Simultaneous detection of sperm membrane integrity and DNA fragmentation by flow cytometry: A novel and rapid tool for sperm analysis
Abstract
Background
Sperm DNA integrity has become one of the most discussed and promising biomarkers for the assessment of male fertility. However, an easy-to-apply method capable of estimating DNA fragmentation in the live fraction of spermatozoa has remained elusive, preventing this parameter from being fully applied in clinical settings.
Objectives
To validate a novel co-staining for the analysis of DNA fragmentation in membrane-intact spermatozoa.
Materials and methods
Normozoospermic semen samples were used to validate the co-staining consisting of acridine orange (AO) and LIVE/DEAD™ Fixable Blue Dead Cell Stain (LD), against established methods for the evaluation of cell viability, propidium iodide stain (PI), and DNA fragmentation, the sperm chromatin structure assay (SCSA), to rule out cross-interference. Furthermore, the accuracy of the method was tested by the evaluation of samples prepared with different amounts of membrane and DNA damage (20, 40, 60, 80, and 100%).
Results
No significant differences were observed between the co-staining and the established staining procedures (membrane integrity, p = 0.755; DNA fragmentation p = 0.976). Moreover, high R square values were obtained from the analysis of samples of known membrane (R2 = 0.9959) and DNA damage (R2 = 0.9843). The simultaneous assaying of sperm membrane integrity and nuclear DNA fragmentation allowed the analysis of four sperm categories and thereby to assess the proportion of membrane-intact spermatozoa with compromised DNA integrity.
Discussion and Conclusion
This new protocol has the potential to provide clinically relevant information about the DNA fragmentation in membrane-intact spermatozoa. Thus, it has the potential of improving the diagnostic of male infertility and enabling a better understanding of sperm dysfunction.
1 INTRODUCTION
In the last decades, sperm DNA integrity has become one of the most discussed biomarkers in basic and clinical andrology due to a push for a deeper understanding of sperm physiology and a need for updating conventional semen analysis.1, 2 This parameter can provide valuable insights into the testicular function and is clinically relevant, not only to diagnose male infertility, but also to provide guidance for treatment options.3 In this regard, it has long been known that higher sperm DNA damage is prevalent among infertile men than in the fertile ones.4-6 Furthermore, it negatively affects fertilization, embryo development, implantation, and pregnancies in natural reproduction.7 Likewise, similar observations have been reported on clinical pregnancies following in vitro fertilization (IVF) and/or intracytoplasmic sperm injection (ICSI).8
Although there are several alternatives, in the andrology field, there is no consensus on the preferred method to assess sperm DNA integrity. The currently available methodologies evaluate different aspects of DNA damage; some are based on the incorporation of acridine orange (AO) as a metachromatic dye (sperm chromatin structure assay—SCSA),9, 10 while others on the integration of labeled nucleotides (terminal deoxynucleotidyl transferase dUTP nick end labeling— TUNEL),6, 11, 12 or differential movement/dispersion of fragmented DNA (comet assay or single cell gel electrophoresis—SCGE for evaluating sperm DNA damage, sperm chromatin dispersion—SCD).13, 14
Among the above-mentioned tests, SCSA is one of the most commonly used9, 15 together with the TUNEL assay.16 SCSA, besides being a simple, fast, and well-established method, its results have shown a consistent correlation with natural and ART outcomes in both animal and human.17 However, as all currently available methods, SCSA is limited by a low specificity and poorpredictive power.18, 19 The primary reason for these limitations is that most of these methods, do not distinguish between live or dead cells in terms of membrane integrity. This is relevant because only live, membrane-intact spermatozoa are of interest in assisted reproductive technologies (ART).20
This limitation not only affects the diagnostic and prognostic power of the assay, but also makes it difficult to reveal the etiology of the sperm DNA fragmentation. Logically, the evaluation of DNA fragmentation in living sperm populations using an accurate and easy-to-apply method would improve the diagnosis of male infertility and guide treatment selection. Moreover, it could help understand diverse cellular mechanisms in spermatozoa such as cell death and oxidative stress.
Here we propose a co-staining in which we combine the principle of the SCSA to evaluate DNA fragmentation with the simplicity of a vital staining to evaluate membrane integrity. To this end, we aimed to rule out any cross-interference between the staining protocols and validate the accuracy of the co-staining through the evaluation of samples prepared with known degrees of membrane and DNA damage.
2 MATERIALS AND METHODS
2.1 Semen collection
Human semen samples were collected from donors (n = 14), ages 25–35 years, by masturbation after 3–5 days of sexual abstinence. The seminal analysis was performed according to the guidelines of the World Health Organization.21 Clinical data on the ejaculate parameters of the donors are presented in Table S1. Written, informed consent was obtained for all the participants. Ethical approval was given by the local ethics committee (4INie).
2.2 Semen processing
After semen liquefaction (30 min), the sample was divided into two aliquots; the first aliquot was diluted (10 × 106 spermatozoa/ml) in human tubal fluid buffer (HTF) (97.8 mM NaCl, 4.7 mM KCl, 0.2 Mm MgSO4, 0.37 mM KH2PO4, 2 mM CaCl2, 21.4 mM Na-lactic acid, 2.8 mM glucose, and 21 mM HEPES; pH 7.35) and divided again for the separate evaluation of membrane integrity by propidium iodide (PI) and DNA fragmentation by SCSA. The second aliquot was prepared for the simultaneous evaluation of sperm membrane integrity and DNA fragmentation, as described below in its respective section. For each condition, the final sperm concentration during the assay was 1 × 106 spermatozoa/ml.
2.3 Evaluation of sperm membrane integrity
For the assessment of membrane integrity using PI-only, 1 ml of sperm suspension (1 × 106 spermatozoa/ml) was stained with 1 µl of PI (Invitrogen™, Thermo Fisher Scientific) stock solution (2.5 mg/ml). The samples were incubated in the dark for 10 min at 37°C before examination via flow cytometry.
2.4 Evaluation of sperm DNA fragmentation
The SCSA protocol has been described in detail elsewhere by Evenson et al.22, 23 Briefly, a 200 µl sperm suspension (1 × 106 sperm/ml Tris-NaCl-EDTA (TNE) buffer (0.01 M Tris, 0.15 M NaCl, 1 mM EDTA) was admixed with 400 µl acid solution (0.1% Triton X-100, 0.15 M NaCl, and 0.08 N HCI, pH 1.20, 4°C) for 30 s, followed by the addition of 1.20 ml of acridine orange (AO) staining solution (containing 6 mg purified AO/L (Invitrogen™, Thermo Fisher Scientific), AO buffer (370 ml of stock 0.1 M citric acid, 630 ml of stock 0.2 M Na2HPO4, 1 mM disodium EDTA, 0.15 M NaCl, pH 6.0, 4°C). When excited at 488 nm, AO intercalated with double-stranded DNA emits green fluorescence and AO associated with single-stranded DNA emits red fluorescence. Non-fragmented (green fluorescence) versus fragmented DNA (red fluorescence) cytograms were used to determine the percentage of DNA fragmentation of the entire sperm population (% DNA fragmentation index or % DFI), which was quantified based on the ratio of red/[red +green] fluorescence. A reference sample (≤15%DFI) was used as control at the beginning of each round of experiments to verify the threshold differentiating non-fragmented DNA spermatozoa (main population of cells) and fragmented spermatozoa.
2.5 Simultaneous evaluation of sperm membrane integrity and DNA fragmentation
A two-step protocol was implemented to achieve the co-staining for the simultaneous evaluation of membrane integrity and DNA fragmentation. First, the specifications of the manufacturer were adapted to the staining of spermatozoa with the LIVE/DEAD™ Fixable Blue Dead Cell Stain kit (Invitrogen™, Thermo Fisher Scientific) (LD); briefly, the sperm suspensions were centrifuged (300 g, 5 min) to remove the seminal plasma. Next, the samples were re-suspended in HTF (10 × 106 spermatozoa/ml) and incubated in the dark for 30 min at 25°C using LD (1 µl/ml). The sperm suspensions were washed 1 × (300 g, 5 min) to remove the excess dye before being re-suspended in 200 µl of TNE Buffer. LD is an amine-reactive dye that reacts covalently with exposed protein amines, external amines when the cell is alive and external and internal when the cell is dead, reflecting, therefore, the membrane integrity by differential fluorescent intensity. This dye with an excitation maximum of ~350 nm, ideal for UV lasers, and emission of ~450 nm,24 leaves free the channels of the blue 488 nm laser for the green and red fluorescence emissions of the AO, avoiding in this way any spectral overlap issues and eliminating the need of color compensation. The second step consists of staining the cells with AO following a similar protocol as the SCSA which was described above.
2.6 Flow cytometry
The analysis by flow cytometry was performed using a high-resolution flow cytometer, CytoFLEX S (Beckman Coulter, Krefeld, Germany), equipped with four diode lasers emitting at 375, 405, 488, and 638 nm; and 15 optical filters. The PI was excited at 488 nm, and the emitted fluorescence was detected using a 690/50 BP filter. The LD dye was excited using a near UV laser (375 nm), and its corresponding emitted fluorescence was detected with a 450/45 BP filter. For the AO, the dye was excited with a 488 nm laser and two different filters, 525/40 BP and 690/50 BP were used to detect green and red fluorescence respectively. Fluorescence information consisting of 10.000 spermatozoa was collected for each sample/condition. Unstained and single-stained control samples were used to set quadrants and to determine background fluorescence. For the co-staining LD+AO, no color compensation was needed. Additional positive controls such as Triton X-100 0.1% (v/v) permeabilized-spermatozoa (for PI and LD) and spermatozoa exposed to UVB light (312 nm) for 30 min (for AO) were also used to confirm the limits of the quadrants. Files were exported and analyzed using FCS Express 6 Plus Research Edition (FCS Express V 6; De Novo Software).
2.7 Experimental design
The co-staining LD +AO requires the prior removal of seminal plasma (300 g, 5 min at 37°C) and 30 min of incubation before the cell nucleus can be stained with AO. In this regard, it is not known whether these differences in the DNA staining protocol could compromise the readout of the DNA fragmentation test, or if the nuclear denaturation with a low-pH (1.2) detergent solution could affect the assessment of sperm membrane integrity. Therefore, to confirm that there is no methodological cross-interference between the two staining steps, native ejaculates, diluted for concentration normalization, were evaluated for membrane integrity and DNA fragmentation independently using PI and SCSA, respectively. These values were compared with those obtained from the co-staining LD +AO. Paired measurements were carried out (n = 10) for this comparison. Furthermore, to test the accuracy and sensitivity of the co-staining, a calibration curve in which sperm suspensions were mixed in different proportions with a positive control sample in which ̴100% DNA and membrane integrity damage was induced by exposure to UVB 312 nm for 10 min. Based on the dilution of the positive control (20, 40, 60, 80, and 100%) and the native values of the samples, theoretical values of membrane integrity and DNA fragmentation were calculated for each condition. Three (n = 3) additional samples were used for this experiment. For both SCSA and the co-staining, the same reference sample was used to set the “High-DNA-fragmentation gate” after its respective processing. In this regard, the gate was defined for the SCSA as described by Evenson and Jost,22 and the DFI value was taken as reference to define the gate used for the co-staining.
2.8 Statistical analyses
The data were assessed using a one-tailed t test for independent samples using Origin 9.1 software (OriginLab). Differences were considered significant when p < 0.05. The results are displayed as columns plots. Bland and Altman plots were constructed to evaluate the similarity of the results as follows: The differences between paired measurements on the same samples were calculated, and the mean of the differences (d) was used to estimate the average bias of one method relative to the other. The 95% limit of agreement was calculated as 2× SD, where SD is the standard deviation of the differences between paired measurements. To evaluate the sensitivity of the method, R square values were calculated after performing linear regression for the experiments using theoretical and experimental values of membrane integrity and sperm DNA fragmentation.
3 RESULTS
3.1 Evaluation of sperm membrane integrity
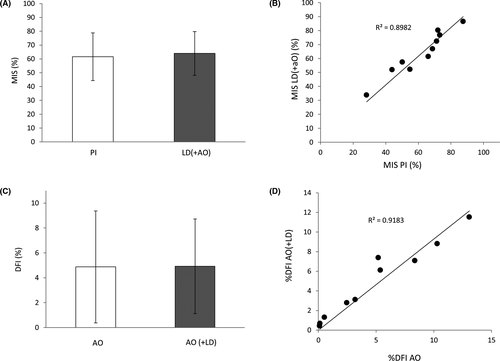
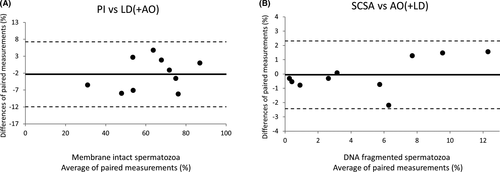
The objective of this experiment was to assess whether there is any difference in the values of membrane integrity when they are evaluated using our co-staining, in contrast to the direct evaluation of this parameter using PI. The results of this comparison can be observed in Figure 1A,B, where the values of membrane integrity obtained with PI and LD, in combination with AO, are represented as box and whiskers plots (Figure 1A). As shown, the ranges between the measurements of both methods are similar and no significant differences were detected (mean ± SD): 61.60 ± 17.24 for the PI staining and 63.95 ± 15.84 for the LD staining together with the AO (p = 0.755); n = 10. Moreover, the Bland and Altman plots also revealed that both assays produced similar results since all paired values fall within the limits of agreement (Figure 2A).
3.2 Evaluation of sperm DNA fragmentation
DNA fragmentation values obtained from the use of SCSA and the co-staining AO +LD were also compared. Again, no significant differences were observed between the values of DNA fragmentation obtained from both methods (mean ±SD): 4.87 ± 4.92 for the SCSA and 4.49 ± 3.79 for the AO staining together with the LD (p = 0.976) (Figure 1B); n = 10. Given that SCSA and our co-staining share the same principle, is not surprising that all values fell within the confidence limits of the agreement analysis (Figure 2B), which translates into a high concordance between both methods.
3.3 Simultaneous evaluation of sperm membrane integrity and DNA fragmentation
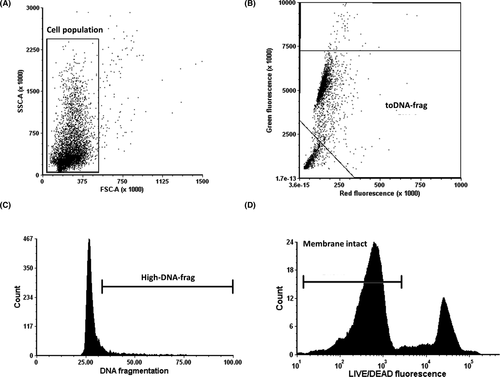
The cell population with a characteristic flame-shaped area25, 26 was selected using their FSC and SSC properties (Figure 3A), this population represents mostly spermatozoa concerning all events within the FSC and SSC region. The FSC threshold and this gate exclude part of the cell debris and other non-sperm elements that can be found out of the flame-shape region. In a subsequent green versus red fluorescence cytogram (Figure 3B), all particles lacking DNA (bottom-left corner) are subtracted from the analysis by applying a gate (toDNA-frag), which was used to determine the levels of DNA fragmentation of only the AO-positive spermatozoa after transformation of the data (Figure 3C). Finally, the values of fluorescence for the LD (Figure 3D) were combined with the levels of DNA fragmentation in a dot plot (Figure 4).
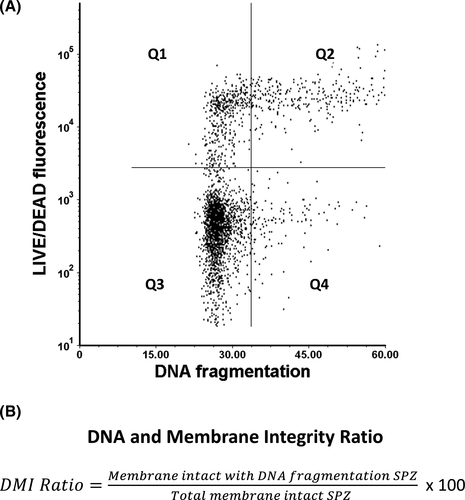
To analyze the values of DNA fragmentation and membrane integrity simultaneously, classical dot plots were used (Figure 4A). Each dot represents a cell, while the limits of each quadrant were established based on single stained positive control samples. In this way, when the values of DNA fragmentation (X-axis) and membrane integrity (Y-axis) are displayed, it is possible to identify four sperm subpopulations: (Q1) membrane non-intact +no DNA fragmentation, (Q2) membrane non-intact +DNA fragmentation, (Q3) membrane intact +no DNA fragmentation, and (Q4) membrane intact +DNA fragmentation. The sum of the percent values of the right quadrants (Q2+Q4) is equivalent to the DNA fragmentation index (DFI) provided by the conventional SCSA. An additional parameter that is possible to assess using SCSA, and our novel co-staining is the percentage of high DNA stainability (%HDS), which mirrors the amount of spermatozoa with nuclear immaturity that are characterized by a high green fluorescence (Figure 3B). However, since the donors used in the study had a very low HDS, this parameter was not included in this study. Anyhow, with the information of the lower quadrants (Q3 and Q4) provided by our method, a new parameter, the DNA, and membrane integrity ratio (DMI) can be derived (Figure 4B). Thus, making possible the assessment and categorization of DNA fragmentation in membrane intact spermatozoa. The power of this new parameter can be more clearly appreciated in Figure 5.
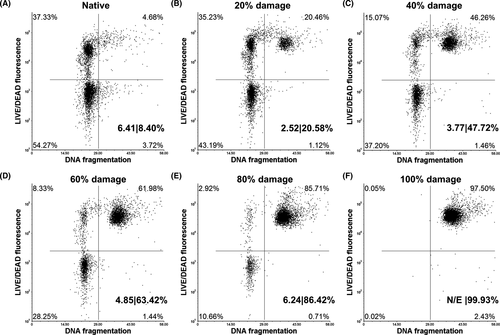
In Figure 5, the efficacy of the method is evident when evaluating samples with increasing values of membrane and DNA fragmentation. Data points corresponding to damaged sperm “migrate” toward the upper quadrants proportionally with the magnitude of damage in the sperm population. Additionally, the utility of the DMI ratio can be better appreciated when compared to the DFI values for each of the conditions (DMI|DFI%): 6.41|8.40% (native), 2.25|20.58% (20% damage), 3.77|47.72% (40% damage), 4.85|63.42% (60% damage), 6.24|86.42% (80% damage), and DMI not evaluable (N/E)|99.93% (100% damage). In this regard, The DMI ratio reveals DNA fragmentation exclusively in the fraction of spermatozoa with intact membranes as opposed to the DFI, which shows the extent of DNA fragmentation in the entire sperm population. Since the DMI ratio focus only on membrane intact spermatozoa, this type of cells is required for its estimation, in the case of extremely low numbers of such cells, like in Figure 5F, the DMI ratio is not evaluable.
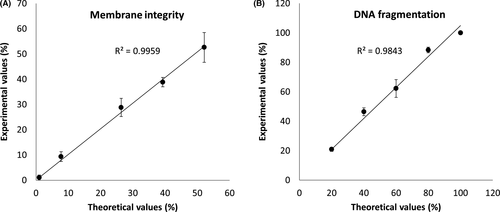
A calibration curve was prepared to further assess the accuracy of the method (Figure 6). Sperm suspensions containing known amounts of membrane and DNA damage were prepared to compare theoretical and experimental values. In the figure, it is possible to observe how theoretical and experimental values are highly correlated in both membrane integrity (R2 = 0.9959) (Figure 6A) and DNA fragmentation (R2 = 0.9843) (Figure 6B).
4 DISCUSSION
To better understand the molecular basis of sperm (dys)function and improve the diagnosis of male subfertility, complementary techniques, and methods to assist conventional sperm analysis are needed.1, 2 Sperm DNA damage has a deleterious effect on natural and assisted reproduction, not only by compromising the success of the fertilization process but also threatening the health of the offspring.7, 8, 27 Therefore, knowing the extent of this parameter can be of special importance for both diagnostic and prognosis when using ART.3
Herein, we proposed the use of a new protocol that augments the ability to determine sperm DNA fragmentation by acridine orange9, 15 with a vital stain, in this case, the LIVE/DEAD™ Fixable Blue Stain. This method distinguishes different sperm populations based on membrane integrity in a simple and easy-to-perform manner. However, the inclusion of this dye implies the previous removal of seminal plasma to avoid its unspecific binding to seminal plasma proteins and its respective incubation time.24 In this regard, it was not known whether these differences in the staining protocol could compromise the readout of the DNA fragmentation test or if the nucleus denaturation with a low-pH (1.2) detergent solution and the posterior staining with AO could also affect the assessment of sperm membrane integrity.
In this work, our co-staining was therefore tested against the independent use of two established staining procedures such as PI staining for membrane integrity and conventional SCSA. As seen in Figure 1A,B, no significant differences were observed between the methods. Moreover, the method agreement analysis shows comparable results among the tested methods (Figure 2). Therefore, we can rule out any cross-interference between the staining protocols of the co-staining, and given the high R square values obtained from theoretical and experimental values (Figure 6), it has been shown that the method is sensitive, accurate, and reliable.
Currently, most of the techniques that are available for the assessment of DNA integrity were introduced decades ago. Since then, few major changes have been implemented for their improvement. In the specific case of the SCSA,9 a method with no commercial kits available that was introduced almost 40 years ago, only minor changes regarding the nuclear denaturation have been applied.10
Similarly, the TUNEL assay,11 introduced in 1993, has undergone several modifications mainly to increase its sensitivity.6, 12 Despite the improvement, the lack of a standard methodology for this test makes it difficult to compare data.17 This approach also offers the possibility of being combined with a LIVE/DEAD fixable dye for the simultaneous assessment of membrane integrity and DNA damage,6, 12 which can provide more information than individual probes. However, it also has its limitations and its use is still restricted to basic research. The TUNEL+LD requires multiple steps, through which the protocol duration can take up to several hours. In the meantime, the accuracy of the values of membrane integrity and DNA fragmentation could be compromised due to excessive manipulation (eg, multiple centrifugations, DTT-induced chromatin de-compaction, fixation, membrane permeabilization, antibody/fluorochrome incubation).6, 28, 29 These limitations are only exacerbated if the person performing the assay lacks the necessary experience.
These methods provide results that associate sperm DNA damage to low fertility success.7, 8, 27 In this respect, Simon et al.30 performed a systematic review and meta-analysis of 92 studies, involving 14,306 IVF and ICSI cycles (5156 IVF, 5972 ICSI, and 3178 mixed IVF +ICSI), and associating sperm DNA fragmentation with ART outcomes. The evidence presented corroborates that sperm DNA fragmentation is associated with male infertility and suggests a negative correlation with the probability of a successful pregnancy following ART. Based on the data, the authors concluded that there is sufficient evidence in favor of using DNA integrity tests as a potentially useful tool in the andrological assessment. In contrast, other authors have found a limited diagnostic and prognostic power. In a meta-analysis performed by Collins et al.,18 the predictive value of sperm DNA integrity tests was evaluated for ART pregnancies in 22 studies and 2162 cycles; the results showed low specificity for all evaluated assays. In this regard, although that there is a statistically significant association between the results of the sperm DNA integrity tests and pregnancy in IVF and ICSI cycles, it is not enough to support the routine use of these tests in clinical praxis. Similar results were reported by Cissen et al.19 in a more recent study.
This discrepancy could be because most DNA fragmentation tests do not offer simple access to the DNA status of sperm fraction that is relevant for ART, namely viable spermatozoa with intact membranes.20 Thus, information concerning the etiology of the problem is limited and the risk of using DNA-damaged spermatozoa during the treatment might be overestimated, resulting in the application of unnecessary, highly invasive, and expensive treatments.
In previous research using TUNEL+LD for the simultaneous evaluation of membrane integrity and DNA fragmentation, it was evident that DNA fragmentation was largely associated with dead spermatozoa.6 Furthermore, using the same method Muratori et al.5 reported that it is possible to discriminate the fertility status of men by evaluating the sperm DNA Fragmentation either in live or dead spermatozoa. This could suggest that the increase of DNA fragmentation is proportional in both fractions, allowing the detection of subfertile patients with the same accuracy when compared to fertile subjects. These observations shed light on the physiology of sperm DNA damage, which is only possible by using an approach to simultaneously evaluate membrane integrity and DNA fragmentation.
On the other hand, one of the factors that can bias the results of the analysis of DNA integrity of spermatozoa by flow cytometry is the presence of apoptotic bodies.31, 32 These membrane-surrounded round elements are frequent in seminal samples from oligoasthenoteratozoospermic patients in which they have similar size and density to sperm heads31-33; therefore, they cannot be gated-out based on their FSC/SSC characteristics, compromising the validity of the analysis. However, one of the main characteristics of apoptotic bodies is that they are virtually depleted of genetic material.31, 32 Thus, due to their scarce DNA content, in other studies, apoptotic bodies were excluded from the analysis by the evaluation of only DNA-positive events (PI positive).12, 26 The acridine orange itself is a DNA stain, and in our method, only AO-positive cells are included; as mentioned before, in the green versus red fluorescence cytogram (Figure 3B), all particles lacking DNA (bottom-left corner) are removed from the analysis. In this way, we subtract potential apoptotic bodies applying the same principle as the PI staining but with a different DNA dye.
A possible limitation of our method is that although powerful benchtop flow cytometers for multi-parameter cell analysis are becoming more affordable, our co-staining requires the use of a flow cytometer equipped with two lasers, which may be still too expensive for most clinical laboratories restricting the use of our test.
Despite this, our co-staining represents a significant improvement that counts with the potential of an increased predictive power compared to other current methods; offering a more accurate and specific readout of the magnitude of the DNA damage in the sperm population. Furthermore, given that the reaction between LD and the protein amines is covalent,24 the samples have the potential to be fixed/frozen, stored, and evaluated at a later time. In this regard, the stability of LD provides reliable values of membrane integrity even after multiple washing steps, fixation, detergent exposure, and several hours of processing time when coupled to other methods such as TUNEL.5, 6, 12 This feature, together with its simplicity and short processing time (⁓45 min), make our method ideal for clinical settings but also in basic research to understand the etiology of sperm DNA damage and to study-specific sperm subpopulations going through oxidative stress/apoptotic-like processes.
Like the TUNEL+LD, our AO+LD staining provides the discrimination of four sperm subpopulations (Figure 4A), but unlike the TUNEL+LD, excessive sample handling is not necessary and the formulation of an accurate “DNA and Membrane Integrity Ratio (DMI)” is possible (Figure 4B). This parameter enables the assessment and categorization of membrane intact spermatozoa with DNA fragmentation. Potentially, these damaged spermatozoa are the most harmful cells for reproductive success of the couple(Rubes et al.34). Using SCSA, this manuscript described a cohort of men exposed to burning coal smoke air pollution, which despite being normozoospermic, had increased levels of DNA fragmentation, correlated with high levels of infertility and miscarriage.34 In such scenarios of heavy pollution and toxic exposures, our improved method may be most useful.
Currently, the only method to evaluate sperm DNA fragmentation with established clinical thresholds is SCSA. In this particular, clinical data revealed that men producing semen samples with a DFI ≥25% are strongly associated with reproductive failures in both natural and assisted reproduction.35-37 These observations were recently validated by Evenson et al.38; however, SCSA does not consider membrane integrity in its DFI calculation; therefore, when it is used, the risk of using spermatozoa with defective DNA during ART can be overestimated if the DNA defective spermatozoa belong to the dead fraction of the ejaculate as shown in Figure 5C–E and as reported by Mitchell et al.6
Our co-staining together with the DMI ratio can accurately reveal the real risk of using DNA-fragmented spermatozoa during ARTs, facilitating decision-making for medical professionals with regard to the most appropriate treatment selection. Thus, treatments such as in vitro fertilization (IVF) or intrauterine insemination (IUI) can be considered instead of intracytoplasmic sperm injection (ICSI), avoiding unnecessary procedures, saving time, money, and relieving stress to the couple by optimizing their ART treatment. Furthermore, our method could provide valuable diagnostic information in cases of unexplained male infertility. Some subfertile normozoospermic men with sperm DNA fragmentation below 25% might still have “high DNA fragmentation levels” among their fraction of live spermatozoa. To reach this potential, first is necessary to apply the method to a larger cohort of patients with a wider range of DNA fragmentation. In this regard, for our study, we used normozoospermic samples of high quality with low DFI% values. Therefore, the comparison between SCSA and the novel co-staining method is limited. Low-quality samples could imply a larger proportion of seminal plasma, apoptotic bodies, cellular debris, and spermatozoa susceptible to damage which could affect the readout. In any case, our protocol is not an alternative to evaluate %DFI, but a new tool to evaluate a novel parameter, the DMI, which as needs an independent validation and the establishment of clinical thresholds in future studies.
In conclusion, the results presented in this work validate the use of our innovative co-staining AO +LD as a feasible tool for the simultaneous evaluation of sperm membrane integrity and DNA fragmentation. This staining method consists of a simple protocol, with no spectral overlap and potential for ethanol fixation or freezing. This easy-to-perform assessment allows the identification of spermatozoa with intact membranes with no DNA fragmentation and the fraction of potentially harmful, membrane-intact DNA-fragmented spermatozoa, providing a more accurate and simple analysis compared to other methods.
This new protocol due to its simplicity has the potential to be applied in clinical settings, opening novel avenues for the evaluation of unexplained andrological conditions, especially after the establishment of clinical thresholds in future studies. In this way, is possible an improvement in the diagnosis in spermatology and in the framework of ART by providing fast and accurate practical information to decide the best treatment to apply.
ACKNOWLEDGMENTS
We would like to acknowledge Samuel Young for proofreading the manuscript. R.DC. during the development of this study was a recipient of a DAAD Research Grant doctoral programs in Germany (91590556). Additional funding was provided by the Deutsche Forschungsgemeinschaft (Clinical Research Unit, CRU 326).
CONFLICT OF INTERESTS
R.DC., K.R., and SS. applied for a patent related to the method for the simultaneous evaluation of sperm membrane integrity and DNA fragmentation (NP-FIL-CONF_LU101881, 22/06/2020) which has recently been granted. The patent grants the marketing rights and their benefits to the University of Münster, in case that any marketable kit derived of our method is developed. The authors declare no conflict of interest.




