Changes in perampanel levels during de-induction: Simulations following carbamazepine discontinuation
Abstract
Objective
To evaluate the time course of changes in perampanel levels when co-administered with carbamazepine, and following carbamazepine discontinuation, using a physiologically based pharmacokinetic (PBPK) model.
Methods
The PBPK model was developed, verified using clinical PK data, and used to simulate the effect of abrupt discontinuation and down-titration (75 mg twice daily [bid]/wk) of co-administered carbamazepine 300 mg bid on the PK of perampanel once daily (qd). Perampanel dose tapering (8-4 mg) and up-titration (2-6 mg) were simulated during abrupt carbamazepine 300 mg bid discontinuation to identify a titration schedule that minimizes changes in perampanel plasma concentrations.
Results
The PBPK model accurately reproduced perampanel plasma concentration-time profiles from clinical studies in single- and multiple-dose regimen simulations, including multiple-dose carbamazepine co-administration. The time course of return to pre-induced perampanel levels occurred more slowly following carbamazepine down-titration (~48 days after first down-titration) vs abrupt discontinuation (~25 days). Perampanel dose tapering (8-4 mg) at abrupt carbamazepine discontinuation produced minimal changes in steady-state concentrations, which returned to the levels observed during carbamazepine co-administration in ~15 days from the time of carbamazepine discontinuation. When perampanel was up-titrated in the presence of carbamazepine, return to steady state occurred more slowly when carbamazepine was down-titrated weekly (~45 days) vs abrupt discontinuation (~24 days).
Conclusion
This PBPK model simulated and predicted optimal perampanel dose tapering and up-titration schedules for maintaining perampanel levels during conversion to monotherapy. These results may guide physicians when managing conversion from perampanel polytherapy with concomitant enzyme-inducing anti-seizure medications to monotherapy.
1 INTRODUCTION
Clinically and for newly diagnosed epilepsy, clinicians often favor prescribing monotherapy over polytherapy1 or look to withdraw concomitant anti-seizure medications (ASMs) to achieve monotherapy.2, 3 ASM polytherapy can increase the frequency of side effects.4 Costs related to side effects (healthcare costs and costs to patients/families),5 an increased cost of ASM treatment,6 and decreased treatment adherence are linked to ASM polytherapy.7 Thus, patients with epilepsy taking monotherapy are preferable where possible.2
Perampanel, an orally active, non-competitive, selective, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor antagonist,8 is indicated for once-daily use in focal seizures (FS; previously known as partial-onset seizures) with or without focal to bilateral tonic-clonic seizures (previously known as secondarily generalized seizures) and generalized tonic-clonic seizures (GTCS; previously known as primary GTCS).9, 10 In the United States, perampanel is approved as a monotherapy for FS in patients aged ≥4 years and as adjunctive therapy for GTCS in patients aged ≥12 years.9 Perampanel is metabolized via primary oxidation mediated by cytochrome P450 3A4/5 (CYP3A4/5) and sequential glucuronidation.9, 11 Pharmacokinetic drug-drug interaction (DDI) assessments of perampanel indicate that plasma concentrations decline twofold to threefold following co-administration of enzyme-inducing ASMs (EIASMs), such as carbamazepine, oxcarbazepine, and phenytoin, which increase perampanel clearance via induction of CYP3A4.9, 12 Thus, perampanel plasma concentrations are expected to increase upon discontinuation of EIASMs, with the time course expected to depend on the half-life of perampanel and the EIASM, and the rate of return of CYP3A enzyme activity to pre-induced state values.11, 13, 14
Adding perampanel to an EIASM regimen, such as carbamazepine (enzyme induction), is well recognized; however, what happens to perampanel levels following EIASM removal (de-induction) is not as clear. This phenomenon is relevant as clinicians may opt to simplify a patient's regimen by removing the ASM that is least helpful/causing adverse effects, or during patient non-adherence. Understanding how perampanel plasma concentrations (a measure of systemic exposure) may change following discontinuation of an EIASM might be important depending on the clinical scenario. The time course to achieve a new steady-state perampanel plasma concentration during monotherapy was not studied/established in clinical trials. Clinical experience in a small number of patients indicates that conversion of ASM polytherapy to perampanel monotherapy is feasible and seizure control is maintained.15, 16 Knowledge of the pharmacokinetic effects discontinuation of co-administered EIASMs has on perampanel levels when converting to perampanel monotherapy is important so that appropriate perampanel plasma concentrations can be maintained.
To systematically evaluate the time course of change in perampanel exposure following cessation of carbamazepine dosing, a physiologically based pharmacokinetic (PBPK) model was developed using the Simcyp® population-based absorption, distribution, metabolism, and excretion (ADME) simulator.17 PBPK models are mechanistic models of the ADME pathways that integrate key input parameters from both in vitro and in vivo resource.18 PBPK modeling and simulation have become an increasingly important tool in the model-informed drug development paradigm,19 with the publication of PBPK guidelines for industry by both the United States Food and Drug Administration and the European Medicines Agency in recent years.20, 21
In contrast to the empiric nature of traditional compartmental pharmacokinetic models, PBPK models aim to describe the phenomena involved in complex ADME mechanisms using mathematical descriptions of anatomical, physiological, and chemical processes behind them.22 As a result, PBPK models are well suited for the evaluation of complex DDI cases that may be too difficult to study in a formal clinical trial or healthy volunteers.23, 24
Simulations were performed with the PBPK model to evaluate changes in perampanel steady-state plasma concentrations when co-administered with carbamazepine, and following discontinuation of carbamazepine. Carbamazepine was selected as it is a strong CYP3A inducer and was the most frequently co-administered EIASM in phase 2/3 clinical trials of perampanel.12
2 METHODS
A PBPK model for perampanel was developed in Simcyp® version 15.1, and simulations developed from this model have been submitted to the United States Food and Drug Administration and European Medicines Agency. The perampanel-specific inputs and model assumptions used to develop the model have been previously described,25 with the exception that the absorption model was changed from the Advanced Dissolution, Absorption, and Metabolism model26 to a first-order absorption model to increase computing speed. The first-order absorption parameters of the model—Fa (fraction of dose absorbed) and ka (first-order absorption rate constant)—were predicted by Simcyp®, with the Mechanistic Permeability (MechPeff) model,27 to be 0.99 and 3.37 h−1, respectively.
The predictive performance of the PBPK model was evaluated in two steps (Table S1). Firstly, a series of single-dose (2, 4, 6, and 8 mg, administered orally) and multiple-dose (2, 4, and 6 mg once daily [qd] for 14 days, administered orally) simulations were performed to compare the predicted perampanel plasma concentration-time profiles with those from four phase 1 studies in healthy adult males (Studies 001, 002, 005, and 006). Secondly, a DDI study was simulated to compare the predicted time course of changes in perampanel steady-state concentrations when co-administered with carbamazepine with those observed in a phase 1 DDI study in healthy adult males (Study 006). Default Simcyp® compound files for carbamazepine and its metabolite carbamazepine-10,11-epoxide (Certara UK Ltd.) were utilized for the simulations.
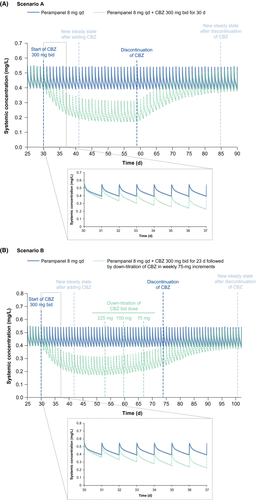
The PBPK model was used to simulate two perampanel 8 mg qd and carbamazepine 300 mg twice-daily (bid) co-administration scenarios (Table S1): an abrupt discontinuation of carbamazepine (Scenario A) and a 75 mg bid weekly down-titration of carbamazepine (Scenario B).
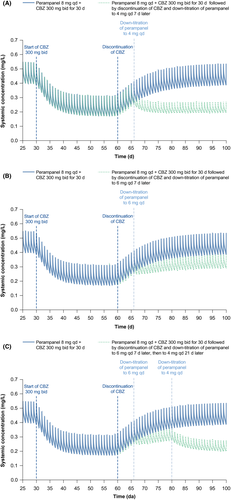
Three simulations of perampanel dose tapering after abrupt carbamazepine discontinuation were performed to investigate dose alterations required for maintenance of pre-discontinuation steady-state concentrations: (a) dose tapering of perampanel from 8 to 4 mg qd, 7 days after carbamazepine discontinuation; (b) dose tapering of perampanel from 8 to 6 mg qd, 7 days after carbamazepine discontinuation; and (c) dose tapering of perampanel from 8 to 6 mg qd, 7 days after carbamazepine discontinuation, then to 4 mg qd, 21 days after carbamazepine discontinuation.
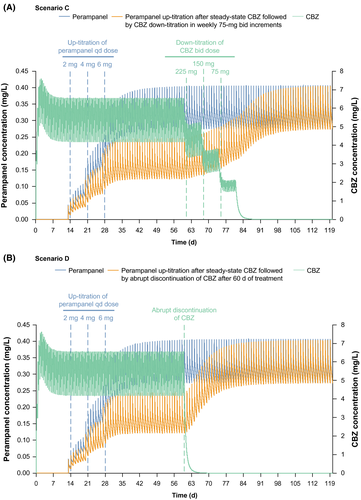
To reflect a common prescribing scenario in clinical practice, the PBPK model was also used to simulate two additional perampanel scenarios (up to 6 mg qd and carbamazepine 300 mg bid co-administration; Table S1): (a) carbamazepine pretreatment followed by up-titration of perampanel from 2 to 6 mg qd in weekly 2-mg increments followed by a 75 mg bid weekly down-titration of carbamazepine (Scenario C); and (b) an abrupt discontinuation of carbamazepine (Scenario D).
3 RESULTS
3.1 Evaluation of the PBPK model
In line with the originally published version of the model,25 the modified PBPK model accurately reproduced perampanel plasma concentration-time profiles from clinical studies in simulations of both single- and multiple-dose regimens, and the majority of observed data fell within the 90% prediction interval of the model (Figures S1 and S2). The PBPK model also accurately reproduced the perampanel plasma concentration-time profiles with and without co-administration of carbamazepine in the phase 1 DDI study (Figure S3). The model was able to replicate the carbamazepine-mediated induction of CYP3A when used to simulate perampanel plasma concentration-time profiles with co-administration of carbamazepine, as seen by the faster terminal elimination phase and decreased overall concentrations of perampanel after dosing on Day 32. Overall, these results support the use of this PBPK model to simulate perampanel plasma concentration-time profiles following carbamazepine discontinuation.
3.2 Simulations of steady-state perampanel plasma concentrations (de-induction)
Following abrupt discontinuation of carbamazepine (Scenario A), the return of perampanel plasma concentrations to pre-induced levels took ~25 days (Figure 1A). Following down-titration of carbamazepine (Scenario B), the rise in perampanel plasma concentrations to pre-induced levels took ~48 days after the first down-titration of carbamazepine (Figure 1B).
3.3 Simulations of steady-state perampanel plasma concentrations (dose tapering)
Dose tapering of perampanel from 8 to 4 mg qd at the time of abrupt carbamazepine discontinuation produced minimal changes in steady-state concentrations, which returned to levels within the range observed during carbamazepine co-administration in ~15 days from the time of carbamazepine discontinuation (Figure 2A). Simulation of perampanel dose tapering from 8 to 6 mg qd 7 days after abrupt carbamazepine discontinuation is predicted to result in the achievement of a new perampanel steady state within ~21 days; this new steady state is in between the range observed during carbamazepine co-administration and the steady state expected for perampanel 8 mg qd (Figure 2B). In this second scenario, simulation of an additional dose tapering to 4 mg qd, 21 days after carbamazepine discontinuation, predicted a fall in steady-state maximum and minimum concentrations of perampanel to within the range observed during perampanel 8 mg qd and carbamazepine 300 mg bid co-administration (Figure 2C).
3.4 Simulations of perampanel up-titration
Following up-titration of perampanel from 2 to 6 mg qd in weekly 2-mg increments after 2 weeks of carbamazepine 300 mg bid pretreatment, perampanel steady state was achieved after ~21 days of perampanel treatment. Following down-titration of carbamazepine in weekly increments of 75 mg bid after 60 days of treatment (Scenario C), the concentration of perampanel increased following carbamazepine discontinuation and perampanel new steady state was achieved ~45 days after carbamazepine discontinuation (Figure 3A). Following abrupt discontinuation of carbamazepine after 60 days of treatment (Scenario D), perampanel concentration increased such that new steady state was achieved ~24 days after carbamazepine discontinuation (Figure 3B).
4 DISCUSSION
A PBPK model was successfully developed for perampanel; the results of the simulations for model evaluation showed good agreement between the model-predicted and observed perampanel plasma concentration-time profiles when dosed alone and when co-administered with carbamazepine, supporting its use for simulations following carbamazepine discontinuation. Simulations of perampanel 8 mg qd with co-administration of the EIASM carbamazepine 300 mg bid reproduced the decreases in plasma perampanel concentrations observed in a formal phase 1 DDI study.
Following abrupt discontinuation of carbamazepine dosing, the increase in perampanel plasma concentrations to levels observed prior to carbamazepine co-administration occurred slowly (~25 days), reflecting both the long half-life of perampanel (~105 hours)9 and the slow recovery of the CYP3A synthesis rate back to pre-induced levels. As expected, the return of perampanel concentrations to pre-induced levels occurred more slowly following down-titration of carbamazepine (~48 days after the first down-titration) vs abrupt discontinuation; this scenario is more likely to reflect the clinical situation whereby the carbamazepine dose would be gradually tapered off. Similarly, following dose escalation of perampanel in the presence of steady-state carbamazepine, steady-state perampanel is achieved more slowly when carbamazepine is down-titrated weekly compared with abrupt discontinuation of carbamazepine at Day 60 (~45 vs 24 days).
Since the increase in perampanel concentrations is slow even during abrupt discontinuation of carbamazepine (due to the long half-life of perampanel and the slow recovery of the CYP3A synthesis rate back to pre-induced levels),9 we do not think it is necessary to monitor plasma concentrations when converting to perampanel monotherapy. If recovery and maintenance of similar perampanel plasma concentrations following carbamazepine discontinuation as those attained during polytherapy are preferable, these simulations suggest that this could be achieved in ~15 days with a single-dose adjustment of perampanel 8-4 mg qd 7 days after carbamazepine cessation. Although clinical studies are required to confirm these findings, in the absence of clinical data, these simulations may inform physicians about possible approaches to take when converting from polytherapy (that includes perampanel and carbamazepine) to perampanel monotherapy. Clearly, dose adjustments should be made on the basis of tolerability and control of seizures in clinical practice,9 and not solely on the basis of pharmacokinetic behavior.
In conclusion, a PBPK model for perampanel was successfully developed, verified, and used to simulate changes in perampanel plasma concentrations during discontinuation of co-administered carbamazepine and to predict down-titration and cessation schedules of carbamazepine that are optimal for maintaining appropriate perampanel levels during this conversion to monotherapy. These results have clinical relevance in guiding physicians in the management of conversion from perampanel polytherapy with concomitant EIASMs to perampanel monotherapy. Further studies are warranted to investigate perampanel concentrations during co-administration and discontinuation of other less potent EIASMs, since clinical data are not available to verify PBPK models for these.
ACKNOWLEDGEMENTS
Funding for these analyses was provided by Eisai Inc. The authors thank Bhaskar Rege (formerly of Eisai Inc.) who was involved in the study design and interpretation of the results.
Medical writing support, under the direction of the authors, was provided by Rebekah Waters, PhD, and Gemma McGregor, PhD, of CMC AFFINITY, McCann Health Medical Communications, funded by Eisai Inc, in accordance with Good Publication Practice (GPP3) guidelines.
CONFLICT OF INTEREST
Edgar Schuck is an employee of Eisai Inc., USA. Jim Ferry is a former employee of Eisai Inc., USA. Barry Gidal has performed consultation for Eisai, Sunovion, UCB Pharma, and Upsher-Smith, and has been a speaker for Eisai, Sunovion, and UCB Pharma. Ziad Hussein is an employee of Eisai Ltd., UK.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




