Effect of graded levels of dietary thiamine on the growth performance, body composition and haemato-biochemical parameters of juvenile Sclizothorax prenanti
Abstract
This study was conducted to determine dietary thiamine requirement of juvenile Sclizothorax prenanti and evaluate the effect of dietary thiamine levels on growth performance, body composition and haemato-biochemical parameters for this fish species. The seven experimental diets were formulated to contain the graded levels of thiamine (0, 10, 20, 30, 40, 60 and 100 mg kg−1 diet, respectively), providing the actual dietary thiamine values of 0.31 (control), 9.82, 21.49, 29.83, 41.66, 62.24 and 114.58 mg kg−1 diet, respectively. Each diet was assigned to three replicate groups of S. prenanti (initial body weight: 13.46 ± 0.28 g, means ± SD) for 60 days. Increasing dietary thiamine level up to 21.49 mg kg−1 diet increased weight gain rate (WGR), specific growth rate (SGR), feed efficiency (FE) and protein efficiency ratio (PER) (P < 0.05), beyond which they remained nearly unchanged. Similarly, hepatic thiamine concentration and several serum biochemical parameters (transketolase activity, triglyceride and total cholesterol contents) increased with increasing levels of thiamine up to 21.49 mg kg−1 diet (P < 0.05) and, thereafter, remained almost constant. However, no significant differences in body composition (moisture, protein, lipid and ash contents) were found among dietary thiamine treatments (P > 0.05). Analysis by the broken-line regression of WGR, SGR, FE, PER, hepatic thiamine concentration and serum transketolase activity indicated that dietary thiamine requirements in juvenile S. prenanti were 18.45–25.91 mg kg−1 diet.
Introduction
Thiamine, known as vitamin B1, is a water-soluble vitamin, which has been demonstrated to be essential for fish (NRC 2011). It functions in all cells as the coenzyme thiamine pyrophosphate (TPP) necessary for several metabolic decarboxylation and transketolation reactions (Lim et al. 2011). Thiamine deficiency may cause poor growth eels (Anguilla japonica) (Hashimoto et al. 1970), high mortality of lake trout (Salvelinus namaycush) (Lee et al. 2009), haemorrhage and ataxia (Hashimoto et al. 1970) as well as anorexia and dark pigmentation (Morito et al. 1986; Masumoto et al. 1987). At present, optimal dietary thiamine requirement has been determined in several species including turbot (Scophthalmus maximus) (Cowey et al. 1975), channel catfish (Ictalurus punctatus) (Murai & Andrews 1978), rainbow trout (Salmo gairdneri) (Morito et al. 1986) and Jian carp (Cyprinus carpio var. Jian) (Huang et al. 2011).
Schizothorax prenanti, a species of cold-water omnivorous fish, is mainly distributed in the upper stream of the Yangtze River (Fang et al. 2014). In recent years, commercial farming of this fish has become of increasing interest in south-west China for its delicious meat and high market value. To date, a few studies have been conducted on morphological and cytochemical studies of peripheral blood cells (Fang et al. 2014) as well as characterization, tissue distribution and regulation of several genes involved in feeding behaviour such as neuropeptide Y (Wei et al. 2013), leptin and cholecystokinin (Yuan et al. 2014) for this fish species. However, limited information was available on the nutrient requirements for this fish species. The purpose of this study was to investigate the dietary thiamine requirement of juvenile S. prenanti and evaluate the effect of thiamine copper levels on growth performance, body composition and haemato-biochemical parameters for the fish species.
Materials and methods
Experimental diets
The composition of the basal diet is given in Table 1. Casein and gelatin, fish oil and soybean oil, and α-starch, maize starch and cellulose were used as protein, lipid and carbohydrate sources, respectively. The basal diet was supplemented with graded levels of thiamine hydrochloride (0, 10, 20, 30, 40, 60, 100 mg kg−1) (purchased from Xiamen Kingdomway Vitamin Incorporation, Xiamen, China) to formulate seven experimental diets. All diets contained 386.2 g crude protein kg−1 diet and 15.57 MJ gross energy kg−1 diet. Diet preparation and storage were similar to those described in our previous study (Zhao et al. 2012). Briefly, diets were prepared by thoroughly mixing all ingredients. Distilled water was included to achieve a proper pelleting consistency, and the mixture was further homogenized and extruded at 40–45 °C through a 2-mm sieve. The noodle-like diets were dried at 40 °C and stored at −20 °C until used. Final dietary thiamine concentrations in the experimental diets were analysed in duplicate using the method of AOAC (1990) to be 0.31, 9.82, 21.49, 29.83, 41.66, 62.24 and 114.58 mg kg−1 diet, respectively.
| Ingredient | Content (g kg-1 diet) |
|---|---|
| Casein | 340.0 |
| Gelatin | 70.0 |
| α-starch | 150.0 |
| Maize starch | 120.0 |
| Cellulose | 100.0 |
| Fish oil | 40.0 |
| Soybean oil | 40.0 |
| Dextrin | 90.0 |
| Vitamin mixturea | 10.0 |
| Mineral mixtureb | 10.0 |
| Ca(H2PO4)2 | 20.0 |
| Choline chloride | 10.0 |
| Nutrition content | |
| Gross energy (kJ kg−1) | 155.7 |
| Crude protein | 386.2 |
| Crude lipid | 85.1 |
| Crude ash | 52.6 |
- a Vitamin mixture (g kg−1 mixture): VA, 30 000 IU; VC, 200 mg; VD3, 25000 IU; VE, 600 mg; VK, 100 mg; riboflavine, 60 mg; niacin, 100 mg; calcium-D-pantothenate, 120 mg; pyridoxine, 40 mg; cyanocobalamin, 0.2 mg; D-biotin, 7 mg; folic acid, 20 mg; and inositol, 250 mg.
- b Mineral mixture (g kg-1 mixture): FeSO4·7H2O (197.0 g kg-1 Fe), 121.83 g; CuSO4·5H2O (250.0 g kg-1 Cu), 7.20 g; MnSO4·H2O (318.0 g kg-1Mn), 5.16 g; ZnSO4·7H2O (225.0 g kg-1Zn), 15.56 g; KI (38.0 g kg-1I), 6.58 g; and NaSeO3 (10.0 g kg-1Se), 2.10 g.
Feeding management
All experimental protocols were approved by the Animal Care Advisory Committee of Southwest University. Juvenile S. prenanti were obtained from Emei Hatchery (Sichuan, China) and transferred to indoor tanks for 2 weeks of acclimatization. Afterwards, 1050 fish (initial weight 13.46 ± 0.28 g) were randomly assigned to 21 glass aquaria (150L × 100 W × 50H cm) in which water depth is 40 cm, with 50 fish for each aquaria. Each diet was assigned to three experimental aquaria in a completely randomized design. A closed recirculating water system and oxygen auto-supplemented system were used to maintain the optimal water quality to S. prenanti. Water was drained through so as to reduce ammonia concentrations and remove solid substance. The fish were fed with the respective diet six times daily (08:00, 10:30, 13:00, 15:30, 18:00, 20:30) and carefully observed to ensure satiation without overfeeding for 60 days. Thirty minutes after the feeding, uneaten feed was removed by siphoning and then air-dried. The experimental units were under natural light and dark cycle. Water temperature, pH and dissolved oxygen were maintained at 22 ± 1 °C, 7.0 ± 0.3 and 6.0 ± 0.2 mg L-1, respectively.
Sample collection and analysis
The procedures of sample collection were similar to those described in the other study from our laboratory (Huang et al. 2011). At the end of the 60-day period, 24 h after the last feeding, all fish were counted and weighed to determine survival, weight gain rate (WGR), specific growth rate (SGR), feed efficiency (FE) and protein efficiency (PER). After obtaining the final total weight of fish in each aquaria, five fish per tank were randomly selected and frozen at −20 °C for subsequent determination of whole-body composition. Another five fishes from each aquaria were randomly selected and anaesthetized with benzocaine (50 mg L−1). Blood samples were obtained from the caudal vein and centrifuged at 1300 g for 15 min at 4 °C. The serum was collected and used for triglyceride (TG), total cholesterol (TCH) and transketolase activity (TKA) determination. The liver was quickly collected and stored at −70 °C until analysis.
The carcass proximate compositions were analysed according to AOAC (1998). The concentrations of serum TG and TCH were measured using the diagnostic reagent kit according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). TKA was determined by the formation (accumulation) of sedoheptulose followed spectrophoto-metrically at 580 nm and 670 nm (Warnock 1970) by colorimetric assays (Bein et al., (1960).; Brin 1964). Hepatic thiamine concentration was determined according to the method of AOAC (1998).
Calculations and statistical analysis
Data on initial body weight (IBW), final body weight (FBW) and feed intake (FI) proximate composition of feed were used to calculate the following parameters:
- Weight gain rate (WGR) = 100 × (mean final weight−mean initial weight)/initial weight.
- Specific growth rate (SGR) = 100 × [ln (mean final weight)−ln(mean initial weight)]/days.
- Feed efficiency (FE) = 100 × weight gain (g)/feed intake (g).
- Protein efficiency ratio (PER) = weight gain (g)/protein intake (g).
All data were presented as means ± SD and subjected to one-way analysis of variance (anova) followed by the Duncan's method to determine significant differences among treatment groups. P < 0.05 was considered to be statistically significant. Thiamine requirement of juvenile S. prenanti was estimated by broken-line analysis (Robbins et al. 1979) based on WGR, SGR, FE, PER, hepatic thiamine concentration and serum TKA.
Results
Growth performance and body composition
As is shown in Table 2, survival showed no significant differences among the treatments (P > 0.05). WGR, SGR, FE and PER increased with increasing dietary thiamine levels from 0.31 to 21.49 mg kg−1 diet and then plateaued above these levels. The equations obtained for WGR, SGR, FE and PER by broken-line analysis were as follows: (YWGR = 387.80 + 6.8417(x−21.47), R2 = 0.9922, Ymax = 387.80), (YSGR = 2.64 + 0.027(x-19.33), R2 = 0.9973, Ymax = 2.64), (YFE = 65.76 + 0.8395(x-20.23), R2 = 0.9977, Ymax = 65.76) and (YPER = 1.66 + 0.0212(x-20.28), R2 =0.9979, Ymax = 1.66), which indicated that optimal dietary thiamine requirements were 21.47 (Fig. 1), 19.33(Fig. 2), 20.23 (Fig. 3) and 20.28 (Fig. 4) mg kg−1 diet, respectively. However, no significant differences were observed in body composition among the treatments (Table 3).
| Dietary thiamine levels (mg kg−1 diet) | |||||||
|---|---|---|---|---|---|---|---|
| 0.31 | 9.82 | 21.49 | 29.83 | 38.66 | 59.24 | 104.58 | |
| IBW | 10.47 ± 0.04 | 10.46 ± 0.05 | 10.45 ± 0.07 | 10.46 ± 0.09 | 10.45 ± 0.11 | 10.47 ± 0.07 | 10.45 ± 0.15 |
| FBW | 36.34 ± 0.27a | 41.92 ± 0.90b | 51.36 ± 1.13c | 49.69 ± 0.34c | 50.90 ± 1.25c | 52.01 ± 2.80c | 51.13 ± 1.05c |
| WGR | 247.17 ± 3.52a | 300.73 ± 8.44b | 391.32 ± 10.28c | 374.90 ± 3.81c | 387.04 ± 11.55c | 396.61 ± 26.07c | 389.15 ± 9.42c |
| SGR | 2.08 ± 0.02a | 2.31 ± 0.04b | 2.65 ± 0.21c | 2.59 ± 0.04c | 2.64 ± 0.09c | 2.67 ± 0.04c | 2.63 ± 0.03c |
| FE | 48.75 ± 1.90a | 57.49 ± 1.32b | 66.58 ± 2.55c | 66.23 ± 0.881c | 66.28 ± 2.42c | 65.40 ± 1.56c | 64.30 ± 2.47c |
| PER | 1.23 ± 0.05a | 1.45 ± 0.03b | 1.68 ± 0.06c | 1.67 ± 0.02c | 1.67 ± 0.06c | 1.64 ± 0.04c | 1.62 ± 0.06c |
| SR | 96.96 ± 3.30 | 97.83 ± 1.57 | 98.37 ± 1.05 | 97.57 ± 2.25 | 97.99 ± 1.56 | 96.90 ± 1.73 | 98.32 ± 1.67 |
- Values are means ± SD (n = 3).
- IBW, initial body weight; FBW, final body weight; WGR, weight gain rate; SGR, specific growth rate; FE, feed efficiency; PER, protein efficiency ratio; and SR survival rate.
- a Values within the same row having different superscripts are significantly different (P < 0.05).
| Dietary thiamine levels (mg kg−1 diet) | |||||||
|---|---|---|---|---|---|---|---|
| 0.31 | 9.82 | 21.49 | 29.83 | 38.66 | 59.24 | 104.58 | |
| Moisture | 800.6 ± 8.2 | 795.5 ± 6.2 | 796.3 ± 6.9 | 797.9 ± 7.4 | 791.6 ± 6.0 | 794.7 ± 6.8 | 794.8 ± 8.3 |
| Protein | 163.2 ± 6.5 | 164.6 ± 1.4 | 171.0 ± 6.2 | 166.2 ± 6.1 | 165.3 ± 4.2 | 165.9 ± 8.8 | 166.0 ± 5.9 |
| Lipid | 15.8 ± 1.0 | 16.5 ± 1.7 | 16.8 ± 0.6 | 17.5 ± 1.4 | 16.4 ± 0.8 | 16.9 ± 1.5 | 16.3 ± 1.1 |
| Ash | 24.7 ± 0.8 | 22.8 ± 1.1 | 23.6 ± 2.3 | 23.3 ± 2.1 | 24.2 ± 2.0 | 22.4 ± 1.7 | 23.1 ± 2.4 |
- Values are means ± SD (n = 3).
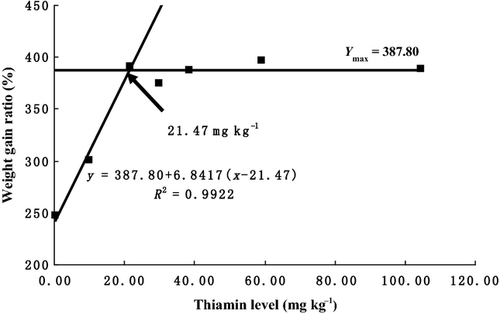

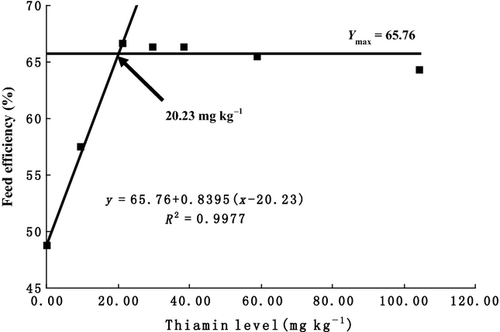
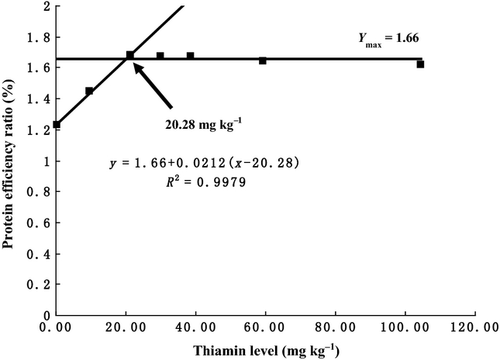
Haemato-biochemical parameters
As shown in Table 4, TKA, TG and TCH in thiamine-supplemented groups were significantly higher than those in the control group (P < 0.05). TKA was the highest for fish with fed with the diet containing 29.83 mg thiamine kg−1 diet (P < 0.05). TG significantly increased with increasing dietary thiamine levels up to 21.49 mg kg−1 diet (P < 0.05), beyond which it remained nearly unchanged (P > 0.05). TCH significantly increased with increasing dietary thiamine levels up to 29.83 mg kg−1 diet (P < 0.05) and then plateaued (P > 0.05). The broken-line regression analysis of TKA against dietary thiamine level indicated that dietary thiamine requirement was 18.45 mg kg−1 diet (Y = 30.45 + 0.425 (X−18.45), R2 = 0.8841, Ymax = 30.45) (Fig. 5).
| Dietary thiamine levels (mg kg−1 diet) | |||||||
|---|---|---|---|---|---|---|---|
| 0.31 | 9.82 | 21.49 | 29.83 | 38.66 | 59.24 | 104.58 | |
| TKA | 21.18 ± 0.93a | 28.14 ± 1.45b | 30.37 ± 1.36bc | 31.08 ± .94c | 30.74 ± 2.31bc | 27.95 ± 1.42b | 29.13 ± 1.67bc |
| TG | 5.64 ± 0.14a | 6.06 ± 0.19b | 6.44 ± 0.21c | 6.61 ± 0.17c | 6.56 ± 0.12c | 6.53 ± 0.14c | 6.49 ± 0.39c |
| TCH | 4.17 ± 0.17a | 4.46 ± 0.18ab | 4.82 ± 0.03bc | 4.95 ± 0.34c | 5.04 ± 0.38c | 4.75 ± 0.24bc | 4.86 ± 0.07bc |
- Values are means ± SD (n = 3).
- TKA, transketolase activity; TG, triglyceride; and TCH, total cholesterol.
- a Values within the same row having different superscripts are significantly different (P < 0.05).
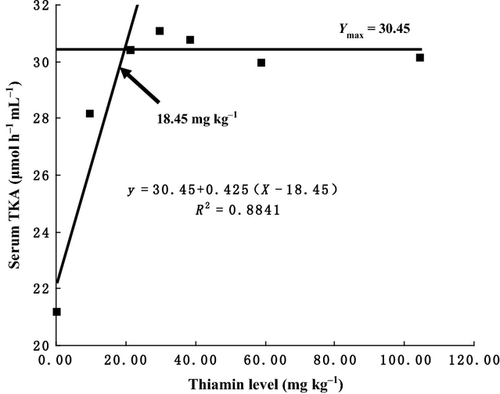
Hepatic thiamine concentration
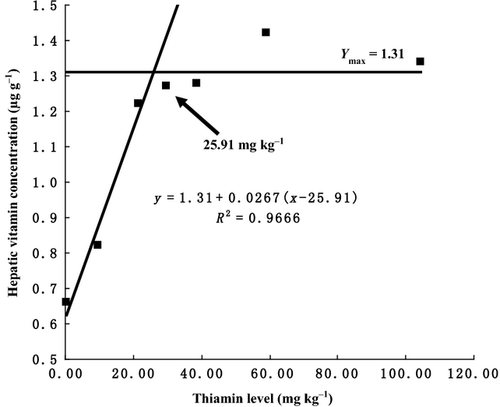
As shown in Table 5, hepatic thiamine concentration increased with increasing levels of thiamine up to 21.49 mg kg−1 diet and, thereafter, remained almost constant. The relationship between hepatic thiamine concentration and dietary thiamine levels was expressed by the broken-line regression model, which indicated that optimal dietary thiamine requirement for S. prenanti was 25.91 mg kg−1 diet (Y = 1.31 + 0.0267(x−25.91), R2 = 0.9666, Ymax =1.13) (Fig. 6).
| Dietary thiamine levels (mg kg−1 diet) | Hepatic thiamine concentration (mg kg−1) |
|---|---|
| 0.31 | 0.66 ± 0.08a |
| 9.82 | 0.82 ± 0.16a |
| 21.49 | 1.22 ± 0.18b |
| 29.83 | 1.27 ± 0.10b |
| 38.66 | 1.28 ± 0.14b |
| 59.24 | 1.44 ± 0.16b |
| 104.58 | 1.34 ± 0.06b |
- Values are means ± SD (n = 3).
- a Values within the same row having different superscripts are significantly different (P < 0.05).
Discussion
In the present study, WGR, SGR, FE and PER in thiamine-supplemented groups were significantly higher than those in the control group, in agreement with the reported in channel catfish (Murai & Andrews 1978) and Jian carp (Huang et al. 2011), indicating that dietary supplementation is necessary for fish growth. In fish, growth rate is related to the digestive and absorptive capacities (Hakim et al., 2006). Thiamine can inhibit cholinesterase activity, which can result in the secretion of digestive juice, gastrointestinal motility and improvement of digestion and utilization of nutrients (Halver & Hardy 2002). On the other hand, thiamine serves as a coenzyme in carbohydrate metabolism and promotes carbohydrate utilization for growth. Therefore, the lower growth rate under thiamine-deficient conditions may be due to the decrease in digestive juice secretion and carbohydrate utilization. Based on the broken-line analysis of WGR, SGR, FE and PER against dietary thiamine level, the optimal dietary thiamine requirement was determined to be 19.33–21.49 mg kg−1 diet, which was higher than those reported in several fish species, such as common carp (0.5 mg kg−1 diet, Aoe et al. 1969) and turbot (0.6–2.6 mg kg−1 diet, Cowey et al. 1975). The difference is probably due to a number of factors, including fish species, basal diet composition, food habits, fish size and age, feeding regime, and environmental and cultural conditions. Although growth performance was significantly affected by the dietary thiamine levels, body composition showed no significant difference among the treatments, similar to that reported in abalone (Zhu et al. 2002). However, in Jian carp, body protein and lipid content were increased by dietary supplemental thiamine, while body moisture and ash decreased with the increment of dietary thiamine (Huang et al. 2011).
Liver vitamin concentration is used as an indicator of vitamin status in fish. In present study, hepatic thiamine concentration significantly increased with increasing dietary thiamine levels up to 21.49 mg kg-1 diet and then remained relatively stable. Similarly, Zhu et al. (2002) found that increasing dietary thiamine yielded positive effects on tissue thiamine levels. Based on thiamine concentration in the liver of S. prenanti, dietary thiamine requirement estimated by the broken line was 25.91 mg kg−1 diet, which was slightly higher than those derived from growth data.
Transketolase activity is very sensitive to thiamine status and has been shown to be the rate-limiting enzyme in the non-oxidative portion of the pentose phosphate pathway (Berthon et al. 1992). In the present study, TKA increased with increasing dietary thiamine levels from 0.31 to 29.83 mg kg−1 diet. A positive correlation between TKA and dietary thiamine levels was also observed in the liver of grouper (Huang et al. 2007), in erythrocytes of rainbow trout (Masumoto et al. 1987), and in viscera and muscle of abalone (Zhu et al. 2002). Lower TKA in thiamine-deficient fish might be attributable to the instability of TKA protein or an inhibition of TKA mRNA translation, as suggested by Sheu et al. (1996). Based on TKA, dietary thiamine requirement estimated by the broken line was 18.45 mg kg−1 diet, which was closed to those derived from growth data.
It has been well documented that thiamine is the cofactor for transketolation reactions in the pentose phosphate cycle, which provides NADPH for fatty acid synthesis (Rindi 1996). TG and TCH concentrations are important indicators of lipid metabolism in animals (Hiraoka et al., 1979). In the present study, the content of TG and TCH in serum from thiamine-supplemented groups was significantly higher in than those from the control group, probably indicating that thiamine supplementation promoted TPP formation in the liver, which enhanced oxidative decarboxylation of α-ketoacids, and consequently increased lipid synthesis. 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), which catalyses the reduction of HMG-CoA to CoA and mevalonate, is a rate-limiting enzyme in the de novo synthesis of cholesterol in fish (Gornati et al. 2005). Study in vitro showed that thiamine-deficient glia reduced cholesterol biosynthesis, accompanied by a comparable reduction in activity of HMGCR (Volpe & Marasa 1978).
In conclusion, the present study indicated that thiamine played an important role in fish growth, and the optimal dietary thiamine requirement for S. prenanti juveniles was 21.47, 19.33, 20.23, 20.27, 25.91 and 18.45 mg kg−1 diet, using WGR, SGR, FE, PER, hepatic thiamine concentration and serum TKA as criteria, respectively. The content of serum TG and TCH was also significantly influenced by dietary thiamine levels.
Acknowledgements
We thank Southwest University for supporting this research. The work was supported by the Fundamental Research Funds for the Central Universities of Southwest University (Grant No. XDJK2009C165).




