated from that of control group. In the meantime, the cluster result elaborated that BA2 and BA4 groups were clustered more tightly as well.

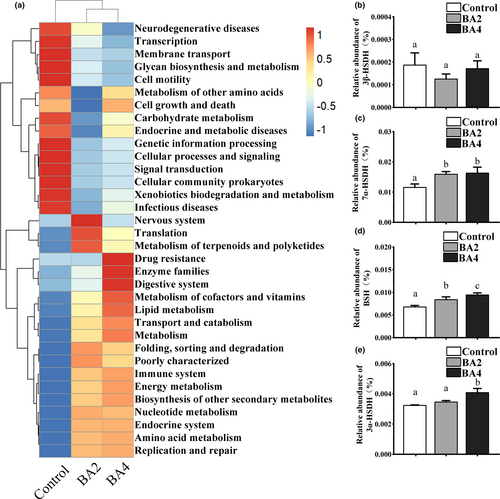
The functional profiling of control, BA2 and BA4 groups was predicted by virtue of Tax4Fun (v1.0). At the KEGG level 3, functional genes related to ‘lipid metabolism’, ‘Amino acid metabolism’, ‘Energy metabolism’, ‘Nucleotide metabolism’ and ‘Metabolism of cofactors and vitamins’ all increased, while ‘Transcription’, ‘Member transport’, ‘Glycan biosynthesis and metabolism’ and ‘Carbohydrate metabolism’ decreased after dietary supplementation of bile acids (Figure 6a). In addition, functional genes associated with the biotransformation of bile acids, the abundance of genes associated with 7α-hydroxysteroid dehydrogenase (7α-HSDH) and bile salt hydrolase (BSH) were increased in the BA2 and BA4 groups, and the abundances of genes associated with 3α-hydroxysteroid dehydrogenase (3α-HSDH) were increased in BA4 group (Figure 6b-e).
4 DISCUSSION
Bile acids are known to have the amphipathic structure and can be regarded as detergent to facilitate the emulsification and digestion of feed by promoting the formation of fat micelles and enhancing the proteolytic cleavage of dietary proteins (Reschly et al., 2008). Dietary bile acids could improve the growth performance and the activities of digestive enzymes of Japanese eel (Maita et al., 1996). Similarly, our result showed that the addition of 0.2 g kg −1 bile acids in feeds could significantly enhance the growth performance together with the activities of lipase and protease of shrimp, indicating that bile acids may be also capable of promoting the emulsification and absorption of feed in shrimp. Ley et al., 2006 have verified that F/B ratio in intestinal microbiota was significantly positively correlated with the host's ability to obtain energy from diet. In the present study, variations of the structure and function in intestinal microbiota might facilitate the digestion and absorption of nutrients in shrimp as well. Support for the viewpoint derives from the significantly increased Firmicutes to Bacteroidetes (F/B) ratio of intestinal microbiota in BA2 and BA4 groups, and functional genes related to metabolism were remarkably increased in bile acids groups. Additionally, Alam et al., 2001 elaborated that bile acid diets increased the activity of lipase of juvenile Japanese flounder, but did not affect the activity of amylase, and in the study, bile acids also exerted no obvious effects on the activity of amylase of shrimp, which may be attributed to the fact that shrimp were poorly efficient for the digestion and utilization of carbohydrate. Together, our study investigating the effects of bile acids on shrimp even on invertebrates for the first time indicated that bile acids could increase the growth performance as well as the activities of digestive enzymes of shrimp, which could lay sound foundations for the future studies.
Dietary bile acids have been reported to affect serum biochemical indexes of juvenile rainbow trout (Adhami et al., 2017). TG is the most crucial ingredients of blood lipids, which can reflect the body's lipid metabolism status to some extent. HDL and LDL play considerable roles in the process of lipid metabolism as well as cholesterol transport in the body. In our results, TG content in serum of shrimp decreased with increasing dietary bile acids level, suggesting that bile acids promoted lipid transport in shrimp. Meanwhile, the levels of dietary bile acids had a significant effect on serum HDL content of shrimp. Therefore, bile acids may regulate the lipid metabolism of shrimp, but the detailed mechanism still needs further exploration.
Under normal physiological conditions, the antioxidant defence system is able to maintain the generation and scavenging of reactive oxygen species (ROS). Once the balance is broken, the accumulated ROS can cause lipid peroxidation and disrupt the structural integrity of cell membranes, leading to the massive release of AST and ALT into the blood. Several studies have demonstrated that bile acids could improve the antioxidant status of organisms not only by directly scavenging hydroxyl radicals (ROS) (Mitsuyoshi et al., 1999), but also by improving the activities of antioxidant enzymes (Ljubuncic et al., 2000). In general, SOD is vital to protect cells and tissues from peroxidation (Wongsasak et al., 2015) and T-AOC is a vital indicator to evaluate the anti-oxidative capacity of organism (Xie et al., 2018). In this study, the BA2 and BA3 groups showed the highest antioxidant capacity during the whole experiment by increasing SOD and T-AOC of shrimp. These results suggested that suitable bile acids might improve the antioxidant capacity of shrimp. On the other hand, the accumulation of bile acids in vertebrates often causes oxidative damage, which is associated with the potential cytotoxicity of bile acids (Poupon, 2012). (Jiang et al., 2018) proved that high concentration of bile acids (1.35 g kg−1) could damage the hepatocytes of tilapia. Analogous results were obtained in the present study; that is, the levels of AST and ALT in the haemolymph all increased in the BA4 group, suggesting that bile acids may cause oxidative injury to hepatopancreas of shrimp. Together, although supplementation of suitable bile acids may produce beneficial effects to shrimp, long-term supplementation should be treated with caution and bile acids of 0.5 g kg−1 may be a concentration threshold beyond which negative effects may occur.
Shrimp without the adaptive immune could merely depend on the non-specific immune to defend against invaded pathogens (Wang et al., 2014), and intestinal immune system is vital to the immune system of shrimp. Specifically speaking, the intestine tract is able to fight against foreign pathogens from surroundings (Xie et al., 2018) and epithelial cells of intestine could sustain the homeostasis of intestine by means of mediating expressions of specific immune factors (Pasparakis, 2008). Growingly increased evidences suggested that bile acids could regulate the intestinal immune system as pleiotropic signal molecules, while information about its regulatory mechanism is only partially defined (Fiorucci et al., 2018). NF-κB-mediated signalling pathways are essential for the immune system of shrimp. Relish and Dorsal are important Rel/NF-κB transcription factors, which regulate the expressions of antimicrobial peptides (AMPs) to protect the organism from diseases (Tassanakajon et al., 2013). Bile acids have been clarified to be capable of regulating innate immunity of bile epithelial cells through nuclear receptor-induced AMPs expression (D'Aldebert et al., 2009). Similarly, in the present experiment, bile acids stimulated the expressions of AMPs through NF-κB-mediated signalling pathway in intestine. Generally, increased expressions of AMPs in the intestine may help to establish the mucosal barrier to hinder the communication between microbes and pathogens (Jin et al., 2018). Furthermore, the increased antibacterial activity and phagocytic activity in all bile acids groups truly certified the significance of bile acids in enhancing the immune capacity of shrimp. Thus, dietary supplementation of bile acids might enhance the immune capacity of shrimp mainly through NF-κB-mediated signalling pathway.
To reveal effects of diets supplemented with bile acids on the intestinal microbiota of shrimp, the structure of bacterial community was investigated. Results demonstrated that bacterial diversity of bile acids groups was higher than the control and UPGMA clustering as well as PCoA analysis also presented that the intestinal microbiota of bile acids groups clustered tightly and separated from the control group, implying that bile acids could indeed affect intestinal microbiota of shrimp. While it should be noted that the changes of intestinal bacterial richness in the BA2 and BA4 groups were opposite, hence, we speculated that bile acids may stimulate the growth of bile acids-metabolizing microorganisms under appropriate concentrations, but prevent the growth of bile acids-sensitive microorganisms at high concentrations (Wahlström et al., 2016). Meanwhile, high concentrations of bile acids could cause the oxidative damage and are associated with potential cytotoxicity (Poupon, 2012), which may also be a possible reason for the significant decrease of intestinal bacterial richness in BA4 group. The composition of intestinal microbiota is closely related with host physiology and could be informative in predicting the health status of host (Ley et al., 2006). In mammals, bile acids could change the composition of intestinal microbiota, improving the abundances of Proteobacteria and Actinobacteria (Zheng et al., 2017). While in our study, dietary supplementation of bile acids also enhanced the abundance of Actinobacteria but reduced the abundance of Proteobacteria of shrimp, which was different from the above experiment. Therefore, it should be recognized that bile acids might have different effects on intestinal microbiota between mammals and shrimp, and this discrepant phenomenon might also relate with different feeding time or experimental species (David et al., 2014) Moreover, it has been elaborated that the more abundant Proteobacteria might pose a threat to the health of shrimp, since the enriched abundance of Proteobacteria was reported in slow-growing (Fan & Li, 2019) and pathogen-infected (Wang et al., 2019) shrimp. Actinobacteria capable of biosynthesizing secondary metabolites against pathogens are regarded as the potential probiotic (Das et al., 2008). Overall, the study suggested that dietary bile acids exerted advantageous effects on the intestinal microbiota of shrimp, and the change of the abundances of Proteobacteria, Bacteroidetes and Actinobacteria in the core intestinal microbiota of bile acids groups may be on account of the higher growth performance of shrimp. At the genus level, the abundances of Stenotrophomonas and photobacterium were decreased in bile acids groups. These two bacterial taxa belong to Gammaproteobacteria, which are generally considered to be opportunistic pathogens of aquatic animals (Brooke, 2012). In addition, the increased abundances of Ruegeria might regulate the composition of intestinal microbiota and improve the immune capability of aquatic animals (Kongnum & Hongpattarakere, 2012). Thus, dietary bile acids might regulate the composition of intestinal community and maintain intestinal homeostasis by enhancing the abundance of probiotics and meanwhile inhibiting the growth of potential pathogens, which is in favour of the health of shrimp.
The functionality and metabolic activities of intestinal microbiota might also be compactly relevant to the health status of the host. For example, the decreased abundance of metabolism-related functional genes of intestinal microbiota seems to be a common feature of diseased shrimp with white spot disease and white faeces syndrome (Hou et al., 2018; Wang et al., 2019). Our findings indicated that dietary supplementation of bile acids induced marked alterations of functionality of intestinal microbiota in shrimp. Among them, consistent with previous investigations about grass carp (Xiong et al., 2018), the lipid metabolism capacity of intestinal microbiota in BA2 and BA4 groups increased, further confirming that bile acids were significant to the emulsification of lipid and the metabolism of shrimp. Meanwhile, functional genes associated with metabolism (including amino acid, energy, nucleotide, cofactors and vitamins) were all increased after dietary supplementation of bile acids. Generally, an increased abundance of metabolism-related functions in intestinal microbiota may signify the adaptation of intestinal microbiota to higher amounts of nutrients in the intestine (Heyman-Lindén et al., 2016). What's more, the increased metabolism not only is conducive to the intestinal homeostasis of shrimp (Zhou et al., 2019), but also supplement the actions of endogenous digestive enzymes of host (Lyons et al., 2017). Consequently, the enhancement of the metabolic capacity of intestinal microbiota may be one of the important mechanisms by which bile acids promoted the growth and nutrient absorption of shrimp. In short, supplementing bile acids diet has rewarding effects on intestinal microbiota function, but deeper study is still needed.
The intestinal microbiota is of great significance to the metabolism of bile acids in mammals, since the secondary bile acids produced by intestinal microbiota could bind to and activate more receptors to a greater extent than primary bile acids (Kawamata et al., 2003). In general, the biological transformation process of bile acids through intestinal microbiota mainly includes deconjugation, 7α-dehydroxylation and epimerization which are mainly carried out by bacteria with the activity of BSH, 7α-dehydroxylation and hydroxysteroid dehydrogenases (HSDs), respectively. Bacteroidetes and Actinobacteria were identified as the main bacteria that could metabolize bile acids (Ridlon et al., 2006). The present study demonstrated that functional genes associated with bile acids metabolism (BSH activity, 7α-HSDH activity and 3α-HSDH activity) were induced to varying degrees in the BA2 and BA4 groups, which may be attributed to the enriched abundances of Bacteroidetes, Firmicutes and Actinobacteria in bile acids groups; in other words, the increased bile acids metabolism capacity of intestinal microbiota exactly coincided with the composition change of intestinal microbiota. All the results clarified that the dynamic interaction between bile acids and intestinal microbiota not only occurred in mammals but also in shrimp. Further investigations are supposed to pay attention to the overall profiles of intestinal metabolites after dietary bile acids, in order to analyse the correlation between various metabolites and intestinal microbiota and evaluate the overall contribution of secondary bile acids to the health status of the shrimp. Overall, the present study provides comprehensive and useful information for the further comprehension of the relationship between bile acids and intestinal microbiota of shrimp.
5 CONCLUSIONS
In conclusion, dietary supplementation of 0.2–0.3 g kg−1 bile acids could promote the growth performance, lipid metabolism and antioxidant capacity in shrimp, and enhance the immune response of shrimp mainly through NF-κB-mediated signalling pathway. High-throughput sequencing analysis showed that bile acids exerted beneficial effects on the structure and function of intestinal microbiota, and the biotransformation process of bile acids completed by the microbiota was also found in the intestines of shrimp. Dietary supplementation with 0.5 g kg−1 bile acids had side effects on shrimp. Considering the feed cost, we recommend the shrimp diet supplemented with bile acids at the 0.2 g kg−1 level. In summary, this study will enrich the knowledge of metabolic mechanism of bile acids in invertebrates and provide a theoretical basis for the development of feed supplements of shrimp.
ACKNOWLEDGEMENTS
We would like to appreciate for all partners’ help during sample collection. This study was supported by the Key Research and Development Program of Guangdong Province (2020B0202010009).
CONFLICT OF INTEREST
There were no conflicts of interest to declare.
ETHICAL APPROVAL
All applicable international, national and/or institutional guidelines for the care and use of shrimp were followed by the authors.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




