Atopic Status Is Associated With Kidney Transplanted Recipients' Outcomes
Funding: This work was carried out with the support of CHU de Nantes thanks to “Appel d'offre interne”, The PULSAR program from Région Pays de La Loire, AGIPI grant (AXA), CENTAURE foundation grants, a Roche Organ Transplantation Research Foundation and European Society for Organ Transplantation grant, and under the auspices of the IHU-Cesti project, which received French government financial support managed by the National Research Agency via the “Investment Into The Future” program (ANR-10-IBHU-005). The IHU-Cesti project is also supported by Nantes Metropole and the Pays de la Loire Region. This work was also supported by the Labex IGO project (ANR-11-LABX-0016-01) funded by the Investissements d'Avenir French Government program, managed by the French National Research Agency.
See Acknowledgments for authors in DIVAT Consortium.
Magali Giral and Sophie Brouard have contributed equally.
Abbreviations
-
- DIVAT
-
- Données Informatisées et VAlidées en Transplantation
-
- DSA
-
- Donor-specific antibodies
-
- IgG-DSA/HLA
-
- Donor-specific antibodies (HLA) of IgG specificity
-
- KTR
-
- Kidney Transplanted Recipients
-
- Phadiatop
-
- ImmunoCAP Phadiatop
-
- T2I
-
- Type 2 inflammation
Kidney transplantation is the best treatment for end-stage renal disease, significantly enhancing survival and quality of life. Advances mainly in immunosuppression have reduced acute rejection rates to 5%–8%, but long-term survival remains unchanged, mostly due to chronic transplant dysfunction. Both immunological and non-immunological (mostly environmental) factors contribute to transplant dysfunction, with atopy—a rising environmental and genetic predisposition—potentially influencing outcomes.
Atopy is characterized by systemic type 2 inflammation (T2I) against environmental antigens at the local and systemic levels. It affects 30% of Europeans and is projected to impact 50% globally by 2050. Beyond allergic diseases, type 2 immunity contributes to autoimmune and alloimmune responses [1], suggesting a significant environmental impact on transplant survival. Here, we performed time-dependent survival models adjusted for confounding factors and competitive risks to explore the role of atopy in a prospective cohort of 516 kidney transplant recipients (KTR), focusing on immune events and transplant loss. Atopy is defined by the presence of a positive Phadiatop at each follow-up (at least 2—see detailed methods in Supporting Information).
Among 516 KTR analyzed, 28.5% are identified as atopic, which mirrors the prevalence in the general population. The atopic phenotype is stable throughout follow-up. Atopic KTR have higher IgE levels, are younger, predominantly male, and exhibit shorter cold ischemia times with higher rates of HLA mismatches (≥ 5) (Table 1 and Tables S1 and S2). There were no differences in serum tryptase levels during follow-up in atopic versus non atopic KTR (Table S5). These individuals demonstrate a 1.5-fold increased risk of immune events, including rejection and IgG-HLA immunization (HR = 1.49, 95% CI = 1.04–2.14). Specifically, the risk of biopsy-proven rejection is 1.7 times higher in atopic KTR (HR = 1.70, 95% CI = 1.08–2.67) (Figure 1a). While atopic KTR exhibit a non-significant trend toward higher de novo DSA IgG immunization (Table S3), their overall transplant and patient-transplant survival is lower. They face a 1.63-fold increased risk of returning to dialysis (HR = 1.63, 95% CI = 0.94–2.82) (Figure 1b and Table S4). Chronic transplant dysfunction emerged as the primary cause of graft failure, while cancer was the leading cause of death in atopic KTR (Table 1).
| Variables | Positive Phadiatop | Negative Phadiatop | p | |
|---|---|---|---|---|
| N = 147 (28.48%) | N = 369 (71.52%) | |||
| Recipient gender | N | 147 | 369 | 0.0034 |
| Female | 41 (27.89%) | 154 (41.73%) | ||
| Male | 106 (72.11%) | 215 (58.27%) | ||
| Recipient age | N | 147 | 369 | 0.0371 |
| Min–Max | (18.00; 79.00) | (20.00; 84.00) | ||
| Mean ± SD | 48.35 ± 14.30 | 51.22 ± 13.96 | ||
| Chronic kidney diseases before transplantation | N | 147 | 369 | 0.6423 |
| Unknown | 12 (8.16%) | 33 (8.94%) | ||
| Chronic glomerulonephritis | 34 (23.13%) | 87 (23.58%) | ||
| Interstial or congenital diseases | 63 (42.86%) | 157 (42.55%) | ||
| Vascular diseases | 16 (10.88%) | 26 (7.05%) | ||
| Diabetes | 22 (14.97%) | 66 (17.89%) | ||
| Transplant type (ref: kidney) | N | 147 | 369 | 0.2567 |
| K | 129 (87.76%) | 336 (91.06%) | ||
| KP | 18 (12.24%) | 33 (8.94%) | ||
| Transplantation rank | N | 147 | 369 | 0.0252 |
| 1 | 133 (90.48%) | 305 (82.66%) | ||
| 2 or 3 | 14 (9.52%) | 64 (17.34%) | ||
| Donor type (ref: SCD) | N | 120 | 326 | 0.3811 |
| SCD | 74 (61.67%) | 186 (57.06%) | ||
| ECD | 46 (38.33%) | 140 (42.94%) | ||
| Cold Ischemia (in hours) | N | 146 | 369 | 0.0023 |
| Min–Max | [0.6–41.7] | [0.6–42.7] | ||
| Mean ± SD | 12.90 ± 7.67 | 15.19 ± 7.66 | ||
| Induction treatment | N | 146 | 369 | 0.5586 |
| Pas d'induction | 3 (2.05%) | 7 (1.90%) | ||
| Non-depleting | 92 (63.01%) | 215 (58.27%) | ||
| Depleting | 51 (34.93%) | 147 (39.84%) | ||
| Immunosuppressive drugs at transplantation | N | 147 | 369 | 0.5881 |
| CNI | 146 (100%) | 369 (100%) | ||
| Antimetabolites | 144 (98.63%) | 366 (99.18%) | ||
| Systemic steroids | 128 (87.67%) | 324 (87.80%) | ||
| HLA incompatibilities (A, B, DR) | N | 147 | 369 | 0.0095 |
| < 5 | 100 (68.03%) | 291 (78.86%) | ||
| ≥ 5 | 47 (31.97%) | 78 (21.14%) | ||
| Donor specific antibodies at transplantation (IgG isotype) | N | 147 | 369 | 0.5812 |
| Negative | 135 (91.84%) | 344 (93.22%) | ||
| Positive | 12 (8.16%) | 25 (6.78%) | ||
| Total immunoglobuline E in serum (KU/L) | N | 147 | 369 | 0.0010 |
| Min–Max | [6.06–11,529] | [0–2701] | ||
| Mean ± SD | 344.86 ± 986.27 | 70.1 ± 196.94 | ||
| Return to dialysis | N | 28 (15.6%) | 35 (10.8%) | 0.0129 |
| Chronic KT dysfunction | 10 (35.7%) | 20 (57.14%) | ||
| Initial disease relapse | 2 (7.1%) | 3 (8.6%) | ||
| Rejection | 6 (21.4%) | 6 (17.1) | ||
| Transplant infection | 2 (7.1%) | 3 (8.6%) | ||
| Unknown | 8 (28.6%) | 3 (8.6%) | ||
- Note: Bold values stands for statistically significant values of p.
- Abbreviations: CNI, calcineurin inhibitors; ECD, extended standard donors; K, kidney; KP, kidney-pancreas; SCD, standard criteria donors.
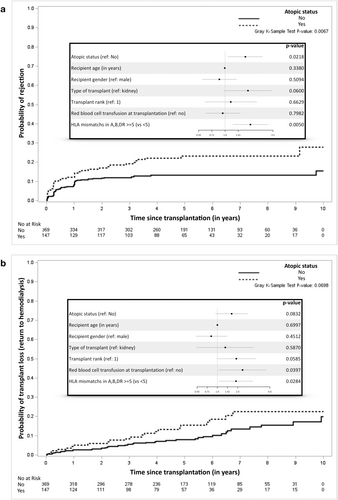
Thus, the stable atopic phenotype in KTR increases the rejection risk by 1.7-fold and an increased tendency toward de novo HLA immunization and transplant failure. Contrastingly, Müller et al. reported better graft survival in atopic KTR. The disparity likely arises from methodological differences and a higher rate of living donors in their cohort. Living donors improve graft outcomes, which could mask atopy's adverse effects [2]. Previous reports show unaltered secretion of type 2-master cytokines (IL4&13) by calcineurin inhbitors [3], which fits with the stable atopic phenotype and T2I mediators that exacerbate rejection. Indeed, HLA-specific IgE [1], IgE deposition, and basophils/mast cell infiltration [4] are associated with kidney rejection. Altogether, those observations suggest that atopy could promote T2I and transplant KT rejection through unclear mechanisms to date. It also questions the impact of atopy/allergy transfer (mostly observed in liver transplantation) on transplant rejection.
Atopy has broader implications beyond transplantation, contributing to T2I in chronic conditions like atherosclerosis. Studies link IgE sensitization against alpha-gal to cardiovascular diseases and unstable atherosclerotic plaques [5]. Observational data also connect asthma to cardiovascular risks, with therapies like anti-PCSK9 reducing asthma exacerbations and IgE-mediated responses [6]. In KTR, accelerated atherosclerosis, compounded by calcineurin inhibitors and steroids, likely contributes to poorer outcomes in atopic patients.
Despite limitations in event numbers, this robust study underscores atopy's negative impact on transplant outcomes. A closer monitoring of atopic KTR with deceased donors appears essential, especially as atopy (1) rises globally; (2) doesn't appear down regulated under conventional immunosuppression. Tailored strategies are essential to mitigate rejection risks.
Author Contributions
L.C. conceptualized, created the methodology, curated data and formal analysis, wrote the original draft, reviewed, and edited. M.L. and F.F. performed multivariate analysis and cox model. C.K. and E.P. managed the data and reviewed the manuscript. C.H. and C.B. performed Phadiatop and serum tryptase and reviewed and edited the manuscript. F.D. performed HLA immunization analysis, reviewed, and edited the manuscript. K.M.R. performed histological analysis of kidney transplant biopsies, reviewed, and edited the manuscript. M.G. conceptualized, created the methodology, reviewed, and edited the manuscript. S.B. conceptualized, created the methodology, curated data, wrote, reviewed, and edited the manuscript. All the authors read and approved this manuscript.
Acknowledgments
We thank the patients and their families, whose trust, support, and cooperation were essential for the collection of the data used in this study. We also thank the “Centre de Ressources Biologiques (CRB)” of CHU de Nantes.
DIVAT Consortium: Pr. Gilles Blancho; Pr. Julien Branchereau; Dr. Diego Cantarovich; Dr. Anne Cesbron; Dr. Agnès Chapelet; Pr. Jacques Dantal; Dr. Clément Deltombe; Dr. Anne Devis; Dr. Lucile Figueres; Dr. Claire Garandeau; Dr. Caroline Gourraud-Vercel; Dr. Christine Kandell; Dr. Aurélie Meurette; Dr. Anne Moreau; Pr. Simon Ville; Dr. Alexandre Walencik.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All relevant data are included in the article and/or its Supporting Information files.