Perioperative complications in patients on low-molecular-weight heparin bridging therapy
Abstract
Background
Patients taking warfarin are often given interim anticoagulation in the perioperative period. Institutional guidelines that use low-molecular-weight heparin (LMWH) ‘bridging’ while the international normalized ratio (INR) is sub-therapeutic are often based on the American College of Chest Physicians Anticoagulation Guidelines.
Purpose
This study aims to identify if patients at a tertiary referral hospital were anticoagulated in line with these guidelines, and the incidence and nature of bleeding and thromboembolic complications.
Methods
A retrospective review of the Alfred Hospital General Surgical and ‘Hospital at Home’ databases was conducted, identifying patients who underwent elective general surgical procedures and received bridging anticoagulation with enoxaparin. Demographics, indication for anticoagulation, bleeding and thromboembolism rates were recorded. Thromboembolic risk was estimated.
Results
The study identified 108 patients. Three-quarters of all patients were anticoagulated with LMWH doses in accordance with the guidelines. Thirty of the 108 patients suffered bleeding complications. This group was younger, weighed less, received higher doses of enoxaparin and were at higher predicted risk of thromboembolism than non-bleeding patients. Wound haematoma, rectal bleeding and intra-abdominal bleeding were the most frequent complications. The peak time of bleeding was 3.5 days after surgery. Twelve patients returned to theatre, 13 were readmitted and 3 received blood transfusion. One patient suffered pulmonary emboli on the first post-operative day.
Conclusion
LMWH bridging therapy when prescribed appropriately is associated with low rates of inpatient thromboembolism in elective general surgical patients within our institution, but an unexpectedly high rate of bleeding complications.
Introduction
Patients taking warfarin for atrial fibrillation (AF), deep venous thrombosis and mechanical cardiac valve prostheses are frequently encountered in surgical practice.1 To prevent perioperative bleeding, warfarin prophylaxis is usually interrupted, which, at least transiently, increases patients' risks of thromboembolic events.2, 3
The American College of Chest Physicians promulgates guidelines (‘ACCP Guidelines’), first published in 20084 and updated in 20125 (Table 1) for managing this risk. The 2008 ACCP Guidelines recommend risk stratification according to CHADS2 score (Table 2), type of cardiac valve prosthesis, thrombophilic disorder and tailor perioperative management regimes (‘bridging’) accordingly. For low-risk patients, either no low-molecular-weight heparin (LMWH) or low-dose LMWH is recommended. The ACCP Guidelines recommend low or therapeutic doses of LMWH for patients at moderate risk and therapeutic doses for high-risk patients.
| Risk stratum | Indication for warfarin | ||
|---|---|---|---|
| Mechanical heart valve | AF | VTE | |
| High |
Any mitral valve prosthesis Any caged-ball or tilting disc aortic valve prosthesis Stroke or TIA within 6 months |
CHADS2 score of 5 or 6 Stroke or TIA within 3 months Rheumatic valvular heart disease |
VTE within 3 months Severe thrombophilia (e.g. deficiency of protein C or S or anti-thrombin, anti-phospholipid antibodies; multiple abnormalities) |
| Moderate† | Bileaflet aortic valve prosthesis and one or more of AF, stroke or TIA, hypertension, diabetes, congestive heart failure, age >75 years | CHADS2 score of 3 or 4 |
VTE within the past 3–12 months Non-severe thrombophilia (e.g. heterozygous factor V or prothrombin gene mutation) Active cancer (within 6 months or palliative) |
| Low | Bileaflet aortic valve without AF and no other risk factors for stroke | CHADS2 score of 0–2 (assuming no prior stroke or TIA) | VTE > 12 months and no other risk factors |
- † These patients now bridged at clinicians discretion; 2008 Guidelines recommended prophylactic or therapeutic-dose LMWH. CHADS2 = Congestive heart failure, hypertension, age ≥ 75, diabetes and stroke or TIA. AF, atrial fibrillation; LMWH, low-molecular-weight heparin; TIA, transient ischaemic attack; VTE, venous thromboembolism.
| Condition | Points | |
|---|---|---|
| C | Congestive heart failure | 1 |
| H | Hypertension (SBP > 140) | 1 |
| A | Age ≥75 years | 1 |
| D | Diabetes mellitus | 1 |
| S2 | Prior stroke or TIA or Thromboembolism | 2 |
| CHADS2 score | Annual stroke risk (%) |
|---|---|
| 0 | 1.9 |
| 1 | 2.8 |
| 2 | 4.0 |
| 3 | 5.9 |
| 4 | 8.5 |
| 5 | 12.5 |
| 6 | 18.2 |
- CHADS2 score > 1 recommends anticoagulation to INR 2.0–3.0.
Several studies have shown that unfractionated heparin and LMWH are associated both with low rates of thromboembolism (TE) and with above average rates of bleeding,6-10 and considerable variation as to how and which patients receive bridging prophylaxis.11
The earliest bridging regimes used unfractionated heparin infusions.12 More recently, bridging regimes have used therapeutic dose enoxaparin,1, 7, 13, 14 dalteparin9, 15 and bemiparin.16 Across these studies, a rate of TE of <1% has been reported, while bleeding rates were between 0.9% and 6.7%.
Guidelines at our institution have been adapted from the 2008 ACCP Guidelines. However, we have noticed a number of patients with perioperative bleeding complications. This study therefore aimed to describe the incidence, nature and timing of bleeding complications and thromboembolic events in patients receiving bridging prophylaxis in a general surgical service in a large Australian teaching hospital, and adherence to the Guidelines to identify opportunities for improvement.
Methods
Patients who had elective general surgical procedures and who received LMWH bridging during perioperative cessation of warfarin anticoagulation between January 2007 and December 2010 were identified from the Alfred Hospital ‘Hospital in the Home’ database.
The reason for anticoagulation was noted, as was the dosage and timing of LMWH administration and recommencement of warfarin. The patient's risk of TE was calculated as being ‘high’, ‘moderate’ or ‘low’ according to the 2008 version of the ACCP Guidelines.4 Each patient's perioperative regimen was described as being consistent or inconsistent with the recommendations of the 2008 ACCP Guidelines.
Anticoagulation regimen
Patients were identified in the pre-admission clinic and were prescribed low or therapeutic dose enoxaparin at the discretion of the treating clinician but not necessarily with prior knowledge of the guideline used in this study. Upon identification, patients were then referred to the hospital in the home service, and warfarin was ceased 5 days preoperatively. Patients were then to receive enoxaparin at the prescribed dose once the international normalized ratio (INR) became sub-therapeutic (less than 2.0 or 2.5) and ceased the day prior to surgery. The day at which warfarin and enoxaparin was recommenced post-operatively was decided by the treating surgeon.
Outcomes
Given that the study was retrospective, all complications were identified from the patient's medical record. Clinically significant bleeding was defined as fatal haemorrhage, return to theatre for haemostasis/evacuation of haematoma, readmission to hospital, interruption of anticoagulation or need for blood transfusion. Minor bleeding was defined as bleeding that was clinically apparent but did not result in altered treatment or additional treatment of the patient. TE was defined as (i) radiologically confirmed deep vein thrombosis (DVT); (ii) radiologically confirmed pulmonary embolism (PE); (iii) transient ischaemic attack (TIA) or (iv) stroke. The latter was defined as new-onset neurological deficits lasting less than 24 or greater than 24 h, respectively, within 1 month of surgery.
Statistical analysis
All data were analysed using the SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). Continuous data were assessed for normality and were expressed as mean ± standard deviation, and categorical data were expressed as count and proportions. Non-normally distributed data were reported as medians and ranges. Comparisons between groups (bleeding versus no bleeding) were performed using the Student's t-test for parametric continuous variables, Mann–Whitney U-test for non-parametric continuous variables, and chi-square or Fisher's exact test as appropriate for categorical variables. Statistical significance was set at a two-sided P-value of 0.05.
Ethics
This study was approved by the Alfred Health Research and Ethics Department prior to data collection.
Results
Study cohort
A total of 108 patients were identified, and their baseline characteristics are presented in Table 3.
| Overall (n = 108) | Low risk (n = 16) | Moderate (n = 43) | High (n = 49) | |
|---|---|---|---|---|
| Age (years) | 68.7 (±11.7) | 70.3 (±11.4) | 69.1 (±11.4) | 68.3 (± 12.2) |
| Male (%) | 70.4 | 73.4 | 64.3 | 72.9 |
| Weight (kg) | 87.4 (±24.1) | 93.6 (±30.7) | 89.3 (±19.5) | 84.2 (± 20.0) |
| Indication for anticoagulation (%) | ||||
| AF | 40.7 | 64.3 | 51.3 | 30.6 |
| PE | 15.7 | 21.4 | 20.5 | 12.2 |
| AVR | 13.9 | 0 | 15.4 | 18.4 |
| MVR | 13 | 0 | 0 | 28.6 |
| DVT | 11.1 | 14.3 | 12.8 | 10.2 |
| Procedures performed† | ||||
| Laparoscopic and open hernia repair (inguinal, incisional) | 38 | 7 | 16 | 15 |
| Bowel resection | 12 | 1 | 4 | 7 |
| Endoscopy (colonoscopy, gastroscopy) | 12 | 1 | 3 | 8 |
| Laparoscopic cholecystectomy | 11 | 1 | 7 | 3 |
| Breast surgery (mastectomy, WLE) | 7 | 0 | 3 | 4 |
| Excision of skin lesion | 7 | 1 | 4 | 2 |
| Thyroid and parathyroid surgery | 5 | 1 | 1 | 3 |
| Laparoscopic gastric banding | 5 | 2 | 1 | 2 |
| Haemorrhoidectomy | 2 | 0 | 2 | 0 |
| Liver resection | 1 | 0 | 0 | 1 |
| Other | 8 | 2 | 2 | 4 |
- † Most commonly performed procedures. Values are mean (±standard deviation), proportion or counts as appropriate. AF, atrial fibrillation; AVR, aortic valve replacement; DVT, deep vein thrombosis; MVR, mitral valve replacement; PE, pulmonary embolism; WLE, wide local excision.
Appropriateness of enoxaparin dose
Of the bleeding patients, the majority were anticoagulated with risk-appropriate doses of enoxaparin (n = 15). Three received doses that were too high and five received doses that were too low. Similar proportions were observed in patients who did not bleed.
Bleeding complications
Thirty patients suffered bleeding complications (27.8%). There were no deaths. Patients who did bleed primarily suffered wound-related bleeding (83%), per-rectal bleeding (13%) and intra-abdominal bleeding (3%).
Patients who bled were at higher risk of TE than non-bleeding subjects (P = 0.003), received higher doses of enoxaparin (120 mg versus 105 mg, P = 0.039) and were more likely to have aortic valve replacements (AVRs) (26% versus 9%, P = 0.02) (Table 4). There were no significant differences in the appropriateness of the dosing of enoxaparin between the two groups (P = 0.83), nor in the proportions who received perioperative anti-platelet therapies such as clopidogrel, aspirin or non-steroidal anti-inflammatory drugs (within 7 days preoperatively 8% versus 7%, P = 1.00; within 7 days post-operatively 12% vs 10%, P = 0.84) (Table 5).
| Non-bleeding (n = 78) | Bleeding (n = 30) | P-value | |
|---|---|---|---|
| Age (years) | 69.1 ± 12.2 | 67.7 ± 10.7 | 0.56 |
| Weight (kg) | 88.4 ± 25.9 | 84.8 ± 19.6 | 0.50 |
| Male | 68% | 76% | 0.42 |
| Risk of TE† | 2 (1–3) | 3 (1–3) | 0.003 |
| Enoxaparin (mg) | 105 (20–240) | 120 (70–200) | 0.039 |
| Reason for anticoagulation | |||
| Aortic valve replacement | 9% | 26% | 0.02 |
| Atrial fibrillation | 45% | 30% | 0.16 |
| Pulmonary embolism | 18% | 10% | 0.39 |
| Deep venous thrombosis | 11% | 10% | 0.82 |
| Mitral valve replacement | 10% | 20% | 0.21 |
- † 1 = low, 2 = moderate, 3 = high as per Table 1. Values are mean ± standard deviation, median (range) or proportions. TE, thromboembolism.
| Non-bleeding (n = 78) | Bleeding (n = 30) | P-value | |
|---|---|---|---|
| Day of first dose enoxaparin (median (range)) | 1 (0–6) | 1 (0–5) | 0.98 |
| Day of first dose warfarin (median (range)) | 1 (0–8) | 1 (0–7) | 0.48 |
| Percentage of patients appropriately anticoagulated | 64.8% | 80% | 0.14 |
| Preoperative anti-platelet agents | 8% | 7% | 1.00 |
| Post-operative anti-platelet agents | 12% | 10% | 1.00 |
| Readmission | 0% | 43% | <0.0001 |
| Return to theatre | 0% | 40% | <0.0001 |
Of the 30 bleeding patients, 28 were clinically significant, as described in Table 6. There were no readmissions or returns to theatre among patients who did not suffer bleeding complications. Patients who suffered bleeding complications had anticoagulation withheld for a median of one additional day.
| Day bleeding identified, median (range) | 3.5 (0–30) |
| Nature of complication (episodes/% all patients in study) | |
| Haematoma | 25 (23.1) |
| Intra-abdominal bleeding | 1 (0.9) |
| PR bleeding | 4 (3.7) |
| Procedure associated with bleeding (number) | |
| Hernia (inguinal) | 11 |
| Hernia (incisional) | 4 |
| Breast | 4 |
| Thyroid | 3 |
| Endoscopy | 3 |
| Other | 2 |
| Haemorrhoidectomy | 1 |
| Laparoscopic cholecystectomy | 1 |
| Bowel resection | 1 |
| Clinically significant bleeding (episodes/% all patients in study) | 28 (25.9) |
| Readmission | 13 (12.0) |
| Return to theatre | 12 (11.1) |
| Transfusion | 3 (2.7) |
- PR, per-rectal.
The median time at which bleeding complications became apparent was 3.5 (0–30) days after surgery, which was 1–2 days after most patients had received both enoxaparin and warfarin (Fig. 1).
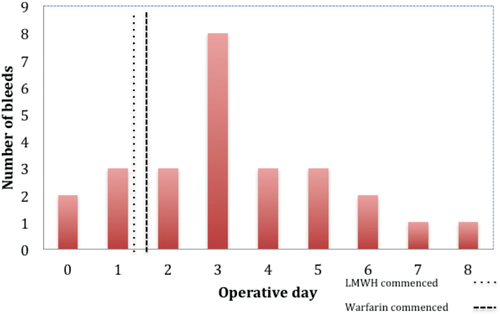
Number of bleeds per operative day.
Bleeding in patients undergoing inguinal hernia repair
The rate of bleeding among patients undergoing inguinal hernia repair was unexpectedly high. Of the 27 patients, 15 (55.6%) had bleeding complications. These patients had mean age of 65.1 years, weight of 84.3 kg and daily enoxaparin dose of 138 mg. Eleven were classified as having a ‘high’ risk of TE, with 4 at ‘moderate’ risk. The most common indication for anticoagulation was mechanical valve replacement (AVR, mitral valve replacement (MVR) = 7). These patients tended to bleed later (median post-operative day 4), with therapeutic enoxaparin and warfarin both commenced on the first post-operative day.
Thromboembolic complications
One patient, assessed to be at high risk for TE, was proven to have suffered a thromboembolic complication during the study period. A 77-year-old man suffered multiple bilateral pulmonary emboli one day after laparoscopic high anterior resection and was transferred to ICU for ventilator and inotropic support. He had been receiving warfarin for AF and MVR and had received preoperative therapeutic-dose enoxaparin, and his anti-coagulation was guideline appropriate.
Discussion
This study highlights that clinically significant bleeding in patients undergoing bridging anticoagulation with LMWH is common. Our finding of clinically significant bleeding rate of 12% is at least double that reported in similar studies elsewhere, as is the overall bleeding rate of 27.8%. The rate of bleeding, especially bleeding defined to be ‘clinically significant’ (12% of all patients treated requiring readmission, 11.1% of all patients treated returning to theatre), was higher than that reported by other authors.6, 8-10 This was particularly unexpected given that many procedures were considered low risk for bleeding (e.g. inguinal hernia repair). Although patients who bled had, overall, higher doses of LMWH and higher risk of TE, most patients who bled received guideline-appropriate regimens.
The timing of bleeding complications in our cohort (median post-operative day 3.5) is likely due to the dual anticoagulation effect of therapeutic anticoagulation with a rising, but sub-therapeutic INR. Many patients had already gone home. Somewhat paradoxically, patients who bled then had their anticoagulation withheld for a further day as a consequence, thus increasing their risk of thromboembolic complications, and possibly making re-anticoagulation more difficult.
The 2012 revision of the ACCP Guidelines (Table 1) changed the recommendation for patients at moderate risk of TE, making the administration of therapeutic dose bridging LMWH at the discretion of the clinician rather than mandatory. Nine of the patients who bled were considered to be at moderate risk, and according to the revised guidelines, would now potentially receive lower doses of LMWH. Most patients who bled in this study would still receive therapeutic-dose LMWH according to the revised guidelines. The risk of bleeding must obviously be weighed against the risk of stroke or PE, particularly when bleeding complications were usually wound-related and unlikely to cause significant long-term morbidity.
It is unclear as to how well treating clinicians were aware of the guidelines. There was slightly lower adherence to the guidelines in the non-bleeding groups, and no significant overall difference in adherence rates between patients who bled and those who did not. We emphasize the importance of institutional guidelines as aids to decision-making for clinicians, but we also urge that practice and outcomes are audited. In light of this study, we are prompted to consider whether there are patients or other institutional factors that influence the effectiveness and local applicability of the ACCP Guidelines for perioperative management of warfarin.
The single instance of TE occurred in a patient predicted to be at high risk. The overall rate of TE of 0.9% is consistent with other studies of bridging LMWH6, 9, 10, 15 and in a study of patients who did not receive any bridging therapy during the reversal of warfarin.17
There may be alternative means of managing perioperative anticoagulation. A recent study investigated the use of intravenous vitamin K without bridging LMWH for the reversal of INR prior to elective surgery, in which no patients appeared to suffer TE, rates of bleeding were low and post-operative restoration of therapeutic INR was timely.18
Study limitations
This study was unable to determine the event rates among patients who did not receive bridging therapy with LMWH, although these are obviously important to understand in a complete picture of the risk of perioperative management strategies. Applying risk stratification criteria to patients retrospectively may have led to the incorrect estimations of thromboembolic risk. Furthermore, the 1-month follow-up is unlikely to miss bleeding complications, but might have under-diagnosed TE.
Relevance
The patients in this study are likely to be similar to patients being considered for bridging therapy in the general surgical population. The study included patients receiving either therapeutic or low-dose enoxaparin bridging for a heterogeneous group of surgical procedures, including both invasive diagnostic-type (e.g. colonoscopy) and major general surgical procedures (e.g. bowel resection). All major indications for warfarin and patients at any risk of TE were included. As the general population continues to age, such patients will be encountered more frequently, and despite the introduction to the market of the direct thrombin inhibitor, dabigatran, and the factor Xa inhibitor, rivaroxaban, their high costs (currently only PBS listed for the prevention of venous thromboembolism post-orthopaedic surgery)19, 20 and lack of reversibility,21, 22 compared with warfarin, suggests warfarin is likely to remain a mainstay of management for some time.
Conclusion
This study described an increased rate of bleeding among patients who received perioperative bridging with LMWH, despite reasonable adherence to established guidelines. Patients who received higher doses of the LMWH were more likely to bleed, as were those who had mechanical aortic valves. Wound haematoma and per-rectal bleeding were the most commonly encountered complications of such a regimen. Prospective studies are required to further evaluate this problem. While there is the need to minimize the risk of perioperative TE, alternative strategies that further reduce the risk of bleeding may need consideration.