One in five patients with chronic spontaneous urticaria has IgE to tissue transglutaminase 2
To the Editor,
Chronic spontaneous urticaria (CSU) is a mast cell-mediated inflammatory skin disease characterized by itchy wheals and angioedema that occur for longer than 6 weeks. IgE antibodies to various autoantigens, for example, thyroperoxidase (TPO) and interleukin 24 (IL-24), are held to drive the occurrence of signs and symptoms in type I autoimmune CSU, also known as autoallergic CSU (aaCSU).1, 2
Expression of tissue transglutaminase 2 (TG2), the autoantigen in celiac disease, was demonstrated to be increased in lesional skin mast cells of CSU patients.3 Furthermore, serum TG2 activity has been proposed as a potential biomarker of CSU severity and response to treatment with omalizumab.4 Here, we assessed the levels of IgE directed against TG2 (IgE-anti-TG2) in CSU patients and the correlation of IgE-anti-TG2 with clinical and serological features of CSU.
We used a human IgE capture ELISA to quantify IgE-anti-TG2 levels in 160 CSU patients and 54 healthy control subjects. We compared CSU patients with high and low serum levels of IgE-anti-TG2 for differences in clinical features and laboratory markers (Table 1). The methodology including statistical tests is described in File S1.
| Parameters | IgE-anti-TG2 | p-Value | |
|---|---|---|---|
| Negative (n = 127) | Positive (n = 33) | ||
| Female, % (n/total) | 72.2 (91/126) | 71.9 (23/32) | 0.969 |
| Age, years | 41.0 (32.0–55.2) | 41.5 (30.2–57.5) | 0.770 |
| Duration of disease, years | 1.7 (1.0–5.0) | 2.0 (0.8–6.7) | 0.863 |
| Presence of angioedema, % (n/total) | 71.5 (88/123) | 62.1 (18/29) | 0.318 |
| UAS7 | 15.0 (5.2–21.0) | 13.0 (4.0–18.0) | 0.443 |
| UCT | 8.0 (4.0–11.5) | 7.0 (5.0–11.0) | 0.925 |
| DLQI | 7.0 (3–12) | 4.5 (1.5–8.7) | 0.205 |
| Positive skin prick tests to inhalant allergens, % (n/total) | 47.4 (54/114) | 65.5 (19/29) | 0.081 |
| Positive ASST, % (n/total) | 42.6 (52/122) | 48.4 (15/31) | 0.564 |
| Total serum IgE, kU/L | 73.2 (22.7–214.7) | 87.5 (30.1–204.5) | 0.728 |
| Total IgA, g/L | 1.6 (1.1–2.1) | 1.8 (1.4–2.2) | 0.321 |
| Total IgG, g/L | 9.5 (7.8–11.9) | 10.8 (9.6–11.7) | 0.124 |
| Total IgM, g/L | 1.1 (0.8–1.7) | 1.3 (0.8–1.8) | 0.772 |
| Blood eosinophil counts, ×109/L | 0.1 (0.1–0.2) | 0.1 (0.1–0.2) | 0.721 |
| Blood basophil counts, ×109/L | 0.03 (0.01–0.05) | 0.02 (0.01–0.05) | 0.639 |
| C-reactive protein, mg/L | 1.8 (0.5–4.9) | 2.2 (0.9–8.2) | 0.297 |
| ESR, mm/h | 8.0 (3.0–16.5) | 14.0 (6.0–20.0) | 0.303 |
| IgG-anti-TPO, kU/L | 10.0 (5.0–17.0) | 10.0 (8.0–18.0) | 0.298 |
| IgE-anti-TPO total, AU | 8.1 (6.4–13.3) | 6.5 (5.9–14.5) | 0.173 |
| IgE-anti-TPO free, AU | 1.1 (0.9–1.4) | 1.1 (0.8–2.0) | 0.928 |
| IgE-anti-IL-24 total, AU | 4.5 (3.8–6.0) | 4.6 (3.3–5.6) | 0.513 |
| IgE-anti-IL-24 free, AU | 3.9 (3.1–4.6) | 3.6 (3.0–4.8) | 0.370 |
| C3 complement, mg/L | 123 (87–126) | 128 (109–148) | 0.173 |
| C4 complement, mg/L | 33.0 (21.5–38.5) | 36.0 (22.0–40.0) | 0.809 |
- Note: Values are given as median (interquartile range).
- Abbreviations: ASST, autologous serum skin test; AU, arbitrary units; C3, complement 3; C4, complement 4; DLQI, Dermatology Life Quality Index; ESR, erythrocyte sedimentation rate; IL, Interleukin; TPO, thyroperoxidase; UAS7, urticarial activity score over 7 days; UCT, urticaria control test.
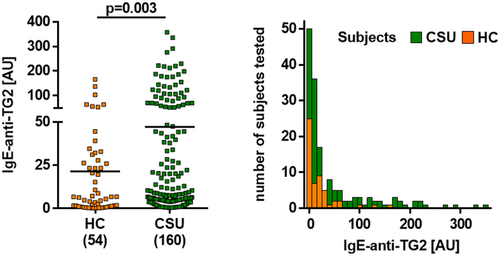
Overall, CSU patients had significantly higher IgE-anti-TG2 serum levels as compared to healthy control subjects (Figure 1). Using a cut-off value of 77.7 AU, as determined by the 95th percentile of respective levels in sera of control subjects, 33 of 160 CSU patients (20.6%) had elevated IgE-anti-TG2 levels (median: 144.6 AU), whereas 79.4% had normal levels (median 7.0 AU, Figure 1). Elevated IgE-anti-TG2 levels were not linked to any demographic, clinical and laboratory features of CSU including the presence of additional, previously described aaCSU-related autoreactive IgE, that is, IgE-anti-TPO and IgE-anti-IL-24 (Table 1 and Table S1).
TG2 and TG3 belong to a family of structurally and functionally related enzymes involved in post-translational modifications of proteins. TG2 along with TG3 are major autoantigens in celiac disease and dermatitis herpetiformis and are associated with the production of TG-targeting IgA autoantibodies.5 Furthermore, TG3 is an autoallergen actively involved in skin inflammation in patients with atopic dermatitis, with higher levels of IgE-anti-TG3 as compared to healthy controls that correlate with disease activity.6
The rate of CSU patients with elevated levels of IgE-anti-TG2, that is, 21%, is lower than the rates previously reported for IgE-anti-IL-24 (80%)2 and IgE-anti-TPO (54%–61%).1 Future studies should assess the levels of IgE and IgG autoantibodies to all three antigens in the same patient population, ideally in a multicenter approach. Interestingly, the presence of IgE-anti-TG2 was not linked to elevated levels of IgE-anti-IL-24 or IgE-anti-TPO, suggesting that different subpopulations of autoallergic CSU patients exhibit distinct profiles of autoallergens targeted by their IgE.
Functional assays have to be performed to confirm that TG2 and IgE-anti-TG2 can activate and degranulate human skin mast cells. Recently, mast cells have been shown to release TG2, leading to enhanced IgE production in B cells by up-regulating CD40L expression.3, 4 This suggests autocrine activation of mast cells by TG2-IgE-anti-TG2 crosslinking of FcεRI. Furthermore, further research should assess CSU patients' levels of IgE-anti-TG3, which were shown to be increased in patients.
Additional studies are needed to compare levels of IgE-anti-TG2 in different responders to omalizumab (fast, slow, non-responders) both at baseline and during follow-up. Finally, CSU patients are shown to have an increased risk of celiac disease and vice versa, and further studies should clarify whether the presence of IgE-anti-TG2 is associated with this risk.
In summary, a distinct and sizeable subpopulation of CSU patients shows increased IgE-anti-TG2 levels. The role of IgE-anti-TG2 and TG2 in the pathogenesis of CSU pathogenesis as well as their clinical relevance should be evaluated in further studies.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: Huichun Su, Scheffel Jörg, Xu Yao, and Sabine Altrichter; Performed the experiments: Huichun Su; Analyzed the data: Pavel Kolkhir, Huichun, Su; Wrote the paper: Huichun Su, Sabine Altrichter, Pavel Kolkhir, Marcus Maurer. Data for IgE anti-TPO, IgG anti-TPO, and IgE anti-IL-24: Yi-Kui Xiang; Statistical analysis: Pavel Kolkhir, Huichun Su.
FUNDING INFORMATION
Natural Science Foundation of Fujian Province (2019J01310), Intramural funds of Fujian Medical University Union Hospital (2021XH026).
CONFLICT OF INTEREST STATEMENT
Huichun Su: No conflicts of interest regarding any aspects of this study. Pavel Kolkhir: No conflicts of interest regarding any aspects of this study. Jörg Scheffel: No conflicts of interest regarding any aspects of this study. Yi-Kui Xiang: No conflicts of interest regarding any aspects of this study. Xu Yao: No conflicts of interest regarding any aspects of this study. MM is or recently was a speaker and/or advisor for and/or has received research funding from Allakos, Aralez, Genentech, GSK, Menarini, Merckle Recordati, Moxie, Novartis, Sanofi, MSD, and Uriach. SA is or recently was a speaker and/or advisor for and/or has received research funding from AstraZeneca, Allakos, CSL Behring, Sanofi, Takeda, ThermoFisher, Moxie, and Novartis.
Abbreviations
-
- ASST
-
- Autologous serum skin test
-
- CSU
-
- Chronic spontaneous urticaria
-
- DLQI
-
- Dermatology Life Quality Index
-
- IQR
-
- interquartile range
-
- TG
-
- tissue transglutaminase
-
- UAS7
-
- urticaria activity score over 7 days
-
- UCT
-
- urticarial control test
ACKNOWLEDGEMENT
Open Access funding enabled and organized by Projekt DEAL.




