Effect of 5′-inosine monophosphate (IMP) and 5′-guanosine monophosphate (GMP) on the growth, immunity and muscle composition of turbot, Scophthalmus maximus (Linnaeus, 1758)
Abstract
We evaluated the effect of different concentrations of 5′-inosine monophosphate (IMP) and 5′-guanosine monophosphate (GMP) on the growth, immunity and muscle composition of turbot Scophthalmus maximus. Eight diets (containing no IMP or GMP, or 0.5 g/kg IMP, 1.0 g/kg IMP, 2.0 g/kg IMP, 0.5 g/kg GMP, 1.0 g/kg GMP, 2.0 g/kg GMP, or 0.5 g/kg IMP plus 0.5 g/kg GMP) were prepared. A total of 360 fish (average body weight of 105 g) were randomly selected and placed in groups into 24 plastic aquaria (8 treatments × 3 replicates × 15 individuals per plastic aquaria). The tanks were maintained at the temperature of 15 ± 2°C. The experimental diets were fed for 60 days. The specific growth rate (SGR) was significantly higher in S. maximus fed with IMP or GMP compared with fish fed neither IMP nor GMP. The highest SGR was observed in fish fed with 1.0 g/kg IMP. Supplementation with these dietary nucleotides had a positive, but not significant effect on the activity of superoxide dismutase, alkaline phosphatase and acid phosphatase. There was a significant difference in the moisture and crude lipid content of muscle from S. maximus fed the different diets compared with control fish. The highest moisture content was 83.44 for a diet of 0.5 g/kg IMP plus 0.5 g/kg GMP, which was also significantly higher when compared to fish fed alternative diets. The crude lipid content of S. maximus fed diets containing either IMP or GMP was significantly higher than those fed diets without IMP or GMP. Thus, according to these results, the optimal level of dietary IMP is 1.0 g/kg, which correlates with the largest increase in growth performance of S. maximus.
1 INTRODUCTION
Scophthalmus maximus (Linnaeus, 1758) is an important economic fish species in northern China because of its rapid growth, high nutritional value, low-temperature resistance and easy domestication. Given its high nutritional value and the accompanying increased market demand, the S. maximus aquaculture industry has developed rapidly since the fish was initially introduced in 1992, making this species the most-abundant farmed marine species in China (Guan et al., 2017). However, the increasing intensity of this aquacultural industry and the low quality of turbot-formulated feed can place substantial physiological stress on the animals, which can result in immune suppression, reduced growth rate, increased susceptibility to disease, and resulting economic losses to the turbot aquacultural industry. To maintain the sustainable development of S. maximus aquaculture, as well as that of other species, there is a need to optimize the feed products used. A proper diet is essential to improve the fish health and to ensure a high-quality and sustainable product for the consumer.
Nucleotides, as novel diet supplements for farmed fish, have attracted much attention in recent years (Burrells, Williams, & Forno, 2001; Li, Lawrence, Castille, & Gatlin, 2007). Reports concerning human and terrestrial animals have indicated that the dietary supplementation with nucleotides can improve the immunity and environmental tolerance of organisms (Carver & Walker, 1995; Grimble & Westwood, 2000). Dietary supplementation with nucleotides can enhance the growth as well as immunity and disease resistance in fish, as reported by Li, Gatlin, and Neill (2007) and Tahmasebi-Kohyani, Keyvanshokooh, Nematollahi, Mahmoudi, and Pasha-Zanoosi (2012). Burrells et al. (2001) demonstrated that the dietary supplementation with nucleotides enhanced the resistance of salmonids to various pathogenic organisms and environmental changes. Research on dietary nucleotides in aquatic animals has focused mainly on improving the immunity, altering intestinal structure and increasing stress tolerance, as well as modulating innate and adaptive immune responses (Li, Lewis, & Gatlin, 2004; Li, Lawrence et al., 2007; Sakai, Taniguchi, Mamoto, Ogawa, & Tabata, 2001). However, our understanding of the effect of nucleotides on the growth performance, immunity and muscle composition of the commercially important S. maximus remains limited. Few studies have investigated the effectiveness of diet additives on the growth performance and health in S. maximus.
In aquaculture systems, maintaining the health (i.e., immunocompetence and disease resistance) of the cultured animals is vital. Immunological enzyme activities can be used to evaluate the immune capacity of S. maximus (Xia et al., 2013). Thus, this study was designed to investigate the influence of 5′-inosine monophosphate (IMP) and 5′-guanosine monophosphate (GMP) on the growth, immunity and muscle composition of S. maximus. The present results of this study will provide the basic information to promote the inclusion of IMP and GMP in fish feed for this economically important species.
2 MATERIALS AND METHODS
2.1 Experimental diets
5′-Inosine monophosphate disodium salt and GMP disodium salt were obtained from the CJ International Trading Co., Ltd. (Liaocheng City, Shandong, China).
Eight diets (containing no IMP or GMP, or 0.5 g/kg IMP, 1.0 g/kg IMP, 2.0 g/kg IMP, 0.5 g/kg GMP, 1.0 g/kg GMP, 2.0 g/kg GMP, or 0.5 g/kg IMP plus 0.5 g/kg GMP) were prepared from the powder mixtures of fishmeal, soybean meal, flour, vitamins and minerals. The composition of the diet was as follows: dry matter 907 g/kg, protein 500 g/kg, lipid 120 g/kg, ash 123 g/kg. Diets with different proportions of IMP and GMP were formulated, as indicated in Table 1. The raw materials were ground and sieved to a particle size <75 μm and then mixed together according to the relevant formula. Eight experimental diets were produced using a single-screw extruder (AHSK215, Buhler, Switzerland). Production parameters were 120°C and 35 standard atmospheric pressure (34 kg/cm2); 2~3 mm diameter extruded diets were selected for the study.
| Ingredient | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| Fishmeal | 600 | 600 | 600 | 600 | 600 | 600 | 600 | 600 |
| Flour | 147.0 | 146.5 | 146.0 | 145.0 | 146.5 | 146.0 | 145.0 | 146.0 |
| Gluten | 82.7 | 82.7 | 82.7 | 82.7 | 82.7 | 82.7 | 82.7 | 82.7 |
| Soybean meal | 82.0 | 82.0 | 82.0 | 82.0 | 82.0 | 82.0 | 82.0 | 82.0 |
| Shell powder | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Lecithin | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Fish oil | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 | 19.3 |
| Soybean oil | 19.0 | 19.0 | 19.0 | 19.0 | 19.0 | 19.0 | 19.0 | 19.0 |
| Vitamina | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Mineralb | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| IMP | 0.00 | 0.5 | 1.0 | 2.0 | 0.00 | 0.00 | 0.00 | 0.5 |
| GMP | 0.00 | 0.00 | 0.00 | 0.00 | 0.5 | 1.0 | 2.0 | 0.5 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
- A group: no IMP or GMP; B group: 0.5 g/kg IMP; C group: 1.0 g/kg IMP; D group: 2.0 g/kg IMP; E group: 0.5 g/kg GMP; F group: 1.0 g/kg GMP; G group: 2.0 g/kg GMP; H group: 0.5 g/kg IMP plus 0.5 g/kg GMP.
- a Vitamin premix contained the following amount which were diluted in cellulose (g/kg premix): L-ascorbic acid, 150; DL-α-tocopheryl acetate, 4; thiamine hydrochloride, 16; riboflavin, 15; pyridoxine hydrochloride, 30; niacin, 90; Ca-D-pantothenate, 36; myo-inositol, 120; D-biotin, 0.6; folic acid, 3; menadione, 8; retinyl acetate, 6.4; cholecalciferol, 2; cyanocobalamin, 0.008; ethoxyquin 24;
- b Mineral premix contained the following ingredients which were diluted in zeolite (g/kg premix): MgSO4 7H2O, 160; Ferric citrate, 24; ZnSO4 H2O, 18; CuSO4 5H2O, 6; AlCl3 6H2O, 12; KIO3, 0.08; MnSO4 H2O, 4; CoCl2 6H2O, 0.08.
2.2 Experimental procedures
Scophthalmus maximus were obtained from a commercial farm in Tianjin (China). Fishes were acclimatized for a week before the experiments at 15 ± 2°C in oxygenated sea water (30‰ salinity). After 24 hr of starvation, 360 fish (average body weight of 105 g) were randomly selected and placed in groups into 24 plastic aquaria (100 L) (8 treatments × 3 replicates × 15 individuals per plastic aquaria). The aquaria contained 80 L of aerated water with a salinity of 30‰ ± 2‰, and a dissolved oxygen level >5 mg/L. The tanks were maintained at the temperature of 15 ± 2°C. During the experimental period, fishes were fed twice a day (at 09:00 and 16:00 hr), for 60 consecutive days. At the end of 60 days, all of the test animals were harvested 24 hr after the final feed was administered, and each S. maximus from each tank was weighed.
2.3 Calculation of growth
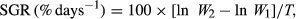 (1)
(1)2.4 Chemical analysis of feed and fish muscle
A standard method of Association of Official Analytical Chemists (Association of Official Analytical Chemists (AOAC), 1990) was used for the analysis of fish muscle (moisture, crude protein, crude lipid and ash) and the experimental diets. The dry matter remaining after the samples had been dried at 105°C was combusted to ash at 550°C for 4 hr (Xia et al., 2012). The total nitrogen content was estimated using the Dumas method (Ebeling, 1968) and a KDY-08 nitrogen/protein determinator (Shanghai Ruizheng Instrument Co., Ltd., P.R., Shanghai City, China). The crude protein content was determined indirectly (nitrogen × 6.25).
2.5 Immunological assays
Blood was collected from the caudal vein of three fish from each replicate tank and pooled for serum analysis. Serum samples were obtained by centrifugation at 4,000× g for 15 min using a high-speed refrigerated microcentrifuge (MX-160; Tomy Tech USA Inc., Tokyo, Japan) and kept at −80°C.
The malondialdehyde (MDA) concentration in serum was detected according to the method of Mendes, Cardoso, and Pestana (2009). Superoxide dismutase (SOD) activity in serum was measured by its ability to inhibit superoxide anions generated by a xanthine and xanthine oxidase reaction system (Marklund & Marklund, 1974). Alkaline phosphatase (AKP) activity was determined by the disodium phenyl phosphate method (Higashino, Hashinotsume, Kang, Takahashi, & Yamamura, 1972). The activity of MDA, SOD, AKP and acid phosphatase (ACP) was measured using a kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (Zhao et al., 2017).
2.6 Statistical analysis
All data were checked for normality and homogeneity of variance before analysis. Growth performance, activity of immune enzyme and muscle composition were subjected to one-way analysis of variance (ANOVA). If any effect was significant, difference between the means was analysed by Duncan's test for unplanned multiple comparison of mean (p < .05). All the statistical analyses were performed using SPSS 19.0 (IBM, Armonk, NY, USA).
3 RESULTS
3.1 Growth performance
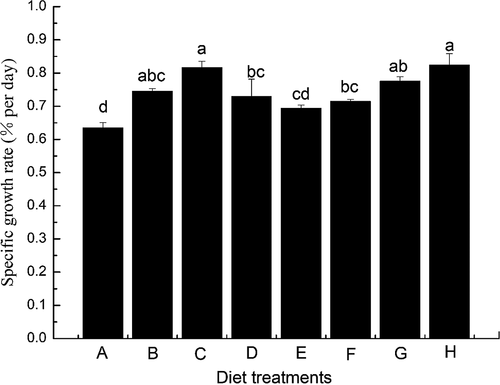
There was a significant difference in SGR of S. maximus fed with the different diets (Figure 1). The highest SGR was 0.82 (% per day), for a diet of 1.0 g/kg IMP, although this value did not differ significantly from those for the diets of 0.5 g/kg IMP, 2.0 g/kg GMP, and 0.5 g/kg IMP plus 0.5 g/kg GMP. Scophthalmus maximus fed on diets containing IMP or GMP showed that the SGR values were significantly higher than those fed neither IMP nor GMP (p < .05).
3.2 Composition analysis of muscle
There was a significant difference in the moisture and crude lipid content of the muscle from S. maximus fed with the different diets, but no significant effect on the crude protein and ash content (Table 2). The highest moisture content was 834.4 g/kg, for a diet of 0.5 g/kg IMP plus 0.5 g/kg GMP, and was significantly higher than for the other diets. Scophthalmus maximus fed on diets containing IMP or GMP had a crude lipid content that was significantly higher than those fed neither IMP nor GMP, except for those fed the 1.0 g/kg GMP diet (p < .05).
| Parameters | Diets treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| Moisture | 80.04 ± 0.10bcd | 80.78 ± 0.52bc | 79.29 ± 0.37d | 80.19 ± 0.27bcd | 79.72 ± 0.37cd | 80.45 ± 0.46bcd | 81.02 ± 0.40b | 83.44 ± 0.38a |
| Crude lipid | 2.28 ± 0.06bc | 2.77 ± 0.17a | 2.70 ± 0.10a | 2.58 ± 0.03ab | 2.57 ± 0.09ab | 2.18 ± 0.09c | 2.46 ± 0.14abc | 2.76 ± 0.03a |
| Crude protein | 15.21 ± 0.21 | 14.89 ± 0.33 | 15.45 ± 0.36 | 15.15 ± 0.09 | 15.23 ± 0.29 | 15.50 ± 0.34 | 15.08 ± 0.21 | 15.13 ± 0.28 |
| Ash | 3.75 ± 0.09 | 3.74 ± 0.16 | 4.08 ± 0.09 | 4.09 ± 0.12 | 3.99 ± 0.07 | 3.92 ± 0.19 | 4.10 ± 0.12 | 4.03 ± 0.31 |
- Values in the same row with different superscripts differ significantly (n = 3; p < .05).
- A group: no IMP or GMP; B group: 0.5 g/kg IMP; C group: 1.0 g/kg IMP; D group: 2.0 g/kg IMP; E group: 0.5 g/kg GMP; F group: 1.0 g/kg GMP; G group: 2.0 g/kg GMP; H group: 0.5 g/kg IMP plus 0.5 g/kg GMP.
3.3 Immunological indicators
There were no significant differences in the activity of SOD, AKP or ACP in fish fed with the different diets (p > .05) (Table 3). The activity of SOD, AKP and ACP was higher in fish fed with either IMP or GMP compared with fish fed the control diet, although the difference was not significant (p > .05). Malondialdehyde activity was lower in fish fed with either IMP or GMP compared with fish fed with control diet, although the difference was not significant (p > .05).
| Parameters | Diets treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |
| MDA (nmol/ml) | 5.56 ± 1.05ab | 4.83 ± 0.81ab | 5.94 ± 1.70a | 4.15 ± 0.40ab | 4.01 ± 0.46ab | 4.15 ± 0.61ab | 3.04 ± 0.33b | 3.77 ± 0.22ab |
| SOD (U/ml) | 108.54 ± 7.22 | 114.99 ± 3.28 | 114.59 ± 4.50 | 114.40 ± 1.52 | 110.69 ± 1.01 | 113.42 ± 4.29 | 115.96 ± 2.89 | 114.59 ± 0.78 |
| AKP (U/ml) | 6.80 ± 0.37 | 7.54 ± 4.21 | 9.75 ± 7.22 | 10.29 ± 2.01 | 12.20 ± 2.02 | 8.32 ± 1.22 | 8.29 ± 1.98 | 8.13 ± 2.26 |
| ACP (U/ml) | 10.05 ± 0.65 | 11.77 ± 3.90 | 13.75 ± 6.20 | 15.18 ± 4.58 | 12.71 ± 2.77 | 14.23 ± 3.51 | 12.36 ± 2.32 | 13.60 ± 4.27 |
- Values in the same row with different superscripts differ significantly (n = 3; p < .05).
- A group: no IMP or GMP; B group: 0.5 g/kg IMP; C group: 1.0 g/kg IMP; D group: 2.0 g/kg IMP; E group: 0.5 g/kg GMP; F group: 1.0 g/kg GMP; G group: 2.0 g/kg GMP; H group: 0.5 g/kg IMP plus 0.5 g/kg GMP.
4 DISCUSSION
5′-inosine monophosphate and 5′-guanosine monophosphate as diet additives have recently attracted extensive attention and investment from the aquaculture researchers and industry. Several studies have focused on the physiological and biochemical functions of IMP and GMP, such as enhancement of immune responses and increasing disease resistance in marine animals (Burrells et al., 2001; Li et al., 2004; Tahmasebi-Kohyani et al., 2012).
Hossain, Koshio, Ishikawa, Yokoyama, and Sony (2016) reported that the SGR was significantly higher in Pagrus major fed a nucleotide-supplemented diet. Yigit et al. (2006) indicated that the nucleotide level of the feed had a significant effect on the SGR of Psetta maeotica. Chen et al. (2017) demonstrated that the GMP supplemented at 0.6 and 1.2 g/kg significantly enhanced the growth of sea cucumber, while Wei et al. (2015) indicated that the SGR was also significantly higher in sea cucumber fed a diet containing 375 mg/kg nucleotides compared with those fed a basal diet without nucleotide supplementation. Added dietary nucleotides significantly improved the SGR of Litopenaeus vannamei, as reported by Oujifard, Abedian Kenari, Taheri, and Ghanizadeh Kazerouni (2012). The results from the present study are similar to those described above. Scophthalmus maximus fed diets containing IMP or GMP showed that the SGR values were significantly higher than those fed no IMP or GMP. However, a clear explanation of how dietary nucleotide and nucleosides enhance the growth has been lacking. Nevertheless, several studies have showed that the dietary nucleotide supplementation can promote animal feeding. Mackie and Adron (1978) showed that the S. maximus preferred diets containing IMP, while Kubitza, Lovshin, and Lovell (1997) reported that the IMP has phagostimulatory effects on Micropterus salmoides. From the above results, we can conclude that the increased ingestion rate and reduced feed conversion ratio contribute to faster growth in fish fed with IMP or GMP.
Wei et al. (2015) indicated that the sea cucumber fed diets with nucleotides showed no significant differences in the activities of SOD and ACP in their coelomic fluid compared with those fed a basal diet. Song, Lim, and Lee (2012) demonstrated that the Paralichthys olivaceus fed a 2 or 4 g/kg IMP diet had significantly higher lysozyme activities compared with fish fed the control diet; however, SOD activity did not differ significantly among the treatments. Tahmasebi-Kohyani et al. (2012) reported that the Oncorhynchus mykiss fed a nucleotide-supplemented diet tended to have lower AKP activity compared with fish fed a control diet. The results from the present study are similar to those described above. No significant differences were detected in the activity of MDA, SOD, AKP or ACP in fish fed different diets. However, Xiang, Zhou, Chen, and Zheng (2011) indicated that the dietary nucleotide supplementation improved the activities of lysozymes, SOD and CAT in Cyprinus carpio. Their results indicated that, depending on the different experimental conditions (e.g., species, dose, time and form of administration,), there might not be the same trend of change in antioxidant enzyme activity (Zhao et al., 2017). In the present study, the dietary nucleotide supplementation had no significant effect on the activity of SOD, AKP or ACP, although there was an increasing trend in that activity. Thus, our results indicated that the adding nucleotides can promote the immune function of turbot, although more indicators are needed to clarify this point.
The addition of nucleotides on feed affects the body composition of turbot. Scophthalmus maximus fed on diets containing IMP or GMP had significantly higher crude lipid contents than did fish fed a control diet. A similar result has been reported by Tan et al. (2009). Their results indicated that the arginine supplementation increased (p < .05) the fat content of longissimus dorsi muscle of pigs compared with controls. However, a clear explanation of why dietary nucleotides can promote the accumulation of fish body fat is lacking. Thus, more research is needed to shed light on this relationship.
5 CONCLUSION
The present study showed that the dietary supplementation with IMP or GMP positively influenced the growth performance, immunity and muscle composition of S. maximus. The optimal proportion of dietary IMP was 1.0 g/kg, which correlated with the optimal growth performance in these fish.
ACKNOWLEDGEMENTS
We thank all members of the research department of Tianjin Lixin Co., Ltd. We are grateful to three anonymous reviewers for their professional revision of the manuscript. This research was supported by National Natural Science Foundation of China (41506192), the Natural Science Foundation of Tianjin, China (14JCQNJC15200), Innovation Team of Tianjin Fisheries Research System (ITTFRS2017002 and ITTFRS2017008) and Tianjin Agricultural Science and Technology Achievement Transformation and Promotion project (201601400).