Multiple sclerosis and COVID-19: The Swedish experience
[Correction added on 30 March 2022, after first online publication: The copyright line was changed.]
Abstract
The COVID-19 pandemic has brought challenges for healthcare management of patients with multiple sclerosis (MS). Concerns regarding vulnerability to infections and disease-modifying therapies (DMTs) and their complications have been raised. Recent published guidelines on the use of DMTs in relation to COVID-19 in MS patients have been diverse between countries with lack of evidence-based facts. In Sweden, there exists a particular interest in anti-CD20 therapy as a possible risk factor for severe COVID-19 due to the large number of rituximab-treated patients off-label in the country. Rapid responses from the Swedish MS Association (SMSS) and the Swedish MS registry (SMSreg) have resulted in national guidelines on DMT use for MS patients and implementation of a COVID-19 module in the SMSreg. Recently updated guidelines also included recommendations on COVID-19 vaccination with regard to the different DMTs. Social distancing policies forced implementation of telemedicine consultation to replace in-person consultations as part of regular MS health care. Patient-reported outcome measures (PROMs) in SMSreg have been useful in this respect. This paper reports our experiences on the progress of national MS health care during the COVID-19 pandemic, in addition to offering an overview of the present scientific context.
1 COVID-19 AND MULTIPLE SCLEROSIS
1.1 Multiple Sclerosis patients – a risk group?
The aberrant regulation of immune responses in Multiple Sclerosis (MS) does not appear to have any prominent effect on host protection against infections, bacterial or viral.1 However, there are studies on MS patients that report a higher frequency of infections compared with the general population.2-4 Also, an increased risk of hospitalization due to infections has been reported and infections are a common cause of death amongst the MS population.5, 6 Further on, infections may trigger relapses in MS, which can cause permanent disabilities. 7, 8 Importantly, infections are well-known complications of some MS disease-modifying therapies (DMTs).9-12 With regard to previous data, the appearance of a new virus has introduced challenging questions on the definition of risk within the MS population.
The neuroinvasive properties of coronaviruses and the detection of COVID-19 virus in the brain of infected patients have raised the question of whether hypoxia/ hypoventilation might be caused by a failed central regulation of the breathing function.13, 14 It has been suggested that COVID-19 enters the brain through the olfactory mucosa in the nasal cavity where it migrates through olfactory axons to other parts of the brain including the respiratory centre.
Furthermore, recent autopsy studies detected a presence of COVID-19 RNA in the epithelial cells of the choroid plexus and ependymal cells in one patient with MS and in a control patient, both of whom died from COVID-19 pneumonia.15 Viral transcripts were not detected in the brain parenchyma and there were no signs of reactivation of MS lesions suggesting that the blood-brain barrier may play a role for COVID-19 CNS entry.15 Viral presence in the CNS may also be involved in COVID-19 associated acute respiratory dysfunction syndrome (ARDS). Given these facts, one may ask; does COVID-19 infection confer specific consequences for patients diagnosed with immune-mediated CNS diseases, such as MS?
Population-based studies on the incidence of COVID-19 in MS patients compared to the general populations are still rare. A Scottish study reported similar rates of COVID-19 infection in MS patients, compared to the general population.16 Similarly, the incidence of COVID-19 in Brazilian MS patients was comparable to that of the general population.17 Accumulating registry and cohort studies have identified neurological disability as an independent clinical risk factor for a more severe COVID-19 disease course.16, 18-20 In addition, progressive disease course and longer disease duration were reported as predictors for a worse COVID-19 outcome.19, 20 The general risk factors old age, male gender and obesity have been confirmed as risk factors also in MS patients.16, 18-21
1.2 DMTs – a risk factor for COVID-19 severity?
It is probable that immunomodulatory therapies may affect COVID-19 disease outcome. To date, several studies have suggested that anti-CD20 therapy may be a risk factor for severe COVID-19. International merged data from 28 countries (including Sweden), found a positive correlation between anti-CD20 treatment and risk for hospital care and intensive care in 2340 MS patients with COVID-19 infection.22 Accordingly, a study from Italy, including 844 MS patients, showed that those treated with anti-CD20 therapy carried a two to threefold risk increase of severe COVID-19 infection.19 Furthermore, a North American registry-based study of 1626 MS patients with COVID-19 described an increased risk for hospitalization in patients treated with rituximab (4.5 fold increase) compared to patients without DMTs.23 Langer-Gould et al.24specifically studied MS patients with rituximab therapy and demonstrated that this group of patients had an increased risk for hospitalization if infected with COVID-19, compared to the general population. This study also showed that shorter time frame post rituximab infusion was a risk factor for severe COVID-19.24 French registry data including nearly 350 MS patients indicated no association between exposure to DMTs and the severity of COVID-19, but sub-analysis of anti-CD20 therapy was not presented.20 In addition, registry data of 129 MS patients from 15 Latin American countries demonstrated no association between COVID-19 severity and any DMT.18 A report on safety data from Roche / Genentech, April 2020, showed that 26 out of 74 cases with confirmed COVID-19 infection in ocrelizumab-treated MS patients were hospitalized, and at least 12 were classified as critical/ serious.25
1.3 Beneficial effects of DMTs?
The most severely affected COVID-19 patients with multi-organ failure have been found with massive immunological hyperactivation and severe cytokine release. Given that the impact on lung function during COVID-19 infection resembles ARDS, which is caused by an overactive immune system, immunologically tailored treatments could be a viable potent option. This hypothesis has been supported by recent preliminary results of improved clinical outcome in COVID-19 patients treated with the interleukin-6 inhibitor tocilizumab.26 The link to current MS immunomodulatory drugs is of interest, as some of these drugs may even be beneficial in COVID-19. A recent study showed that combination therapy with interferon beta-1b, lopinavir, ritonavir and ribavirin improved clinical outcome in COVID-19 infection, maybe secondary to antiviral effects of beta interferon.27 In line with the aforementioned, data analyses from US electronic health records of approximately 30 000 MS patients showed that treatment with beta interferons or glatiramer acetate was associated with a lower risk of contracting COVID-19, compared to those treated with other DMTs.28 Another example of interest in antiviral effects is the current clinical trials of fingolimod as a treatment for ARDS in COVID-19.29 Further, a potential positive effect correlated with the selective blocking of adhesion molecules mediated by natalizumab, has been proposed as the virus might use integrins, in addition to the ACE2 receptor, for cell entry.30 On the other hand, blocking CNS lymphocyte entry could be problematic in relation to the risk for COVID-19 encephalitis. The properties of teriflunomides to induce tolerogenic immune cell profiles and possible anti-viral effects have been highlighted as potentially beneficial in preventing hyperinflammation in complicated COVID-19 infection.31 A study on COVID-19 in teriflunomide-treated MS patients reported mild disease courses in patients that continued DMT treatment during the active infection.31 It has also been discussed whether the mechanisms of action of dimethyl fumarate may confer protective effects against severe COVID-19 infection. However, the potency of this drug is complicated by the risk of lymphopenia which is a well-recognized side effect. Mild courses of COVID-19 disease, without the need for hospitalization, were reported in seven dimethyl fumarate-treated MS patients in Italy, of whom only one had lymphopenia.32
1.4 COVID-19 vaccination and DMTs
The immunosuppressive mechanisms of high-effective DMTs do most likely hamper the development of effective immune responses against vaccines.33 It is recognized that it takes at least four weeks to acquire an initial optimal antibody response and a couple of months to complete a vaccination programme. This poses a challenge for MS patients as treatment with DMTs should be initiated promptly to induce suppression of inflammation and avoid neuronal damage, and perment disabilities. There are currently limited studies on vaccine responses in MS patients with different DMTs. Protective immune responses following vaccinations with inactivated viruses have been demonstrated in MS patients treated with interferons and fumarates.33, 34 Adequate seroprotection after vaccination for influenza H1N1, H3N2 and B antigens has been observed in one study on teriflunomide-treated MS patients.35 A few reports indicate that fingolimod reduces immune responses to certain vaccines.36, 37 Olberg et al. showed diminished seroprotection after seasonal influenza vaccination in MS patients receiving fingolimod or natalizumab while another study showed sufficient humoral response to influenza vaccine in natalizumab-treated patients.38, 39 Reduced antibody production after vaccination against pneumococcal and tetanoid vaccine 12 weeks after initiation of ocrelizumab in MS patients has been reported.40 The same study also showed absence of protective immune response to vaccination with a new antigen in ocrelizumab treated individuals.41 A similar decrease in vaccine response was found in patients with neuromyelitis optica spectrum disorders (NMOSD) patients who received influenza vaccination 3–5 weeks after rituximab treatment.41 In patients with immune reconstitution therapies, proper vaccination response is expected when T and B-cell reconstitution is completed (3–6 months after autologous haematopoetic stem cell transplantation (AHSCT), >6 months after alemtuzumab, 3–9 months after cladribine).42
2 COVID-19: THE SWEDISH EXPERIENCE
Early on, in spring 2020, the Swedish National Board of Health and Welfare addressed risk groups for COVID-19: patients with MS, Parkinson's disease (PD) and Alzheimer's dementia were included. This information was later revised: only those with severe MS-related motor disabilities, particularly those affecting the respiratory function, were considered to belong to a risk group.43 The Swedish MS Association (SMSS) and the Swedish MS registry (SMSreg) have surveyed the control and medical care of MS patients in relation to the COVID-19 outbreak in Sweden.42, 44, 45 The SMSreg implemented a COVID-19 registration module in March 2020, as a framework of national and international collaborative efforts. The aim was to assess how MS patients have been affected by COVID-19, especially in relation to DMTs.
SMSS pointed out at an early stage that severe disability may be a potential risk factor for worse outcome of COVID-19 disease, in addition to the general risk factors outlined by the National Board of Health and Welfare.46 These circumstances mentioned above were reported by our group in a previous article, in the Swedish language.45
Updated information on COVID-19 cases amongst the Swedish MS population has been available on the webpages of SMSreg and SMSS, respectively, since spring 2020.42, 44 The COVID-19 module within the SMSreg allows for several parameters to be entered, including onset date of COVID-19 symptoms, information on clinical evaluation of COVID-19 infection (if performed by a neurologist, other physician or through patient history), viral tests (PCR or antibody serology) and test date. In cases where tests were missing, at least two of the following symptoms; fever, fatigue, dry cough and loss of appetite were required to confirm an infection. Additional optional parameters in the COVID-19 module include; information about co-morbidities, assessment of disease course (if the patient required hospitalization, intensive care, ventilation and/or extracorporeal membrane oxygenation) and outcome (survival/death).
As of 25 January 2021, a total of 501 MS patients have been reported as COVID-19 positive, according to the SMSreg. Of these patients, 79 were hospitalized including 20 patients that required intensive care. This corresponds to a rather low proportion of cases with severe COVID-19 disease in relation to the total number of approximately 18 000 registered MS patients in the country.44 The total number of confirmed COVID-19 cases as of the beginning of February 2021, in the Swedish population (approx. 10 million), was 566,957 including 4772 intensive care cases.47, 48 Subsequently, at present, there is no indication that MS per se would be associated with a higher risk for a more severe COVID-19 outcome in the absence of convincing data to support a firm conclusion.
2.1 Recommendations on DMT use during the COVID-19 pandemic
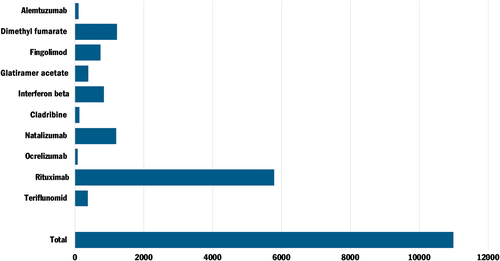
DMTs are currently prescribed to approximately 10,000 MS patients in Sweden, most of whom do not have severe disabilities that affect the respiratory system. According to SMSS guidelines (as of 5 January 2021), interferon beta, glatiramer acetate and teriflunomide are classified as ‘possible non-risk’ therapies for severe COVID-19. Other therapies such as dimethyl fumarate (without lymphopenia), natalizumab, fingolimod, siponimod are considered to mediate a ‘possible low risk’. Cladribine is also considered as ‘possible low risk’ therapy but increased infection control precaution is recommended within 2–3 months following drug administration. Immune reconstitution therapies including alemtuzumab and AHSCT pose ‘a possible increased risk’ for severe COVID-19 in patients up to two years after AHSCT and alemtuzumab, provided that the B and T lymphocyte counts are normalized. Considerable attention has been given to rituximab, which at present is the most commonly prescribed DMT for MS patients in Sweden (Figure 1). It should be noted that rituximab is prescribed off-label in Sweden at a much higher rate compared to other nations.49 In the earliest recommendations from SMSS, rituximab and other anti-CD20 therapies were considered to entail a ‘possible increased risk’. Rituximab had previously been associated with a higher incidence of respiratory infections in MS patients compared to other DMTs.12 The original classification remains in the latest recommendations, with support from SMSreg (as of 25 January 2021). Data from SMSreg showed that 55 out of 5927 rituximab-treated patients had been hospitalized for COVID-19 infection, of whom 13 patients needed intensive care.44 These data (adjusted for age, gender, disease duration, EDSS and progressive disease course) correspond to a nearly threefold risk increase (OR = 2.89, p < .001) for the need of hospital care in COVID-19 infected MS patients with anti-CD20 therapy which are in line with other studies.19, 22 A recent study with detailed data on 476 of the COVID-19 infected (confirmed and suspected) Swedish MS cases reported that the mean age at infection was 44 (range 19–78) years and 84% of the patients had a relapsing-remitting MS disease course.50 It is worth noting that the availability of COVID-19 tests was limited in Sweden for several months after the onset of the pandemic. Individuals with COVID-19 symptoms were initially recommended to stay at home and avoid seeking health care to limit spread of infection. This might have resulted in an underreporting of positive COVID-19 cases with less severe symptoms amongst Swedish citizens, including MS patients. A possible increased tendency to seek health care and to perform tests in MS patients with immunomodulatory treatments, including rituximab, can however not be ruled out which might also be the case for other DMTs.
Extended time intervals between dosing of rituximab have been advised and introduced in several MS clinics across the country since spring 2020. This regimen aims to enable repopulation of circulating B-cells, as well as diminish the risk for severe COVID-19. This has been supported by a recent study that indicated an absence of disease activity in patients despite prolonged dosing intervals of rituximab.51 An additional advantage of this strategy has been a reduction in hospital visits, from the perspective of infection prevention.45
2.2 COVID-19 vaccine recommendations in MS
National general recommendations for vaccination strategies in relation to DMTs has been developed within the SMSS, (published online in January 2020). Complementary information about COVID-19 vaccination has been available since January 2021. The guidelines are yet general and individual strategies are advised. With regard to the choice of DMTs, age and co-morbidities must be assessed to evaluate the need to protect the patient from infection through vaccination. Following the general recommendation of SMSS (as per the 20th of January 2020) treatment with interferon beta, glatiramer acetate and natalizumab are in most cases considered as safe alternatives for adequate vaccination responses, regardless of the type of vaccine (despite for live-attenuated vaccines that should be avoided with natalizumab).42 These drugs may therefore be an option if vaccination is to be started at the same time as DMT initiation. As for all other available DMTs for MS, common vaccines (except for live-attenuated), may be administrated safely, although the antibody response may be decreased with risk for insufficient protective immunity.42 As reported by SMSS, COVID-19 -vaccination is advised to start at 3 months, but preferably after 6 months following anti-CD20 therapy.42 The current recommendation of prolonged time interval dosing of rituximab may favour vaccine responses. Long-term therapy with rituximab induces a sustained low level of naïve B-cells and memory B-cells with faster repopulation of naïve B-cells compared to memory B-cells.52, 53 There may be a possible time window for vaccination during a prolonged interval between rituximab infusions. Preferably, when the pathogenic memory B-cells are still suppressed while the naïve B-cells have re-emerged, possibly giving rise to effective vaccine responses.53 This might be a successful strategy in order to avoid termination with B-cell depleting therapies in patients with active disease. Furthermore, T-cell responses are likely important contributors for the protective immune mechanisms following vaccination against COVID-19.54 The possible future clinical implementation of analyses of COVID-19 T-cell responses may further improve surveillance of immune response after vaccination in MS. With respect to the current limited knowledge, a rigorous assessment of risk-benefit balance regarding initiation/ disruption of DMTs and COVID-19 infection/vaccine response is recommended for each MS patient.
2.3 COVID-19 sick leave in the MS population
Following the Swedish National Board of Health and Welfare's initial statement that MS patients constitute a risk group, requests about the adaption of work tasks and the possibility to work from home were raised from patients.45 The patients were advised to reach agreements with their respective employers about possible work-related changes to favour work from a distance and temporary sick leave has been applied in a few specific cases.
In spring 2020, the National Board of Health and Welfare announced that a preventive sickness compensation (‘smittskyddspenning’), as part of Swedish Social Insurance, was to be established for patients in risk groups. In summer 2020, the government announced approval of an amended COVID-19 budget to reimburse patients belonging to risk groups unable to work from home, in terms of temporary sickness compensation. The qualifying period is due at the end of April 2021 and it was recently announced that the requirement for a medical certificate, when applying for sickness compensation, has temporarily been removed. Sickness compensation can also be obtained by relatives who are obliged to refrain from work in order to avoid infecting a close relative who belongs to a risk group. In the event of confirmed COVID-19 infection there is also a possibility of disease carrier compensation (‘smittbärarpenning’).55
2.4 Telemedicine and patient-reported outcome measures during COVID-19
Major changes in MS patient care have been implemented across Sweden with a focus on telemedicine to replace in-person visits. Regular annual follow-up consultations with MS patients have been converted into phone or video consultations while visits with new patients are mostly maintained in person. Monitoring of patients through patient-reported outcome measures (PROMs) in the SMSreg has also gained increased interest for caregivers as a method to enhance surveillance of the patients. Application of telemedicine had to a certain degree already been started as part of neurological patient care in Sweden in recent years. Consequently, previous experiences in the field facilitated the procedure. Studies on the feasibility and efficacy of internet-based health care in neurological diseases have reported promising outcomes.56, 57 Interestingly, a recent study from Norway found that neurologists considered teleconsultations as better suited for patients with headache and epilepsy compared with MS and PD.58 Furthermore, telephone consultations were regarded as being more professionally suitable for follow-up consultations consistent with the current clinical praxis at MS centres in Sweden. However, future studies assessing patient-reported outcomes related to telemedicine will provide more knowledge on the efficacy of this type of medical care.
3 CONCLUSION
Recent reports have shown that patients with anti-CD20 therapies have a higher risk of a worse COVID-19 disease course. Taken into account disease type, age, gender, and other host factors, individual risk assessment needs to be applied. Future research on COVID-19-related immune system effects will improve risk assessment tools to monitor MS patients in the current pandemic. Further experience is warranted in order to establish comprehensive guidelines for COVID-19 vaccination in relation to DMTs in MS patients.
Sickness compensation from the Swedish Social Insurance may enhance national care and management of MS patients. Strategies in Sweden to manage COVID-19 in MS patients so far include an increased telemedicine approach which is facilitated by PROMs in the SMSreg. Also increased intervals between rituximab infusions have been introduced, because of immunological effects of the drug, but also to protect against COVID-19 infection in the hospital environment.
4 MAIN MESSAGE
- Recent studies indicate an increased risk for severe COVID-19 disease in MS patients treated with anti-CD20 therapy.
- Previous research and current observations do not suggest that patients with MS have an increased risk to contract COVID-19 infection.
- Extended time interval dosing of anti-CD20 therapy may diminish the risk of severe COVID-19 infection and favour COVID-19 vaccine response.
- Telemedicine and the use of PROMs in SMSreg are important instruments in MS care in a time of pandemic.
- Individual risk versus benefit in relation to disease activity and COVID-19 vaccination should be carefully evaluated before initiation of anti-CD20 therapy.
- Future updates from the SMSreg, SMSS and research will provide knowledge to support the care and management of MS patients in Sweden during the ongoing COVID-19 pandemic.
CONFLICT OF INTEREST
E.I. has received honoraria, for advisory boards and lectures, from Sanofi, Biogen and Merck Serono. A.M.L. has received honoraria for lectures or advisory boards from Merck Serono, Sanofi, Teva, Biogen, Celgene. S.B. and I.B. have nothing to disclose. No funding has been received for this study.
AUTHOR CONTRIBUTIONS
A.M.L contributed to conceptualization, methodology, performing literature search, writing original draft preparation, reviewing and editing. S.B. contributed to conceptualization, manuscript writing—reviewing and editing. I.B. contributed to conceptualization, methodology and manuscript writing–reviewing and editing. E.I contributed to conceptualization, methodology, performing literature search, and writing original draft version and reviewing and editing.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.




