Effects of nano-Selenium supplementation in plant protein-rich diet on reproductive performance and egg and larval quality of female Arabian yellowfin sea bream (Acanthopagrus arabicus)
Abstract
This study was conducted to examine effects of supplementing nano-selenium in a plant protein-based diet on reproductive performance of female Acanthopagrus arabicus. Two basal diets were formulated including a fishmeal (FM)-based diet and a plant protein (PP)-rich diet. FM-based diet served as a positive control (control) and the PP-rich diet was used as the basal diet for supplementing with 0, 0.5, 1, 2 and 4 mg N-Se Kg−1. Six groups of fish were fed one of the test diets over three months to spawning. Relative fecundity was higher in females fed 4 mg Kg−1 N-Se diet (p < .05). The greatest fertilization rate (FR) was found in broodfish fed 4 mg N-Se Kg−1 diet, but it was not significantly different from the FR in groups fed the control and 2 mg N-Se Kg−1 diets. Eggs from the females fed the control or 2 mg N-Se Kg−1 diets displayed higher hatchability (p < .05). The highest length and survival rate at 3-days post-hatch were observed in larvae of females fed 2 mg N-Se Kg−1 diet (p < .05). The results indicated that administration of 2–4 mg N-Se Kg−1 in PP-rich diets has beneficial effects on reproduction of female A. arabicus.
1 INTRODUCTION
Selenium (Se) is an essential micronutrient in fish nutrition required for optimal growth, development, and antioxidant defence (Watanabe et al., 1997). It is known that Se combines with amino acid selenocysteine, which is then incorporates into selenoproteins. Increasing scientific reports indicate that selenoproteins have important roles in different biological processes such as antioxidant protection, decrease of inflammation, DNA synthesis, fertility and reproduction (Lobanov et al., 2009; Rayman, 2000).
In commercial aquafeeds, fishmeal (FM) has been commonly utilized as the major source of Se (Sissener et al., 2013). FM-based feeds can supply adequate Se to cover the nutritional demands of carnivorous fish (Watanabe et al., 1997), but can represent the main portion of the production cost. Currently, FM is increasingly substituted in fish feeds by plant ingredients (Ilham et al., 2016; Tacon & Metian, 2008; Wischhusen et al., 2021). However, replacement of FM by plant ingredients with low Se content can reduce Se levels in aquafeeds (Domínguez et al., 2020).
Suboptimal dietary Se intake can decrease the expression of selenoproteins (Elia et al., 2011; Hesketh, 2008; Mariotti et al., 2012). In fish, dietary Se deficiencies, in addition to the effects at the cellular level, have increasingly been reported to result in growth reduction and increased mortality (Deng et al., 2007; Jaramillo et al., 2009; Lin & Shiau, 2005; Wang et al., 2013). On the other hand, extra dietary Se can have harmful consequences for the fish such as decreased growth and food efficiency, impaired immunological functions, increased mortality, and reduced egg viability (Berntssen et al., 2018; Domínguez et al., 2020; Gatlin & Wilson, 1984; Schultz & Hermanutz, 1990; Watanabe et al., 1997; Zee et al., 2016). However, the consequences of dietary Se deficiency or excess vary according to species, life stage, and bioavailability of the Se form, and environmental conditions that may induce stress (Khan et al., 2017).
Commercial animal feeds can be supplemented with 0.2 mg Kg−1 according to European Food Safety Authority, and 0.3 mg Kg−1 as recommended by US Food and Drug Administration. However, the chemical form of Se is shown to considerably influence its bioavailability and its effect on metabolism (Fairweather-Tait et al., 2010). In fish, organic Se compounds have been demonstrated to have more bioavailability and tissue bioaccumulation rate compared to inorganic form (Fontagné-Dicharry et al., 2015; Le & Fotedar, 2014; Le & Fotedar, 2014; Prabhu et al., 2016). Recently, nano-Selenium (N-Se) has been reported to have more bioavailability and lower toxicity when fed to fish (Saffari et al., 2017, 2018; Wang et al., 2007; Zhou et al., 2009). Moreover, N-Se enhanced growth rate, feed efficiency and the antioxidant protection capacity of various fish species (Ashouri et al., 2015;2019a ; Longbaf Dezfouli et al., 2019; Naderi et al., 2017; Pour et al., 2020; Saffari et al., 2017, 2018).
According to the current literature, most of the investigations exploring the effects of supplemental Se on the performance and well-being in fish, have been undertaken with fry, fingerlings and young fish, and knowledge on the effect of Se in broodstock nutrition is scarce (Hardy et al., 2010; 2014a ; Penglase, Hamre, Rasinger, et al., 2014b; Wischhusen et al., 2019). The reproduction stage is known to be greatly different to other stages of life as nutrients and energy are transferred to the reproduction organs and not allocated to somatic growth. As Se required by developing embryo is provided by the female during oogenesis (Wischhusen et al., 2021), maternal nutrition is of fundamental importance in fish. Recently, Wischhusen et al. (2019) demonstrated that supplementation of Se in broodstock diets increased the number of spawning females and also affected the amount of Se transferred to their progeny.
Arabian yellowfin sea bream (Acanthopagrus arabicus) is a perciform fish belonging to the family Sparidae. This species is a marine carnivorous fish with great potential for cultivation in the Indo-Pacific area due to its high economic value, and good adaptation to husbandry conditions (Zakeri et al., 2009, 2011). Searching the literature, the knowledge available for Se nutrition in sparids is limited to Pagrus major (2019a, 2019b), Acanthopagrus schlegelii (Wang et al., 2019), and Sparus aurata (Domínguez et al., 2020), while optimal Se dietary levels for Arabian yellowfin sea bream have not been studied up to now. This study was carried out to examine the effects of dietary N-Se supplementation on reproductive performance as well as egg and larval quality of Arabian yellowfin sea bream females fed diets with high amounts of plant feedstuffs.
2 MATERIALS AND METHODS
2.1 Ethical statement
All the experimental conditions and sampling procedures have been performed according to the ethical standards of Animal Protection and Use Committee of Khorramshahr University of Marine Science and Technology.
2.2 Husbandry trial
Two months (from late October to late December) before the beginning of the feeding trial wild brooders of A. arabicus were caught with hook from the Persian Gulf and were stocked (10 fish m−3) in rectangular concrete tanks (10 m3) connected to a flow-through system (10 L min−1) in a private marine fish hatchery (Sarbandar, Khuzestan, Iran). Tanks were supplied with sand filtered and disinfected (chlorination, 10 ppm and neutralization with sodium thiosulfate, 5 ppm) sea water and each tank aerated with aquarium air stones. During the acclimation, the broodfish were fed with a commercial feed (21 Beyza, Iran; particle size: 6 mm, 420 g Kg−1 crude protein, 150 g Kg−1 crude fat, 100 g Kg−1 ash, 90 g Kg−1 moisture, and 20 KJ g−1 gross energy) at visual satiation once a day. Two weeks before starting the feeding trial, 288 brooders were first weighed then distributed in 18 10-m3 concrete rectangular tanks. Sixteen brooders (2–3 years old) at a sex ratio of 1:1 (males: 195.3 ± 10.8 g; females: 237.5 ± 8.1 g, mean ±standard error) were stocked in each tank. Males had running milt and generally were smaller than the female fish. Tanks filled with eight m3 disinfected and sand filtered seawater in a flow-through system (50% water exchange day−1). The feeding trial consisted of six treatments and each treatment performed in three replicates (tanks). Fish were fed with the experimental feeds once a day (12:00 h) for 90 days (from mid-January to mid-April) at visual satiation to make sure that feed was not left uneaten. The natural spawning season of A. arabicus in the Northwest of the Persian Gulf occurs from mid-March to mid-April, when the water temperature is between 19 and 23℃. Thus, in this study broodfish were fed with the test diets 60 days before the first spawning and during the spawning period (30 days). The photoperiod was artificial and included 8L:16D (light: darkness) during the pre-spawning period and 11L:13D during the spawning period (Zakeri et al., 2009). The average values for water quality parameters during the experiment period including temperature, pH, dissolved oxygen and salinity were 20.5 ± 0.4℃, 7.8 ± 0.4, 7.6 ± 0.5 mg L−1 and 40.2 ± 0.4 ppt respectively.
2.3 Experimental diets
For evaluating the effects of N-Se on reproductive performance of A. arabicus female, two basal diets were designed including: a fish meal-based diet (FM-based diet) and a plant protein-rich diet (PP-rich diet) (Table 1). Fish meal-based diet served as a positive control (control) because of high bioavailability of Se in FM. In the PP-rich diet about 60% of FM was substituted by blends of PP sources (wheat gluten, corn gluten and soybean meals). L-lysine and dl-methionine were incorporated in the PP-rich diets. Also, inorganic phosphorus in the form of dicalcium phosphate was added in diets with PP sources to enhance their nutritional value. For increasing the bioavailability of Se in the PP-rich diet, N-Se (30–45 nm particle size, 99.95% purity, 3.89 g cm3 true density, Iranian Nanomaterials Pioneers, Iran) was included in the diet at five levels including 0, 0.5, 1, 2 and 4 mg Kg−1 to produce experimental feeds. All dry ingredients were completely mixed for 20 min. The oils and soybean lecithin were added to them, and they were then blended for 10 min. Distilled water was poured on the mixed ingredients to make a dough, and then, a meat grinder was used for pelleting the dough. For supplementing experimental diets with the prescribed N-Se dosages, N-Se was dissolved in distilled water then added to the mixed feedstuffs. The produced dough was wet-extruded (6 mm), dried at 25℃ and kept in a freezer (−18℃) until use. The analysed total Se concentrations of the experimental diets were 1.34 ± 0.1 mg Kg−1 (control), 0.67 ± 0.08 mg Kg−1 (0 mg Kg−1), 1.09 ± 0.05 mg Kg−1 (0.5 mg Kg−1), 1.48 ± 0.18 mg Kg−1 (1 mg Kg−1), 2.60 ± 0.14 mg Kg−1 (2 mg Kg−1), and 4.82 ± 0.10 mg Kg−1 (4 mg Kg−1).
| FM-based diet | PP-rich diet | |
|---|---|---|
| Ingredients (g kg−1)a | ||
| Fish meal | 750 | 300 |
| Soybean meal | - | 100 |
| Wheat gluten meal | - | 200 |
| Corn gluten meal | - | 200 |
| Beef gelatin | 25 | 25 |
| Wheat middling | 120 | 30 |
| Fish oil | 20 | 31 |
| Canola oil | 20 | 31 |
| Soy lecithin | 20 | 20 |
| DL-methionine | - | 3 |
| L-lysine | - | 5 |
| Se-free premixb | 40 | 40 |
| Butyric acid | 2.5 | 2.5 |
| Vitamin C | 2.5 | 2.5 |
| Di-calcium phosphate | - | 10 |
| Proximate composition (g/kg) | ||
| Crude protein | 500.6 | 502.9 |
| Crude lipid | 163.5 | 150.4 |
| Ash | 112.5 | 106 |
| Moisture | 77 | 64 |
- a Composition of ingredients [fish meal (620 g kg−1 crude protein, 150 g kg−1 crude lipid), soybean meal (410 g kg−1 crude protein, 42 g kg−1 crude lipid), wheat gluten meal (500 g kg−1 crude protein, 40 g kg−1 crude lipid), corn gluten meal (714 g kg−1 crude protein, 41 g kg−1 crude lipid), beef gelatin (850 g kg−1 crude protein, 42 g kg−1 crude lipid), wheat middling (120 g kg−1 crude protein, 30 g kg−1 crude lipid).
- b Included: (as mg kg−1 of premix): vitamin A, 50,000 (IU kg−1); vitamin D3, 10,000 (IU kg−1); vitamin E, 30; vitamin B1, 20; vitamin B2, 10; vitamin B6, 3; vitamin K3, 15; nicotinamide, 150; calcium pantothenate, 40; Copper (Cu++), 30; iron (Fe++), 100; zinc (Zn++), 150; manganese (Mn++), 200.
| N-Se (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| Control | 0 | 0.5 | 1.0 | 2.0 | 4.0 | |
| BWi (g) | 237.2 ± 12.4 | 242.8 ± 13.4 | 230.4 ± 16.0 | 242.3 ± 18.2 | 233.5 ± 19.1 | 238.6 ± 19.8 |
| BWf (g) | 260.5 ± 17.5 | 267.5 ± 27.6 | 245.6 ± 14.1 | 297.8 ± 25.4 | 246.2 ± 7.5 | 275.8 ± 38.3 |
| Survival (%) | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 90.0 ± 3.2 | 91.0 ± 2.5 | 100 ± 0.0 |
| HSI (%) | 2.79 ± 0.22bc | 1.72 ± 0.14c | 3.02 ± 0.15bc | 2.42 ± 0.72bc | 5.33 ± 0.68a | 3.22 ± 0.27b |
| VSI (%) | 2.69 ± 0.14b | 3.10 ± 0.25b | 2.70 ± 0.39b | 4.18 ± 0.71ab | 4.18 ± 0.49ab | 6.10 ± 1.80a |
| K (%) | 1.96 ± 0.03 | 1.87 ± 0.08 | 1.82 ± 0.08 | 1.89 ± 0.07 | 1.85 ± 0.04 | 2.07 ± 0.07 |
- Abbreviations: BWf, final body weight; BWi, initial body weight; HSI, hepatosomatic index; K, Fulton condition factor; VSI, viscerosomatic index.
| N-Se (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| Control | 0 | 0.5 | 1.0 | 2.0 | 4.0 | |
| Relative fecundity (×103, eggs kg−1 female) | 175.3 ± 18.4d | 411.2 ± 27.7b | 409.0 ± 2.1b | 347.9 ± 0.7c | 458.6 ± 21.2b | 633.5 ± 14.8a |
| Spawning period (day) | 18.0 ± 0.6b | 28.0 ± 1.2a | 20.0 ± 1.2b | 21.0 ± 0.6b | 20.0 ± 1.7b | 31.0 ± 1.7a |
| Average spawned eggs per day (×103) | 38.1 ± 1.9c | 51.1 ± 1.9b | 35.2 ± 0.7c | 51.8 ± 1.8b | 56.5 ± 3.4b | 78.9 ± 0.8a |
| Number of eggs per mL | 1199.5 ± 11.6b | 1302 ± 13.3a | 1296 ± 19.5a | 1177.5 ± 15.7b | 1162.8 ± 18.0c | 1207.3 ± 15.7b |
| Egg diameter (µm) | 794.3 ± 2.28bc | 777.3 ± 4.32d | 773.9 ± 3.48d | 788.6 ± 3.57c | 801.13 ± 3.48ab | 805.8 ± 4.63a |
| Egg's oil globule diameter (µm) | 185.83 ± 2.22abc | 184.58 ± 2.44bc | 180.66 ± 2.16c | 189.17 ± 2.05ab | 191.66 ± 1.83a | 189.17 ± 2.14ab |
| Eggs weight (mg) | 0.416 ± 0.02 | 0.389 ± 0.0 | 0.387 ± 0.02 | 0.403 ± 0.01 | 0.422 ± 0.03 | 0.435 ± 0.02 |
| N-Se (mg kg−1) | ||||||
|---|---|---|---|---|---|---|
| Control | 0 | 0.5 | 1.0 | 2.0 | 4.0 | |
| Abnormal embryogenesis (%) | 8.75 ± 0.91b | 13.75 ± 1.93a | 9.55 ± 1.54ab | 10.15 ± 1.7ab | 8.95 ± 1.49b | 9.15 ± 1.53b |
| Newly hatched larvae length (µm) | 1776.0 ± 13.42b | 1681.3 ± 11.20c | 1697.1 ± 6.83c | 1686 ± 8.30c | 1749.0 ± 10.55b | 1822 ± 8.20a |
| Yolk sac length (µm) | 595.0 ± 9.12ab | 529.2 ± 10.3c | 568.3 ± 11.0b | 599.0 ± 12.6ab | 611.0 ± 17.3a | 593.0 ± 12.5ab |
| Oil globule diameter (µm) | 182.0 ± 5.3a | 165.6 ± 3.3b | 172.1 ± 4.0ab | 179.0 ± 3.1a | 179.0 ± 3.4a | 181.0 ± 3.3a |
| Larval length at 3 DPH1 | 2603.7 ± 23.1bc | 2520.6 ± 27.6e | 2461.3 ± 22.4de | 2547.7 ± 24.5cd | 2777.1 ± 24.1a | 2639.7 ± 24.4b |
| Oil globule diameter in 3 DPH larvae (µm) | 71.0 ± 3.12b | 75.0 ± 4.05ab | 68.75 ± 3.34b | 85.0 ± 5.20a | 69.5 ± 4.0b | 79.9 ± 4.63ab |
| Survival rate at 3 DPH larvae (%) | 37.80 ± 4.9c | 17.7 ± 3.4d | 23.0 ± 4.43d | 50.1 ± 4.41c | 88.2 ± 4.19a | 63.8 ± 4.9b |
2.4 Hatchery techniques and sampling
During the spawning period, the inlet water of the experimental tanks was closed in the afternoon (05:00 pm) and then it was disclosed in the morning (07:00 am) to avoid the exit of flouting eggs from the tank's outlet. Generally, the spawning behaviours of A. arabicus occur from evening to midnight. They naturally spawn during spawning season under captivity without hormone injection. All tanks were checked every day for the presence of the eggs. Eggs were collected into a funnel net (300 µm mesh size) that was securely placed under the tank's outlet while the tank was gradually being drained. Any debris mixed with the collected eggs was screened out with a net (1000 µm) and gently transferred into a 1-L graduated cylinder and remained for 5 min for separating the buoyant eggs (normal eggs) and the sink eggs (abnormal eggs). The buoyant eggs (30 mL) from each tank were stocked in three 20-L buckets (10 mL per each bucket). The eggs were incubated in the buckets up to three days post-hatch (DPH) of larvae. Each bucket filled with disinfected marine sea water (20–21℃) with mild aeration. These procedures carried out for three days for each tank (3 days ×3 tanks ×3 buckets = 27 samples for each treatment). After that, three samples of 100 buoyant eggs from each bucket were inspected using a microscope to determine fertilized eggs with cellular division compared with unfertilized eggs without any cell division. In addition, three samples of 100 fertilized eggs from each bucket were transferred into three 1-L beakers equipped with mild aeration to calculate hatching and survival rates of larvae up to three DPH. Hatching rate was calculated by dividing the total number of larvae to the total number of fertilized eggs. To evaluate the abnormal embryogenesis rate, three samples of 100 fertilized eggs from each bucket were examined under the microscope and the average proportions of eggs exhibiting abnormal cleavage was determined according to Pavlov and Emel’yanova (2008). The total number of eggs was determined volumetrically by counting the number of eggs per one mL of the collected buoyant eggs (n = 6 samples per tank). In addition, for determining egg's weight, the number of eggs was counted in one gram of the collected buoyant eggs (n = 6 samples per tank). For determining the relative fecundity, total number of spawned eggs in each tank during the spawning period was divided by the total weight (kg) of the females (n = 3). For determining the average spawned eggs per day, total number of spawned eggs in each tank was divided by the spawning period (day) (n = 3).
As it was mentioned, the collected buoyant eggs (30 ml) from each tank were transferred in three 20-L buckets (10 ml per each bucket) and eggs were incubated up to three DPH of larvae. For biometry, samples of eggs and larvae (newly hatched and 3 DPH) were fixed in 10% buffer formalin (pH = 7.0). Egg's diameter, oil globe diameter, newly hatched larval length, yolk sac length and larval length at three DPH were measured by examining 100 specimens from each bucket by a binocular microscope equipped with ocular micrometre. In addition, for evaluating Se concentration in the eggs and three DPH larvae, 1 g biomass was collected, transferred into a cryotube then kept into liquid nitrogen for further analysis (n = 3 per tank).
At the end of the feeding experiment, the individual weight and length of broodfish in each tank were recorded. Three female brooders from each tank were sacrificed with an overdose of an anaesthetic (2-phenoxyethanol, 1000 ppm) for determining the liver and visceral weights. For evaluating Se concentration in the liver and ovaries, 1 g of each tissue was dissected, transferred into a cryotube then kept into liquid nitrogen for further analysis (n = 3 per tank). For determining brooders performance, the following standard equations were used:
Survival (%) = number of fish in each group remained on day 90/initial number of fish) × 100.
Hepatosomatic index (%, HSI) = (liver weight (g) / BWf (g)) × 100.
Viscerosomatic index (%, VSI) = (visceral weight (g) / BWf (g)) × 100.
Fulton's condition factor (%, K) = (BWf (g)/ standard length (cm)3) × 100.
The initial and final body weight was denoted by BWi and BWf, respectively. The visceral weight contained the gonads and the liver.
2.5 Proximate composition and total Se content
Proximate composition analysis of the experimental diets was performed according to the standard procedures (AOCA, 2005). To evaluate the Se content in biological samples and experimental diets, the samples were first digested in concentrated HNO3 and H2O2, and then measured in the digests using inductively coupled plasma mass spectrometry (Agilent 7500; Yokogawa Analytical Systems, Japan) as reported previously (Fontagné-Dicharry et al., 2015).
2.6 Statistics
Data were analysed using the SPSS software version 16.0. The normality and homogeneity of data were confirmed by Shapiro-Wilk and Leven tests, respectively. The effects of dietary N-Se on reproduction of A. arabicus female brooders were then analysed using a One-way ANOVA. Duncan's post hoc analysis was performed following significant differences between groups. The Pearson product-moment correlation test was conducted to determine probable correlations among parameters. The p < .05 was regarded significant for all statistical tests.
3 RESULTS
3.1 Se content and somatic indices
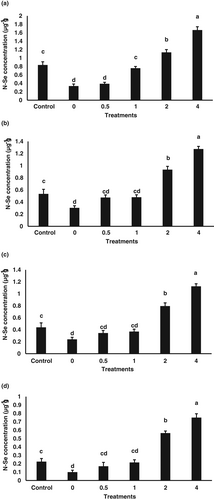
Effects of dietary N-Se on total Se content in liver, ovary, eggs and 3DPH larvae of the female brooders are shown in Figure 1. Total Se content in the liver and ovary tissues, eggs, and larvae of females exhibited an increasing trend in response to elevated dietary N-Se supplementation levels. The Pearson product-moment correlation analysis, also showed that the Se content in the liver, ovary, eggs and 3DPH larvae was positively correlated with the dietary Se level (Table 5).
| Dietary Se level | HSI | Egg's diameter | Spawned Egg's day−1 | Egg's OGD | Egg's weight | Egg's mL−1 | RF | EAR | NHL length | YSL | OGD in NHL | 3DPH larvae length | SR at 3DPH larvae | Se level in the liver | Se level in the ovary | Se level in the eggs | Se level in 3DPH larvae | FR | HR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary Se level | 1 | 0.445 | 0.829* | 0.876* | 0.581 | 0.870* | −0.465 | 0.745 | −0.479 | 0.829* | 0.503 | 0.574 | 0.565 | 0.680 | 0.960** | 0.980** | 0.982** | 0.968** | 0.635** | 0.656** |
| HSI | 0.445 | 1 | 0.580 | 0.213 | 0.553 | 0.545 | −0.580 | 0.273 | −0.636 | 0.402 | 0.687 | 0.467 | 0.823* | 0.847* | 0.511 | 0.609 | 0.588 | 0.644 | 0.224 | 0.365 |
| Egg's diameter | 0.829* | 0.580 | 1 | 0.700 | 0.835* | 0.990** | −0.818* | 0.332 | −0.593 | 0.845* | 0.750 | 0.811* | 0.844* | 0.851* | 0.949** | 0.860* | 0.871* | 0.876* | 0.126 | 0.063 |
| Spawned Egg's day−1 | 0.876* | 0.213 | 0.700 | 1 | 0.659 | 0.705 | −0.353 | 0.817* | −0.041 | 0.569 | 0.225 | 0.281 | 0.490 | 0.577 | 0.816* | 0.809* | 0.827* | 0.829* | 0.501* | 0.072 |
| Egg's OGD | 0.581 | 0.553 | 0.835* | 0.659 | 1 | 0.751 | −0.873* | 0.289 | −.0299 | 0.418 | 0.674 | 0.594 | 0.851* | 0.894* | 0.721 | 0.612 | 0.623 | 0.676 | 0.146 | 0.116 |
| Egg's weight | 0.870* | 0.545 | 0.990** | 0.705 | 0.751 | 1 | −0.746 | 0.364 | −0.617 | 0.911* | 0.712 | 0.808* | 0.790 | 0.794 | 0.969** | 0.894* | 0.904* | 0.897* | 0.203 | 0.370 |
| Egg's mL−1 | −0.465 | −0.580 | −0.818* | −0.353 | −0.873* | −0.746 | 1 | 0.043 | 0.641 | −0.466 | −0.918** | −0.864* | −0.764 | −0.846* | −0.670 | −0.519 | −0.516 | −0.553 | 0.533** | 0.135 |
| RF | 0.745 | 0.273 | 0.332 | 0.817* | 0.289 | 0.364 | 0.043 | 1 | 0.035 | 0.319 | −0.012 | −0.069 | 0.240 | 0.391 | 0.555 | 0.705 | 0.703 | 0.712 | 0.619** | 0.053 |
| EAR | −0.479 | −0.636 | −0.593 | −0.041 | −0.299 | −0.617 | 0.641 | 0.035 | 1 | −0.621 | −0.881* | 0.870* | −0.449 | −0.581 | −0.582 | −0.565 | −0.536 | −0.528 | −0.142 | −0.046 |
| NHL length | 0.829* | 0.402 | 0.845* | 0.569 | 0.418 | −0.911* | −0.466 | 0.319 | −0.621 | 1 | 0.524 | 0.711 | 0.580 | 0.530 | 0.878* | 0.842* | 0.854* | 0.811* | 0.176* | −0.053 |
| YSL | 0.503 | 0.687 | 0.750 | 0.225 | 0.674 | 0.712 | −0.918** | −0.012 | −0.881* | 0.524 | 1 | 0.928** | 0.664 | 0.812* | 0.666 | 0.581 | 0.560 | 0.585 | 0.103 | 0.053 |
| OGD in NHL | 0.574 | 0.467 | 0.811* | 0.281 | 0.594 | 0.808* | −0.864 | −0.069 | −0.870 | 0.711 | 0.928** | 1 | 0.567 | 0.667 | 0.744 | 0.607 | 0.598 | 0.589 | 0.080 | 0.044 |
| 3DPH larvae length | 0.565 | 0.823* | 0.844* | 0.490 | 0.851* | 0.790 | −0.764 | 0.240 | −0.449 | 0.580 | 0.664 | 0.567 | 1 | 0.918** | 0.703 | 0.679 | 0.688 | 0.735 | 0.214* | 0.210* |
| SR at 3DPH larvae | 0.680 | 0.847* | 0.851* | 0.577 | 0.894* | 0.794 | −0.846** | 0.391 | −0.581 | 0.530 | 0.812* | 0.667 | 0.918** | 1 | 0.783 | 0.770 | 0.763 | 0.816* | 0.211 | 0.431 |
| Se level in the liver | 0.960** | 0.511 | 0.949** | 0.816* | 0.721 | 0.969** | −0.670 | 0.555 | −0.582 | 0.878* | 0.666 | 0.744 | 0.703 | 0.783 | 1 | 0.959** | 0.965** | 0.956** | 0.501* | 0.603* |
| Se level in the ovary | 0.980** | 0.609 | 0.860* | 0.809* | 0.612 | 0.894* | −0.519 | 0.705 | −0.565 | 0.842* | 0.581 | 0.607 | 0.679 | 0.770 | 0.959** | 1 | 0.999** | 0.995** | 0.501* | 0.534* |
| Se level in the eggs | 0.982** | 0.588 | 0.871* | 0.827* | 0.623 | 0.904* | −0.516 | 0.703 | −0.536 | 0.854* | 0.560 | 0.598 | 0.688 | 0.763 | 0.965** | 0.999** | 1 | 0.995** | 0.0515* | 0.654* |
| Se level in 3DPH larvae | 0.968** | 0.644 | 0.876* | 0.829* | 0.676 | 0.897* | −0.553 | 0.712 | −0.528 | 0.811* | 0.585 | 0.589 | 0.735 | 0.816* | 0.956** | 0.995** | 0.995** | 1 | 0.516* | 0.620* |
| FR | 0.635** | 0.224 | 0.126 | 0.501* | 0.146 | 0.203 | 0.533** | 0.619** | −0.142 | 0.176* | 0.103 | 0.080 | 0.214* | 0.211 | 0.501* | 0.501* | 0.515* | 0.516* | 1 | 0.320** |
| HR | 0.056 | 0.365 | 0.063 | 0.072 | 0.116 | 0.370 | 0.135 | 0.053 | −0.046 | −0.053 | 0.053 | 0.044 | 0.210* | −0.431** | 0.103 | 0.034 | 0.154 | 0.200 | 0.320** | 1 |
- Abbreviations: DPH, days post hatch; EAR, Embryogenesis abnormality rate; FR, fertilization rate; HR, hatching rate; HSI, hepatosomatic index; NHL, newly hatched larvae; OGD, oil globule diameter; RF, relative fecundity; SR, survival rate; YS, yolk sac length.
- * Correlation is significant at the 0.05 level (two tailed).
- ** Correlation is significant at the 0.01 level (two tailed).
The somatic indices of the females fed on diets supplemented with varying concentrations of dietary N-Se are shown in Table 2. No significant differences in initial or final body weight, survival, and Fulton condition factor (K) were found in females fed different test diets. However, the HSI index was significantly higher in females fed diet with 2 mg N-Se Kg−1 compared to other experimental groups. Moreover, HSI positively correlated with survival and total length of 3DPH larvae (Table 5). The highest VSI existed in broodfish fed diet containing 4 mg Kg−1 N-Se. However, it was not significantly (p > .05) different from the VSI value in groups receiving 1 and 2 mg N-Se Kg−1 diets.
3.2 Reproductive performance
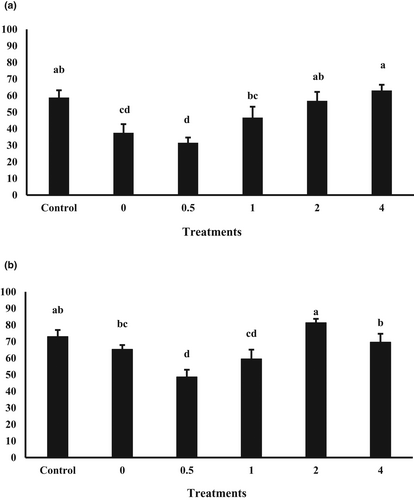
The females that were fed 4 mg N-Se Kg−1 diet had significantly highest relative fecundity (RF), followed by the 0, 0.5, and 2 mg Kg−1 N-Se groups, then the 1 mg Kg−1 N-Se fish, and lowest in females fed on the control diet (Table 3). The spawning period was longest in females fed diet supplemented with 4 mg Kg−1 N-Se. However, it was not significantly (p > .05) different from the spawning period in females fed the non-supplemented diet (0 mg Kg−1 N-Se diet). The average number of spawned eggs per day was significantly greater in females fed diet with 4 mg Kg−1 N-Se in comparison with other groups. In addition, a positive correlation was found between average number of spawned eggs per day and total Se content in liver and ovary of the females (Table 5). The highest number of eggs per mL was detected in broodfish fed diets with 0 and 0.5 mg N-Se Kg−1. Egg diameter significantly increased in females receiving 2 and 4 mg N-Se Kg−1 diets. As shown in Table 5, a negative correlation was found between the number of eggs per mL and the egg diameter parameters. The greatest oil globule diameter (OGD) of eggs was observed in females fed on diet containing 2 mg N-Se Kg−1, however it was not significantly different from the OGD in broodfish fed the control, 1, and 4 mg N-Se Kg−1 diets. Also, OGD of eggs was positively correlated to the length and survival of 3DPH larvae (Table 5). Egg weight was not significantly different between the six dietary groups (Table 3). The highest fertilization rate (FR) was detected in females fed on 4 mg N-Se Kg−1 diet. However, it was not significantly different from the fertilization rate in broodfish fed the control and diet containing 2 mg N-Se Kg−1 (Figure 2). Hatchability was affected by dietary treatment, with eggs from the females fed the control or 2 mg N-Se Kg−1 diets showing higher hatching rates (HR) as compared to other experimental groups (Figure 2). Also, positive correlations were found between both FR and HR with total Se contents in the liver, ovary and eggs of broodfish (Table 5).
3.3 Larval quality
Results on qualitative parameters of hatchlings and 3DPH offspring of female breeders fed different dietary N-Se levels are given in Table 4. The broodfish fed on diets with lesser Se supplementation (0, 0.5, and 1 mg Kg−1) showed higher percentage of abnormal embryogenesis. The lowest yolk sac length (YSL) was observed in larvae hatched from breeders fed the non-supplemented diet (0 mg N-Se Kg−1). Also, a negative correlation was detected between abnormal embryogenesis rate and YSL (Table 5). Total length of hatchlings was highest in larvae descended from females fed 4 mg N-Se Kg−1 diet, followed by the control and 2 mg Kg−1 N-Se, and lowest in 0, 0.5, and 1 mg Kg−1 N-Se groups. The OGD of hatchlings was lowest in larvae hatched from females fed 0 and 0.5 mg Kg−1 N-Se diets. The highest total length and survival rate at 3DPH was observed in larvae hatched from broodfish fed 2 mg N-Se Kg−1 diet, followed by those hatched from females fed 4 mg N-Se Kg−1 diet. The survival rate of larvae at 3DPH was positively correlated with their Se content and total length at 3DPH (Table 5).
4 DISCUSSION
In our study, Se deposition in the liver and ovary tissues of the broodfish augmented linearly in response to increasing concentrations of dietary N-Se. This linear bioaccumulation trend, regardless of the Se source, has been previously reported for various fish species (Ashouri et al., 2015; Berntssen et al., 2018; Domínguez et al., 2020; Lee et al., 2016; Lin, 2014; Liu et al., 2010; Tashjian et al., 2006; Wang et al., 2019). In the current study, there was also a linear elevation of Se content in the eggs and 3DPH larvae of the broodfish responding to the elevated dietary N-Se concentrations. These results are in line with data obtained in rainbow trout (Wischhusen et al., 2019) and arctic grayling (Brix et al., 2021) describing the maternal transfer of Se to the eggs and offspring.
In our study, supplementation of N-Se to a plant protein-rich (PP-rich) diet had no significant effect on the final body weight of female Arabian yellowfin sea bream broodstock. It could suggest that the PP-rich diet with a basal Se level of 0.67 mg Kg−1 did not cause a dietary Se deficiency to result in growth reduction. On the other hand, broodstock fish have been reported to use the main portion of food energy for reproduction instead of somatic growth (Zakeri et al., 2009). Similarly, Wischhusen et al. (2019) reported that inclusion of Se in a plant-ingredient based feed did not exert any beneficial effect on the growth rate of rainbow trout broodfish.
Nutrition can influence somatic indices including HSI and VSI (Dawood et al., 2017). In the current study, the highest HSI was observed in females fed diet supplemented with 2 mg N-Se Kg−1 diet. Liver is the primary organ for storage, nutrient metabolism and detoxifying processes in both males and females. In female fish, in addition to these functions, the liver plays a vital role in the synthesis of vitellogenin, which is then taken up by oocytes during vitellogenesis (Lubzens et al., 2010). Therefore, the increased HSI found in the present study may indicate a metabolic strategy in females to meet the metabolic requirements for intense hepatic activity during vitellogenesis. In analogy, the hepatosomatic modifications during reproduction period have been reported for other fish species studied (Nunes et al., 2011; Rinchard & Kestemont, 2003). Furthermore, HSI showed a positive correlation with survival and total length of 3DPH larvae in our study. These results indicate that increased HSI in female broodfish can improve the larval quality characteristics. In the present study, the VSI index was also increased with increasing dietary N-Se level. Such data show that N-Se supplementation can increase the lipids reserves in the abdominal area of females that can be utilized as a source of nutrients for ovarian growth during reproduction period (Lal & Singh, 1987).
Fecundity, as an important indicator of reproductive performance of broodfish, is demonstrated to be influenced by the nutritional status of fish (Izquierdo et al., 2001; Zakeri et al., 2009). The data of our study showed that both the relative fecundity (RF) and the average number of eggs spawned per day were significantly higher in 4 mg Kg−1 N-Se group as compared to other experimental groups. Our data are not similar to Wischhusen et al. (2019) who could not observe significant difference in absolute and relative fecundity of rainbow trout in response to Se supplementation in a plant-protein based diet. Also, similar to the mentioned rainbow trout trial, Penglase, Hamre, Rasinger, et al. (2014b) could not find any significant difference in number of eggs between zebrafish fed diets containing 0.09 mg Se Kg−1 or 0.65 mg Se Kg−1. With regard to the positive correlation found between the average number of spawned eggs per day and total Se content in liver and ovary tissues of the females, it can be concluded that a plant protein-rich (PP-rich) diet supplemented with 4 mg N-Se Kg−1 can have beneficial effect on Arabian yellowfin sea bream reproduction by increasing the egg production.
Both RF and average spawned eggs per day were significantly lesser in the control group compared to those in fish fed on 0 mg Kg−1 N-Se diet. These observations could suggest that PP-rich diet without N-Se supplementation had favourable effects on reproductive performance of A. arabicus. However, when these findings are combined with the fertilization rate (FR) data, such conclusion cannot be drawn. As shown in Figure 2 of the present study, FR was significantly lower in females fed basal PP-rich diet as compared to that in the control group.
The highest number of eggs per mL and the lowest egg diameter were found in broodfish fed diets contained 0 or 0.5 mg N-Se Kg−1. The negative correlation between these parameters indicates that PP-rich diets with low Se levels result in reduction of egg size in A. arabicus. Our data also showed that supplementation of PP-rich diets with 2–4 mg N-Se Kg−1 can positively affect the egg size of broodstock fish. Moreover, no significant difference was detected in egg weight among the experimental groups. These data are similar to findings in rainbow trout, where no significant difference in egg weight could be found between experimental groups in response to dietary supplementation of Se (Wischhusen et al., 2019).
Among the fish that were fed PP-rich diets in the current study, the groups receiving above 0.5 mg N-Se Kg−1 showed the greatest OGD of eggs. Furthermore, OGD of eggs had a positive correlation with the length and survival of 3DPH larvae. These findings may be related to altered vitellogenesis and levels of blood steroid hormones as these factors were reported to be influenced in female rainbow trout fed dietary selenomethionine at a high concentration of 4.5 mg Kg−1 (Wiseman et al., 2011). Further studies are required to investigate this possibility in A. arabicus.
Both the fertilization rate (FR) and the hatchability of eggs are the key parameters to assess the reproduction performance of broodfish and are known to be influenced by broodstock nutrition (Izquierdo et al., 2001; Zakeri et al., 2009). We found that FR was significantly lesser in the groups fed PP-rich diets with low N-Se level (0 and 0.5 mg N-Se Kg−1) in comparison to that in the control group. Also, among the fish that were fed PP-rich diets, the fish fed diets supplemented with 2 or 4 mg N-Se Kg−1 displayed the highest FR. These findings suggest that substitution of fishmeal by plant ingredients in fish feeds can have detrimental effects on A. arabicus reproduction by decreasing the FR, which can be removed by supplementing N-Se. Based on our data, there was a positive correlation between the FR and Se content of eggs, indicating that FR is improved by Se content of oocytes provided by maternal nutrition during oogenesis (Wischhusen et al., 2021).
The present study showed that hatching rate (HR) was comparable in fish fed the control and 2 mg N-Se Kg−1 diets and significantly higher in comparison with other groups. Similarly, Wischhusen et al. (2019) reported that dietary Se (selenomethionine) in rainbow trout broodstock nutrition influences the HR. Moreover, in our study, HR was positively correlated with Se content of eggs. These observations suggest that the amount of Se transferred to the oocytes provides the available pool needed for the embryonic development in A. arabicus.
We observed higher percentage of embryogenesis abnormality in broodstock groups fed on PP-rich diets with lesser N-Se supplementation (0–1 mg N-Se Kg−1). The published literature shows that interactions between genetic, environmental and nutritional factors are involved in occurrence of abnormality (Babaheydari et al., 2016; Fraser et al., 2012; Lall & Lewis-McCrea, 2007). Fish at the early developmental stages are known to be more susceptible to oxidative stresses due to lack of efficient antioxidant defence system (Fontagné et al., 2008; Fontagné-Dicharry et al., 2014). Therefore, the Se supplementation of the maternal diet has received considerable attention for protection of fish during embryonic and larval stages (Pappas et al., 2008). Moreover, the percentage of abnormal embryogenesis in our study showed a negative correlation with the yolk sac length (YSL). The egg yolk is full of proteins, lipids, vitamins and minerals to supply the nutritive compounds for nutrition of developing embryos and hatchlings (Díaz et al., 2010). Thus, the prevalence of embryo abnormality in groups with little Se supplementation in this study may be associated with decreased maternal investment of Se in the yolk sac.
The results of this study showed that the length of hatchlings and 3DPH offspring were influenced by N-Se supplementation of broodstock diet. The greatest lengths of hatchlings and 3DPH larvae were recorded in 4 and 2 mg N-Se Kg−1 groups, respectively. Data from the present literature on the impact of maternal Se intake on growth (length or weight) of progeny are inconsistent. Similar to our findings, some works have shown that addition of Se in the broiler breeder diets could have beneficial effects on growth of the offspring (Pappas et al., 2005, 2006a, 2006b). On the contrary, Wischhusen et al. (2019) reported that mean weight of rainbow trout swim-up fry was not significantly affected by supplementing a plant-protein based broodfish diet with different sources of Se. Some selenoproteins are known to be involved in growth performance by promoting proliferation and differentiation of the cells (Park et al., 2012). Also, it is demonstrated that the expression of selenoprotein genes in the liver or muscle of rainbow trout were significantly upregulated in response to elevated dietary Se levels (Wang et al., 2018). Thus, the greater length of hatchlings and 3DPH larvae originating from the broodstock fed dietary N-Se at high levels of 2–4 mg Kg−1 could be connected to the upregulation of those selenoproteins involved in cell proliferation.
Vertical transmission of immune factors and antioxidants from mother to progeny is known to play a crucial role in protecting the susceptible progeny against pathogenic invasions and oxidative stresses at early stages of life (Pappas et al., 2008; Yue et al., 2013). In the present study, 3DPH larvae descending from fish fed replete plant-protein based diets (2 and 4 mg N-Se Kg−1) had higher survival rates than those originating from broodfish fed deficient plan-protein based diets (0 and 0.5 mg N-Se Kg−1). Similar to these results, administration of Se (selenomethionine) in a plant-protein based rainbow trout broodstock diet enhanced the survival rate of swim-up fry (Wischhusen et al., 2019). Moreover, the survival rate in our study was positively correlated with the Se content in 3DPH larvae. These results support the theory that maternal transfer of Se provides the crucial protection to the developing offspring.
5 CONCLUSION
In conclusion, the results of our study indicate that addition of 2–4 mg N-Se Kg−1 in plant protein based diets with low basal Se concentrations can have beneficial effects on reproductive performance of female A. arabicus by increasing egg production, egg size, fertilization rate and egg hatchability. It was also demonstrated that supplementation of plant-based diets with N-Se for A. arabicus broodfish improves the larval quality by reducing the percentage of embryo abnormality, and increasing the length and survival of hatchlings and 3DPH larvae. Moreover, the present work confirms that maternal Se intake influences the total Se content in eggs and larvae.
ACKNOWLEDGEMENTS
This research was funded by grant no. 435816 from Khorramshahr University of Marine Science and Technology.
CONFLICT OF INTEREST
I wish to confirm that there are no known conflicts of interest associated with this publication.
AUTHOR CONTRIBUTIONS
Sadegh Saffari: Methodology and Review; Saeed Keyvanshokooh: Conceptualization, Writing—Review and Editing and Supervision; Mansour Torfi Mozanzadeh: Methodology, Statistical analysis, Writing and Review; Ali Shahriari: Methodology.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




