Role of mechanotransduction mediated by YAP/TAZ in the treatment of neurogenic erectile dysfunction with low-intensity pulsed ultrasound
Yang Liu and Xiao-ying Pan have contributed equally to this work.
Abstract
Background
Erectile dysfunction (ED) and weakness of the penis are processes related to hemodynamic alteration. Low-intensity pulsed ultrasound (LIPUS), as a new mechanical modality for the treatment of ED, deserves to be explored in depth for the biomechanical mechanisms it exerts.
Objective
The aim of this study was to explore the role of YAP/TAZ-mediated mechanotransduction in mechanical therapy for the treatment of neurogenic erectile dysfunction (NED).
Materials and methods
Forty-two male SD rats (12 w old) were randomly divided into sham-operated (n = 14), bilateral cavernous nerve injury (BCNI, n = 14), and LIPUS-treated (n = 14) groups. Intracavernosal pressure/mean arterial pressure (ICP/MAP) was measured 14 and 28 days after treatment. Penile tissue specimens were collected for pathological examination, and the changes in YAP, TAZ, connective tissue growth factor (CTGF), CYR61, LATS1, and p38 mitogen-activated protein kinase expression levels were assessed by Western blot, real-time quantitative polymerase chain reaction (RT–qPCR) and immunological staining.
Results
Compared with BCNI, LIPUS significantly improved ICP/MAP levels and enhanced histopathological changes. The penile expression levels of YAP, TAZ, CTGF, and CYR61 were significantly downregulated in the BCNI group (p < 0.01), and LIPUS upregulated the expression levels of these proteins (p < 0.05). The expression levels of p-LATS1 and LATS1 were not significantly different among the groups (p > 0.05). Interestingly, the expression level of p-p38/p38 significantly increased in BCNI rats (p < 0.05), which was reversed by LIPUS treatment (p < 0.05). However, the p38 inhibitor SB203580 did not change the expression of YAP/TAZ in rat primary smooth muscle cells or mouse MOVAS cells (p > 0.05).
Discussion and conclusion
LIPUS can effectively improve penile erectile function in NED rats. The underlying mechanism may be related to the regulation of YAP/TAZ-mediated mechanotransduction. However, the upstream regulatory signal may differ from the classical Hippo pathway.
1 INTRODUCTION
Penile erection is essentially a complete hemodynamic process. During an erection, a large amount of blood flows into the penis through relaxation of the cavernous smooth muscle, increased arterial blood flow and obstruction of the venous return; this blood flow is reduced when the penis returns to a flaccid state.1 Penile erection ability begins at the fetal stage, and healthy individuals experience spontaneous penile erections throughout their lives, which is essential to maintain the structural and functional integrity of the penis. Healthy men experience three to six erections during 8 h of sleep that last for 1.5 to 3 h.2 Periodic erection and weakness of the penis (for example, at night time) are characterized not only by changes in oxygen partial pressure but also by frequent changes in mechanical parameters such as pulling deformation, intercellular sparseness, and tissue tension.3 Yes-associated protein (YAP)/Transcriptional coactivator with PDZ-binding motif (TAZ) is a signaling protein that mediates mechanomechanical signals in the nucleus and plays a central role in the regulation of the Hippo pathway, which regulates tissue homeostasis, organ development, cell proliferation, tissue repair, and mechanotransduction.4-6 Mechanomechanical signaling regulates cellular function, and YAP/TAZ plays a key role in regulating cellular behavior in response to various internal and external stimuli.7 For instance, YAP/TAZ has been identified as a conserved mechanosensor for signals such as shear stress, cellular deformation, and extracellular matrix rigidity, translating them into cell-specific transcriptional programs.8-10
YAP and TAZ are well known for their synergistic transcriptional activity in the nucleus. However, cytoplasmic YAP/TAZ also plays an important role in angiogenesis and vascular remodeling.11 With advances in vascular molecular biology, it has been recognized that the vasculature itself is a complex and complete organ that autonomously produces many locally active factors that alter the tone, structure and function of the vasculature. These changes maintain the blood pressure state and gradually alter hemodynamic properties. The penile corpus cavernosum is also a vascular structure in nature; the diastole of the penile corpus cavernosum smooth muscle causes engorgement and erection as a biomechanical process.12 The incidence of erectile dysfunction (ED) is as high as 70% 12 months after radical surgery for prostate cancer.13 When penile weakness is treated, the penis regains the ability to complete the process of cyclic engorgement. Therefore, we suggest that YAP/TAZ-mediated mechanistic signaling may have important effects on the maintenance of penile function and repair after injury.
Recently, the influence of energy-related mechanical biomechanics on biological phenomena has received worldwide attention from the scientific community. Low-intensity pulsed ultrasound (LIPUS) is a convenient mechanomechanical treatment method with a much lower energy output intensity than conventional ultrasound (<3 W/cm2) and is commonly used in rehabilitation medical science.14 In recent years, LIPUS has been increasingly used in urology and male surgery.15 Due to its low intensity and pulsed output mode, LIPUS does not produce cavitation effects on pressure levels, and the thermal effects are negligible. It has been suggested that ultrasound, as a convenient, noninvasive mechanical force, can induce mechanical movement of molecules during the periodic alternating phases of erection and flaccidity.16 Previous studies have revealed that LIPUS has a good therapeutic effect on ED.17, 18 Meanwhile, another biomechanical modality, low-intensity extracorporeal shock waves, can promote recovery in rats with sciatic nerve injury and may be related to the mechanosensitive YAP/TAZ signaling pathway for nerve regeneration.19 This finding also prompted us to further believe that LIPUS treatment of ED may be related to the mechanosensitive YAP/TAZ signaling pathway.
In previous studies, researchers have focused on the effect of tissue oxygen supply on penile function and repair after injury, and the study of mechanistic signaling in related mechanisms has not been reported. Therefore, the present study was designed to investigate the safety and efficacy of LIPUS treatment in a rat model of neurogenic erectile dysfunction (NED) and the possible mechanisms regulating the mechanistic signaling pathways in penile pathological repair.
2 MATERIALS AND METHODS
2.1 Animal groups and study design
Healthy 12-week-old specific pathogen-free male Sprague Dawley rats (42 in total) weighing 270 ± 15 g were used for this study. The animals were provided by the Changsheng Animal Breeding Center with experimental animal production license number (SCXK [Liao] 2020-0001). The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Changchun University of Chinese Medicine (protocol code 2021048, January 14, 2021). The rats were divided into three groups according to the random number method: 14 rats in the sham-operated group, in which only the bilateral cavernous nerves (CNs) were dissected and exposed without injury; 14 rats in the nerve injury group, in which the bilateral CNs were exposed and clamped at a distance of 5 mm from the emanation of the autopelvic ganglion for 2 min each; and 14 rats in the LIPUS treatment group, in which the CNs were clamped and injured. After 14 days of treatment, seven rats from each of the three groups were randomly selected for erectile function testing and sampling. The remaining rats continued to be normally housed for 14 days before testing; penile tissues were obtained and subjected to histopathological staining and molecular biology experiments, and the detailed mechanism was evaluated with primary cultured penile cavernous smooth muscle cells from rats and verified in mouse MOVAS cells.
2.2 Surgical procedures
Twenty-eight 12-week-old SD rats were randomly selected to establish an animal model of neuropathic ED. The model was constructed as follows20: SD rats were anesthetized with an intraperitoneal injection of 3% pentobarbital sodium solution (30 mg/kg); after proper fixation and disinfection, the pelvic ganglion was located on the dorsolateral side of the prostate through a hypogastric midline incision; the left and right CNs were clamped at 5 mm from the site of pelvic ganglion emission for 2 min; after CN injury, the abdominal cavity was closed layer by layer, and the abdominal incision site was disinfected with iodophor.
2.3 LIPUS treatment of rats
All 14 rats in the treatment group were treated with ultrasound using a multimodal low-intensity pulsed ultrasound therapy instrument (WBL-ED, WanBeiLi, Beijing, China) as follows 17: the rats were anesthetized (same method as above), the ultrasound probe was applied uniformly with a coupling agent close to the penis with a sound intensity of 300 mW/cm2 and a frequency of 1.7 MHz per treatment. The duration of each treatment was 3 min, and the ratio of the pulse duration to the pulse resting time was 1:4 (200 μs:800 μs). The treatment period was 2 weeks, with 3 treatments per week.
2.4 Determination of erectile function
Erectile function was measured in rats 2 and 4 weeks after surgery, as previously described.21 Briefly, SD rats were first anesthetized intraperitoneally (as above) and placed in the supine position, the abdominal hair was removed, and an inferior median incision was made to expose the bladder and prostate and locate the CN. Then, two 24G puncture needles filled with heparinized saline (200 IU/ml) were taken; one was connected through a PE-50 tube, and the other was connected to a multichannel physiological recorder (MP150, Biopac Systems, Goleta, CA, USA) through a PE-50 tube. The rat was then anesthetized with 3% sodium pentobarbital solution (30 mg/kg), the left common carotid artery was freed, and a puncture needle was inserted to monitor the mean arterial pressure (MAP). Finally, the intracavernous pressure (ICP) and MAP were recorded simultaneously by comparing the maximum ICP with the MAP in different groups. The ICP/MAP values were compared among the groups to assess changes in penile erectile function.
2.5 Masson's trichrome staining
The rats were sacrificed after penile erection function was assessed, and the mid-penis was fixed in 4% paraformaldehyde for 48 h, dehydrated in gradient alcohol, paraffin embedded and sectioned at a thickness of 5 μm. The staining was performed according to the Masson kit directions (Beijing Solaibao Technology Co, China), and Masson-stained images were acquired by image processing software (Olympus Image Viewer). The smooth muscle was stained red, and collagen fibers were stained blue; the smooth muscle/collagen ratio was semiquantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to assess interstitial collagen deposition in the penile cavernous tissue.
2.6 Immunohistochemical staining
Paraffin sections of rat penises with 5-μm thickness were stained by the SP method according to the procedure shown in the assay kit (Beijing ZhongShan JinQiao Biotechnology Co., Ltd.), primary antibodies against alpha smooth muscle actin (α-SMA, 1:1000, Abcam, Cambridge, MA, USA), YAP (1:500, Proteintech), and TAZ (1:500, Atlas) were applied overnight at 4°C. Images were acquired by image processing software (Olympus Image Viewer), and image analysis was performed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
2.7 Immunofluorescence staining
YAP/TAZ nuclear transfer was detected in the smooth muscle of the cavernous body by immunofluorescence. The sections were pretreated according to the manufacturer's instructions for Abcam IF staining. Primary antibodies, including α-SMA (1:500, Abcam, Cambridge, MA, USA) and YAP/TAZ (1:25, Santa Cruz, 63.7:sc-101199), were incubated at 4°C overnight. Then, secondary antibodies, Alexa-488 and Alexa-594-conjugated antibodies (1:250; Invitrogen, USA), were applied for 1 h. Finally, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, C1006). Images (600 ×) were observed and acquired with a confocal microscope (NIKON ECLIPSE TI, Japan), and ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to analyze the images.
2.8 Cell culture
Fresh penises were taken from untreated SD rats, and rat primary penile cavernous smooth muscle cells were extracted using the digestion method. Cell lines were identified by analysis and tested for mycoplasma contamination. Cells were cultured in dulbecco's modified eagle medium containing 10% fetal bovine serum and 1% penicillin/streptomycin (100 units/ml). All cell culture reagents were purchased from Sigma–Aldrich Company, USA. We divided primary penile cavernous smooth muscle cells into two groups and added equal amounts of DMSO and a p38 inhibitor (SB 203580, 10 μM, ApexBio Technology LLC; Catalog No: A8254). SB 203580 was prepared in DMSO. Cells were maintained in a 37°C incubator at 5% CO2. Cells were inoculated at a density of 1.0 × 104 cells/cm2 in six-well plates and cultured for 14 days. Briefly, cells were isolated on days 3, 7, and 14 of culture and used for experiments after reaching 70%−80% confluency; the inhibitor SB203580 was added 1 h before sample collection. The medium was changed every 2 days. Repeat experiments were performed with cells of different passaging times. In addition, a mouse aortic vascular smooth muscle cell line was used for validation (MOVAS, The Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China).
2.9 Western blot
Liquid nitrogen-preserved penises were milled and lysed by adding RIPA buffer (containing protease buffer and phosphatase buffer, Beyotime, Beijing, China) to extract proteins; harvested cells were lysed with RIPA buffer, and samples were sonicated and centrifuged at 12,000 × g for 10 min at 4°C. The protein concentration was measured with a bicinchoninic acid protein assay kit (Beyotime, Beijing, China). The samples were then denatured at 99°C for 5 min, and gel electrophoresis was performed with lysates containing 30 μg protein, after which the proteins were transferred to PVDF membranes. The transfer was performed at 200 mA for 2.5 h, and the membranes were blocked with 5% nonfat milk powder in Tris-buffered saline (pH=7.5) for 2 h at room temperature and then incubated overnight with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:20,000, Millipore, #3241215), β-tubulin (1:15,000, Cell Signaling, #2146), p-LATS1 (Thr1079) (1:1,000, Cell Signaling, #8654), LATS1 (C66B5) (1:1,000, Cell Signaling, #3477), p38 mitogen-activated protein kinase (p38 MAPK) (1:1,000, Cell Signaling, #8690), phospho-p38 MAPK (Thr180/Tyr182) (1:1,000, Cell Signaling, #4511), YAP/TAZ (1:1,000, Santa Cruz, 63.7: sc-101199), and p-YAP (S127) (1:1,000, Cell Signaling, #4911S) primary antibodies. The membranes were then incubated with horseradish peroxidase-conjugated anti-IgG for 2 h at room temperature, and the protein bands were visualized using the enhanced chemiluminescence method (Millipore, Billerica, MA, USA). Western blot bands were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to determine the integration density values of each protein band.
2.10 RNA extraction and quantitative real-time polymerase chain reaction
Total RNA from penile tissues was extracted with TRIzol reagent (Invitrogen Life Technologies) according to the manufacturer's instructions and then reverse transcribed using PrimeScript RT Master Mix (Vazyme, Nanjing, China). All reverse-transcribed RNA was purified using C (Vazyme, Nanjing, China) and quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, NC, USA). Rapid quantitative polymerase chain reaction was performed using SYBR Premix Ex Taq reagent (Vazyme, Nanjing, China) and a 7500 real-time polymerase chain reaction system. The cycling conditions were 95°C for 15 s, 60°C for 60 s, and 40 cycles. The calculated values of target gene expression were normalized to the GAPDH (GAPDH level as the internal control). The results were calculated with the 2-ΔΔCt method. All primer sequences were synthesized by GenScript (GenScript, Nanjing, China), and the primer sequences are shown in Table 1.
| Gene | Forward (5′−3′) | Reverse (5′−3′) |
|---|---|---|
| R-GAPDH | TATCGGACGCCTGGTTAC | CTGTGCCGTTGAACTTGC |
| R-YAP | CTGCCCGACTCCTTCTTCAA | TGGAGACGAGTGAGCTCGAA |
| R-TAZ | ACCACATGGCAAGACCCT | CTGCTCCCGTGAATGATAG |
| R-CTGF | TCTTCGGTGGGTCCGTGTA | CGGTCCTTGGGCTCATCAC |
| R-CYR61 | AGAGGCTTCCTGTCTTTGG | CAGTATTTGGGCCGGTAT |
| R-p38 MAPK | ACCTAAAGCCCAGCAACC | CCACGGACCAAATATCCA |
| m-GAPDH | ATCCTGCACCACCAACTGCT | GGGCCATCCACAGTCTTCTG |
| m-CTGF | CTGCCTACCGACTGGAAGAC | CATTGGTAACTCGGGTGGAG |
| m-CYR61 | GCTCAGTCAGAAGGCAGACC | GTTCTTGGGGACACAGAGGA |
- Abbreviations: CTGF, connective tissue growth factor; CYR61, cysteine-rich protein 61; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; m, Mouse; p38 MAPK, p38 mitogen-activated protein kinase; RT–qPCR, real-time quantitative polymerase chain reaction; R, Rat; TAZ, transcriptional coactivator with PDZ-binding motif; YAP, yes-associated protein.
2.11 Statistical analysis
SPSS 26.0 statistical software (SPSS Inc., Chicago, IL, USA) was used to analyze the statistical significance of the data. All data conformed to a normal distribution, and the chi-square test and cell experiments were independently repeated at least three times. All data are expressed as the mean ± SD. Student's t test was used to analyze two groups. One-way Analysis of Variance was used to compare means between multiple groups with Tukey's multiple comparisons test, both two-sided, with test level α = 0.05. p < 0.05 was considered a statistically significant difference (*p value < 0.05; **p value < 0.01; ***p value < 0.001; ns, no significance.)
3 RESULTS
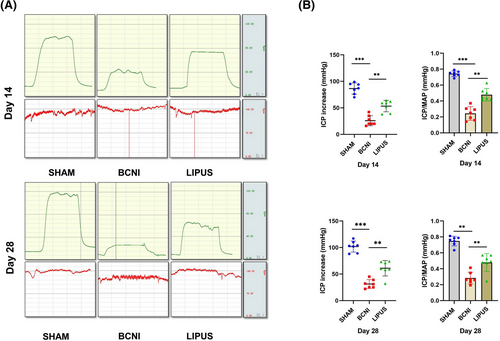
3.1 LIPUS improves erectile function in neurologically damaged ED rats
The ICP/MAP was significantly lower at 14 days in the bilateral cavernous nerve injury (BCNI) group (0.24 ± 0.08) than in the SHAM group (0.74 ± 0.03, p < 0.001) and higher in the LIPUS-treated group (0.48 ± 0.08) than in the BCNI group (p < 0.01); at 28 days, ICP/MAP was significantly lower in the BCNI group (0.28 ± 0.07) than in the SHAM group (0.75 ± 0.06, p < 0.001), and LIPUS (0.48 ± 0.11) had a significant effect (p < 0.01); there was no significant difference in MAP among the groups (p > 0.05) (Figure 1).
3.2 LIPUS can repair pathological damage in neurologically damaged ED rats
Masson trichrome staining of penile paraffin sections is shown in Figure 2A. The smooth muscle/collagen ratio was higher in the SHAM group (0.12 ± 0.01) than in the BCNI group (0.07 ± 0.01, p < 0.01) on day 14. After treatment, the smooth muscle/collagen ratio was higher in the LIPUS group (0.10 ± 0.01) than in the BCNI group (p < 0.05). On day 28, the smooth muscle/collagen ratio was significantly lower (p < 0.01) in the BCNI group (0.07 ± 0.01) than in the SHAM group (0.11 ± 0.01, p < 0.05) and slightly lower in the LIPUS group (0.09 ± 0.02). (Figure 2B)
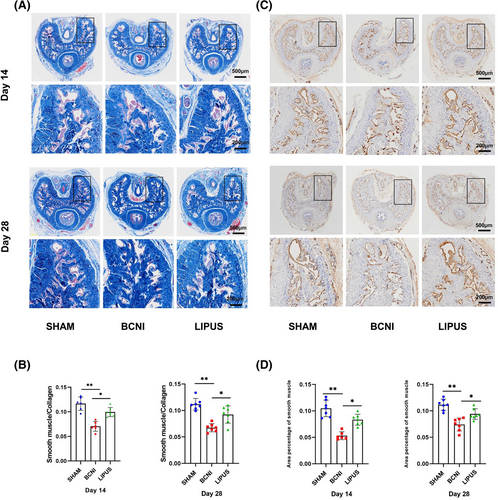
At the same time, we performed immunohistochemical staining of penile paraffin sections (Figure 2C). At 14 days, the smooth muscle/cavernosal ratio was higher in the SHAM group (0.10 ± 0.02) than in the BCNI group (0.05 ± 0.01, p < 0.01). After treatment, the smooth muscle/cavernosal ratio was higher in the LIPUS-treated group (0.08 ± 0.01) than in the BCNI group (p < 0.05). At 28 days, the smooth muscle/cavernosal ratio was higher in the SHAM group (0.11 ± 0.01) than in the BCNI group (0.07 ± 0.01, p < 0.01). After treatment, the smooth muscle/cavernosal ratio was significantly higher in the LIPUS group (0.09 ± 0.01, p < 0.05) (Figure 2D).
3.3 YAP/TAZ is a key protein that regulates LIPUS to improve erectile function
We performed immunohistochemical staining for YAP/TAZ on all rat penile tissues in the 14-day and 28-day groups (Figure 3A). At 14 days, YAP and TAZ expression levels were significantly lower in the BCNI group (0.13 ± 0.01; 0.09 ± 0.02) than in the SHAM group (0.18 ± 0.03; 0.18 ± 0.03) (both p < 0.01), and compared with the BCNI group, the LIPUS group (0.16 ± 0.02; 0.14 ± 0.02) had significantly higher YAP and TAZ expression (both p < 0.05). At 28 days, YAP and TAZ expression levels were significantly lower in the BCNI group (0.14 ± 0.02; 0.11 ± 0.02) than in the SHAM group (0.18 ± 0.03; 0.17 ± 0.03) (both p < 0.05), and compared with the BCNI group, the LIPUS group (0.17 ± 0.02; 0.16 ± 0.02) YAP and TAZ expression levels were significantly higher (p < 0.05). (Figure 3B)
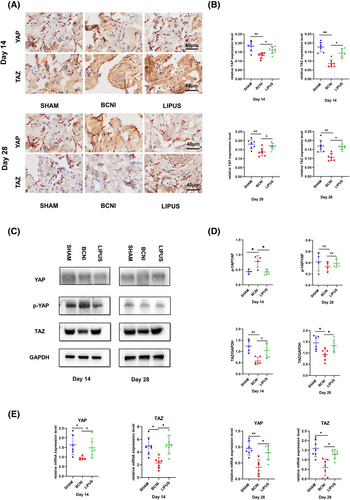
The Western blot results (Figure 3C) showed that TAZ expression was reduced in the BCNI group (0.58 ± 0.16; 0.92 ± 0.24) compared to the SHAM group (1.24 ± 0.27; 1.40 ± 0.31) at both 14 and 28 days, with a more pronounced reduction in the 14-day group (p < 0.01). The expression of TAZ was slightly increased in the 28-day group (p < 0.05), and the expression of YAP at 28 days was not statistically significant among the three groups (p > 0.05). Therefore, it is reasonable to assume that TAZ may be the main gene controlling this mechanistic change, while YAP plays a secondary role, and their mechanistic effects decrease over time. TAZ levels were elevated after LIPUS treatment (1.04 ± 0.31; 1.34 ± 0.28) compared to those in the BCNI group at both 14 and 28 days (p < 0.05) (Figure 3D). In the RT‒qPCR assay, the trends of YAP and TAZ expression levels were the same (Figure 3E).
3.4 Localization study of YAP/TAZ in cells
In immunofluorescence experiments (Figure 4A,B), we found that the positive expression of YAP/TAZ in smooth muscle was significantly lower in the BCNI group (21.50 ± 3.08,%) than in the SHAM group (59.83 ± 4.95,%) at 14 days; meanwhile, the localized expression of YAP/TAZ in cells was significantly higher in the LIPUS group (33.83 ± 3.66,%). We also found that at 28 days, the expression level of YAP/TAZ localization in smooth muscle was similar to that at 14 days.We also found that at 28 days(Figure 4C,D), the expression level of YAP/TAZ localization in smooth muscle was similar to that at 14 days, and that the expression level of α-SMA was the same as the immunohistochemical outcome trend.
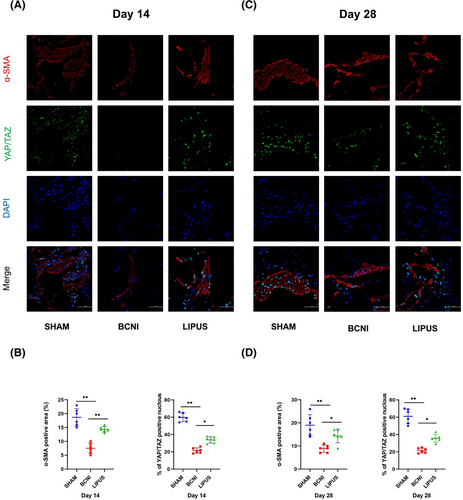
3.5 Study of the classical upstream and downstream pathways of YAP/TAZ
In the Western blot assay (Figure 5A), the expression level of CTGF was lower in the BCNI group (0.44 ± 0.12) than in the SHAM group (0.78 ± 0.18, p < 0.05) at 14 days and lower in the BCNI group (0.49 ± 0.03) than in the SHAM group (0.79 ± 0.06, p < 0.05) at 28 days. After LIPUS treatment, the expression of CTGF improved (0.81 ± 0.19; 1.28 ± 0.53) (p < 0.05) (Figure 5B). Our statistical analysis of the RT‒qPCR results revealed (Figure 5C) that at 14 days, both CTGF and CYR61 levels were significantly reduced in the BCNI group (0.27 ± 0.12; 0.20 ± 0.11) compared to those in the SHAM (0.88 ± 0.19; 1.16 ± 0.39) and LIPUS-treated (0.65 ± 0.26; 0.57 ± 0.19) groups. LIPUS treatment significantly recovered CTGF and CYR61 levels (all p < 0.01). We found that the decrease was most pronounced in the BCNI group compared with the SHAM group at 14 days, and at 28 days, CTGF and CYR61 expression levels in rats in the BCNI group (0.79 ± 0.20; 0.86 ± 0.34) were damaged more than in the SHAM group (0.30 ± 0.07, p < 0.01; 0.24 ± 0.08, p < 0.05), and the levels in the LIPUS group (0.59 ± 0.25; 0.53 ± 0.32) improved after treatment (p < 0.05).
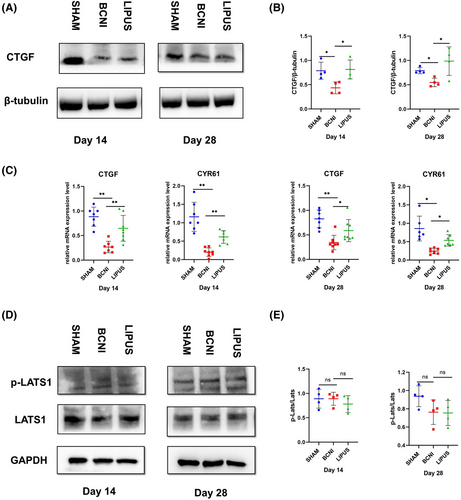
In addition, we failed to find a significant trend in p-LATS/LATS expression by Western blot (Figure 5D) in all three groups at both 14 and 28 days (p > 0.05) (Figure 5E).
3.6 The role of p38 protein in the improvement of ED by LIPUS treatment
The levels of p-p38/p38 were significantly higher in the BCNI group (1.58 ± 0.05; 2.01 ± 0.51) at 14 and 28 days than in the sham group (0.56 ± 0.18; 0.76 ± 0.17) by Western blot (p < 0.01), demonstrating that p38 may be a key regulating factor of neurological damage in ED. The expression levels of p-p38/p38 decreased in the LIPUS group after treatment (0.62 ± 0.39, p < 0.01) at 14 days compared with those in the BCNI group; this trend was similar at 28 days (1.45 ± 0.39, p < 0.05) (Figure 6A,B). Our statistical analysis of the RT‒qPCR results revealed that p38 expression was elevated in the BCNI group (0.16 ± 0.05; 0.37 ± 0.14) compared with the SHAM group (0.06 ± 0.03; 0.12 ± 0.07) at both 14 and 28 days (p < 0.05). After treatment, no significant change in p38 expression levels was found in the LIPUS group (0.20 ± 0.09) compared to the BCNI group at 14 days (p > 0.05), while there was a decrease in p38 expression in the LIPUS group (0.13 ± 0.05) at 28 days (p < 0.05). (Figure 6C)
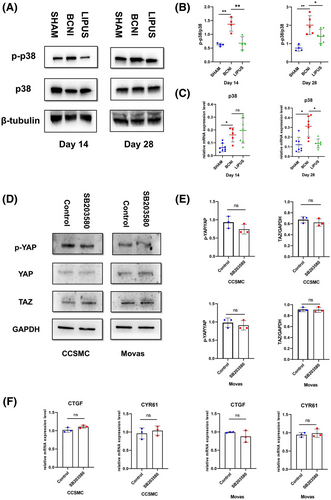
3.7 Exploring the link between p38 and YAP/TAZ activation
To investigate the relationship between p38 and YAP/TAZ activation and mechanical tension improvement in ED, we extracted primary penile cavernous smooth muscle cells from rat penile tissue and added SB203580, an inhibitor of p38, to the cultured cells and compared these cells to cells treated with only DMSO to determine whether p38 affected YAP/TAZ expression. We found no significant changes in CTGF and CYR61 levels by RT‒qPCR (p > 0.05), and no significant differences in TAZ and p-YAP/YAP levels were found by Western blot (p > 0.05). We verified these findings in the MOVAS cell line. (Figure 6D,E)
4 DISCUSSION
The vasculature is a complex system of cells and extracellular matrix, which macroscopically includes tangential stresses generated by blood flow, tensile stresses generated by blood pulsation, and compressive stresses generated by blood flow. Changes in the vascular mechanical environment affect vascular development by influencing the morphology and arrangement of vascular cells, causing changes in the cytoskeletal structure and intercellular connections of vascular cells and affecting the migration, proliferation and apoptosis of adult vascular cells. The blood supply of penile blood vessels plays a vital role in erection. Mechanical signals are known to regulate embryonic development, tissue homeostasis and regeneration.22, 23 The penis develops during gestation and maintains a regular nocturnal erection cycle after birth. Since the cavernous sinus of the penis is a vascular structure, it can be considered a single hemodynamic system in terms of physiological function, and the physiological response of erection and withdrawal is represented by a substantial change in the hemodynamics of inflow and outflow in the penis.24 When arterial inflow is diminished and balanced with venous outflow, the penis is in a flaccid state. When arterial inflow increases and venous outflow decreases, the penis swells and becomes erect due to increased blood content. Morphological changes in erection cause frequent mechanical state changes, and such mechanical changes may play an important role in penile vascular development, functional maintenance, and injury repair.
In this experiment, we found that the ICP/MAP of rats in the BCNI group was significantly lower than that of the sham-operated group at both 14 and 28 days, demonstrating that their erectile function was obviously impaired. The smooth muscle of the penile corpus cavernosum controls arteriovenous blood flow in the penile corpus cavernosum and plays an extremely critical role in erectile function. Immunohistochemistry and Masson staining showed that the smooth muscle content of the penile corpus cavernosum was significantly lower in the BCNI group than in the sham-operated group, demonstrating impaired erectile function in the BCNI group. It has been shown that cell culture on hard substrates leads to the aggregation of YAP/TAZ in the nucleus; in contrast, culture on soft substrates leads to the migration of YAP/TAZ out of the nucleus, and its function is inhibited, suggesting that different mechanical stimuli can alter the activity and expression of YAP/TAZ.25 In the BCNI group, erectile function was impaired, the penis was in a flaccid state, blood flow to the cavernous sinus of the penis was significantly reduced, and mechanical force strength was decreased. In this experiment, the immunohistochemistry and Western blot results showed that the expression level of TAZ was significantly reduced, and the phosphorylation level of YAP was slightly decreased in the BCNI group rats at either 14 or 28 days. At the same time, the immunofluorescence results showed that the percentage of positive expression of YAP/TAZ in the nucleus was significantly lower in smooth muscle in the NED model, and the level increased significantly after LIPUS treatment. We further verified the expression levels of CTGF and CYR61, direct downstream target genes of YAP/TAZ, by RT–qPCR. We found that the expression levels of both CTGF and CYR61 were reduced, demonstrating low expression of YAP/TAZ in BCNI rats. CYR61 was found to stimulate eNOS-Ser(1177) phosphorylation and an acute increase in NO through activation of integrins, suggesting an important role of CYR61 in vasodilation.26
The mechanical effect of LIPUS was evaluated in this study. This nonthermal effect ensures the transmission of acoustic energy to the target tissue. LIPUS is a noninvasive, portable ultrasound instrument that delivers therapeutic effects directly to the injury site and has been widely used to promote tissue healing, inhibit inflammation and pain, and stimulate nerve regeneration and angiogenesis.27 Some researchers speculate that the mechanism of action of LIPUS in improving penile erectile function in NED animal models may be related to the promotion of neurotrophic factor secretion levels.28 We found in this study that ICP/MAP levels were increased and erectile function was significantly restored in BCNI rats after LIPUS treatment, and the repair of pathological tissue in the penile corpus cavernosum was also confirmed by different histological tests. Some researchers have shown that LIPUS promotes neovascularization and regulates blood flow in tissues.29 We suggest that in the cellular mechanics microenvironment, LIPUS, as an external biomechanical signal, causes molecular vibrations and collisions in penile tissues by propagating in penile tissues, which can affect cell adhesion, proliferation, activity and other behaviors, thus causing a series of biological response events in the mechanical signaling pathway of YAP/TAZ.
The Hippo signaling pathway responds to various upstream stimuli from the cellular microenvironment, including mechanical signals, cellular stress, extracellular stimuli, cell polarity, and interactions through upstream regulatory factors. It has recently been revealed that YAP/TAZ, a transcriptional coactivator downstream of the Hippo signaling pathway, plays a key role in the proliferation and differentiation of stem cells of different tissue origins, including the heart, kidney, liver and skin.30 As a core component of the classical Hippo signaling pathway, changes in YAP/TAZ are often thought to be regulated by the upstream Hippo signaling pathway. The literature suggests that the Hippo-YAP/TAZ signaling pathway plays an important role in angiogenesis.25 However, in our experimental results, no changes in the phosphorylation level of LATS1, the upstream core kinase of the Hippo signaling pathway, were found in any of the three groups of rats at different times, which further demonstrates that the changes in erectile function and the effects of LIPUS treatment may not be regulated through the classical kinase cascade Hippo pathway.
p38 MAPK is a major member of the MAPK signaling pathway family, which plays an influential role in the cellular response to extracellular stimuli and regulates cell proliferation, growth, differentiation, and apoptosis.31, 32 Moreover, several studies have shown that p38 MAPK phosphorylation is elevated in neurological injury ED models.33 In the streptozotocin-induced diabetic ED model, similar changes in the expression of p38 MAPK phosphorylation levels were observed.34, 35 Unlike the findings of Chen et al. in a study of neurologically injured rats,36 in the present experiment, we found by RT–qPCR and Western blot that in the BCNI group of rats, at both 14 and 28 days, the phosphorylation level of p38 MAPK was elevated, and this change was partially reversed after LIPUS treatment. Therefore, we can reasonably speculate that p38 MAPK is a potential upstream regulatory signal of YAP/TAZ that regulates the general mechanism of penile tissue repair after injury. p38 MAPK can inhibit tumor cells from upregulating the expression of the proto-oncogene transcriptional regulator YAP1.37, 38 It has been revealed that mechanical signaling through activation of p38 MAPK can affect actin remodeling and cell migration and that p38 MAPK signaling is involved in a wide range of cellular responses to mechanical stimuli. The p38 MAPK signaling pathway is activated in response to stretch, and inhibition of p38 MAPK disrupts stretch-induced cytoskeletal reorganization. It has also been shown that the p38 MAPK signaling pathway is an important link between F-actin cytoskeleton remodeling and YAP activation.39 SB203580 is an inhibitor of p38-MAPK signaling and has been widely used in experimental studies to explore the role of the p38-MAPK signaling pathway.40 However, there was no significant difference in YAP/TAZ expression and expression of its target genes (CTGF and CYR61) among the cell groups after the application of the p38 MAPK inhibitor SB203580 in our rat primary smooth muscle cell and mouse MOVAS cell experiments. Our results failed to confirm that MAPK/YAP signaling is necessary to promote penile smooth muscle regeneration in response to mechanical tension. Our findings differ from the previously reported pathway of mechanical tension-induced MAPK signaling changes through activation of YAP/TAZ activity,39 which indicates that other cellular signaling pathways also regulate YAP/TAZ expression.
5 CONCLUSIONS
In summary, LIPUS is safe and effective as a mechanical force-mediated ED treatment. The mechanism of action is likely to be the regulation of a mechanistic signaling pathway with YAP/TAZ as the core, whose upstream regulatory mechanism does not rely on the classical Hippo signaling pathway.
AUTHOR CONTRIBUTIONS
Methodology: Yang Liu. Formal analysis: Xiaoying Pan and Jilei Sun. Data curation: Xiangxiang Zhang. Writing—original draft preparation: Yang Liu and Yinhui Mao. Writing—review and editing: Zhitao Wei and Yong Yang. Visualization: Xiangxiang Zhang. Supervision: Zhitao Wei and Yong Yang. Project administration: Yong Yang and Zhitao Wei. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Department of Science and Technology of Jilin Province (grant number: 20200201419 and YDZJ202301ZYTS138).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, Yong Yang and Zhitao Wei, upon reasonable request.