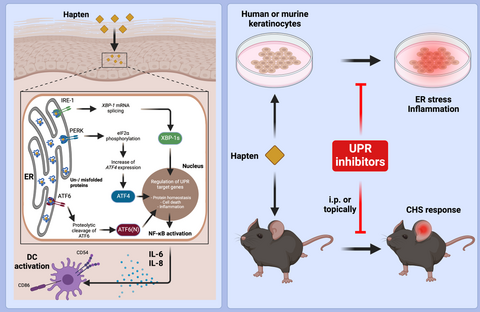
IRE1 and PERK signaling regulates inflammatory responses in a murine model of contact hypersensitivity
Funding information
DSD/ADF stipend and a DFG grant (ES 431/1-4) to Esser P.R. and a DFG grant (MA 1567/14-1) to Martin S.F.
Abstract
Background
Contact sensitizers may interfere with correct protein folding. Generation of un-/misfolded proteins can activate the IRE-1 or PERK signaling pathways initiating the unfolded protein response (UPR) and thereby determine inflammatory immune responses. We have analyzed the effect of sensitizers with different potencies on the induction of UPR activation/inhibition and the subsequent generation of a pro-inflammatory micromilieu in vitro as well as the effect of UPR modulation on the inflammatory response in the murine contact hypersensitivity (CHS) in vivo.
Methods
Semi-quantitative and quantitative PCR, fluorescence microscopy, ELISA, NF-κB activation and translocation assays, DC/keratinocyte co-culture assay, FACS, and in vivo CHS experiments were performed.
Results
Sensitizers and irritants activate IRE-1 and PERK in murine and human keratinocytes. Synergistic effects occur after combination of different weak sensitizers / addition of irritants. Moreover, tolerogenic dinitrothiocyanobenzene can be converted into a strong sensitizer by pre-activation of the UPR. Blocking UPR signaling results in decreased NF-κB activation and cytokine production in keratinocytes and in activation marker downregulation in a HaCaT/THP-1 co-culture. Interestingly, not only systemic but also topical application of UPR inhibitors abrogates CHS responses in vivo.
Conclusion
These observations highlight an important role of the UPR in determination of the inflammatory response in vitro and in vivo further underlining the importance of tissue stress and damage responses in the development of ACD and provide mechanistically based concepts as a basis for the development of new therapeutic approaches to treat allergic contact dermatitis.
Graphical Abstract
UPR in murine and human keratinocytes is activated by contact sensitizers and irritants; combining weak sensitizers results in synergistic effects. UPR inhibition reduces NF-kB activation and pro-inflammatory cytokine production thereby preventing ER stress–mediated DC activation in a keratinocyte/DC co-culture assay in vitro. Systemic or topical inhibitor application prevents CHS; UPR pre-activation enhances CHS to the weak allergen/tolerogen DNTB.
Abbreviations: ATF-4 /-6, activating transcritption factor 4/ -6; CHS, contact hypersensitivity; DC, dendritic cell; DNTB, 2,4-dinitrothiocyanobenzene; eIF2, elongation initiation factor 2; ER, endoplasmic reticulum; i.p., intraperitoneal; IRE, inositol-requiring transmembrane kinase/endonuclease; PERK, protein kinase RNA-like ER kinase; UPR, unfolded protein response; XBP-1, X-box binding protein 1
Abbreviations
-
- ACD
-
- allergic contact dermatitis;
-
- ATF6
-
- activating transcription factor 6
-
- CHS
-
- contact hypersensitivity
-
- DC
-
- dendritic cell
-
- DNTB
-
- 2,4-dinitrothiocyanobenzene
-
- IRE1
-
- inositol-requiring transmembrane kinase/endonuclease 1 alpha
-
- PERK
-
- protein kinase RNA-like ER kinase
-
- TNCB
-
- 2,4,6-trinitrochlorobenzene
-
- UPR
-
- unfolded protein response
-
- Xbp1
-
- X-box binding protein 1
1 INTRODUCTION
Allergic contact dermatitis (ACD) is a chemical-induced, T cell–mediated inflammatory skin disease. It results in local to systemic erythema and eczema formation.1 In Europe, ACD has a high socio-economic impact2-4 with a prevalence of ~20% of the population.2-4 It is caused by contact with one of >43505 electrophilic, low molecular weight (up to ~2.2 kDa), organic chemical or metal sensitizers.6 The lack of causative treatments for ACD often necessitates long-term therapy with immunosuppressive drugs like corticosteroids with significant side effects.7, 8
ACD is often caused by mixtures and formulations like cosmetic products containing combinations of weak sensitizers and irritants resulting in additive or synergistic effects with respect to the innate immune and stress responses and thereby enable or facilitate the development of ACD.9-12
Therefore, a better understanding of mechanisms determining the strength of the sensitizing potential of the chemicals is necessary to allow for better risk assessment. Despite recent efforts to understand the underlying mechanisms13, 14 and the acknowledgement that sensitizers have adjuvant effects, the nature of tissue stress and tissue damage responses in the development of ACD remains ill-defined.
To exert their immunogenic potential, sensitizers need to bind covalently or non-covalently to proteins.15 Keratinocytes are the first cells to encounter exogenous chemicals. Malmberg et al16 showed that penetration of sensitizers into the deeper skin layers might be even limited/restricted to the upper epidermis, indicating that keratinocytes might be the major cell type coming into contact with sensitizers in their protein reactive form. Therefore, keratinocytes act as sensor cells for potential danger, contribute critically to the cutaneous cytokine micromilieu,17 and are paramount for the full activation of dendritic cells (DCs) and finally sensitization in ACD. The clinically inapparent sensitization phase in ACD involves activation of both innate and adaptive immune responses after the first encounter of the skin with a sensitizer. Importantly, to facilitate priming and activation of naïve T cells, DCs need to initially receive a “tissue stress and tissue damage” signal provided by the sensitizers’ chemical reactivity.1, 13, 14, 18, 19 Renewed contact with the same sensitizer initiates the elicitation phase, leading to T cell–mediated killing of hapten-modified keratinocytes and subsequent clinically relevant erythema and eczema formation.
Here, we hypothesized that direct binding of sensitizers to proteins (haptenization process) and sensitizer-meditated reactive oxygen species (ROS) generation20, 21 would lead to changes in cellular protein structures, generating and accumulating misfolded proteins in the endoplasmic reticulum (ER). This may lead to the activation of an ER stress response, the unfolded protein response (UPR), which has been linked to the generation of pro-inflammatory conditions.22
Several pathologic conditions result in UPR activation.23, 24 Under both physiological25-27 and pathological conditions, newly synthesized proteins are processed within the ER. Accumulation of un- or misfolded proteins activates the UPR to restore correct protein folding. Three distinct stress sensors in the ER membrane are described: inositol-requiring transmembrane kinase/endonuclease 1 alpha (IRE1α protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6).28 Activation of IRE1α leads to an unconventional splicing of a 26-nucleotide fragment from the unspliced X-box binding protein 1 (uXbp1) mRNA to the spliced sXbp1 mRNA.29 The resulting protein Xbp1 acts as a potent transcription factor.30 Activation of the PERK branch by trans-autophosphorylation ends in subsequent translational arrest of most proteins and cell cycle arrest. Activation of ATF6 exposes a Golgi localization sequence and allows translocation of ATF6 into the Golgi, where it is sequentially cleaved. The ATF6 fragment translocates to the nucleus where it acts as a transcription factor activating several UPR genes including Xbp1.29
Here, we analyzed the impact of different sensitizers and irritants on the activation of the UPR in keratinocytes and the subsequent effect of the UPR on the sensitization and elicitation phase in CHS. Importantly, we show that sensitizers activate all three UPR branches and modulate the generation of a pro-inflammatory milieu in vitro. This is in line with previous work by Luís et al. showing that the extreme sensitizer DNFB activates the ATF-4 pathway in the monocytic cell line THP-1.31 Interestingly, in our system combinations of weak sensitizers result in synergistic UPR activation. Concomitant activation of the UPR by tunicamycin application before sensitization enhances the CHS response. Using ER stress reporter mice,32 we show that small molecule inhibitors can suppress the UPR when applied topically. In addition, modulation of the UPR by systemic or topical application of small molecule inhibitors for IRE1α or PERK abrogates CHS responses in vivo.
This indicates that the UPR is a crucial element of the inflammatory response to sensitizers and is involved in the modulation of their sensitizing potency. This provides mechanistically based concepts for the development of novel causative therapies.
2 MATERIALS AND METHODS
2.1 Ethics statement
Experimental procedures were in accordance with institutional, state, and federal guidelines on animal welfare, approved by the Regierungspräsidium Freiburg and supervised by the Animal Protection Representatives of the CEMT Freiburg. Primary human keratinocytes were obtained from biopsies after informed donor consent as approved by the local Ethics committee and in compliance with the declaration of Helsinki.
2.2 Mice
Female C57BL/6 mice were purchased (Charles River Laboratories) or provided by the breeding facility of the CEMT in Freiburg, Germany. ERAI mice32 were provided by Prof. R. Zeiser from a breeding stock at CEMT Freiburg. Mice were used at the age of 6–10 weeks.
2.3 Statistical analysis
Unless noted otherwise, all experiments were performed at least three times and data show results from all three independent experiments. Animal experiments used 3–5 mice per group and experiment; again, results from all three experiments are shown. Analyses were done using Graph Pad Prism 5.0 and one-way ANOVA with Dunnet´s post-test (untreated set as controls) or unpaired Student´s t test unless indicated otherwise. Error bars represent standard deviation (SD) as indicated. p < .05 was considered to be statistically significant.
All procedures used in this study are fully described in the Methods section in this article's Supplementary Material and Methods S1.
3 RESULTS
3.1 Sensitizers and the irritant SDS induce Xbp1 splicing, differentially induce PERK phosphorylation, and modulate Atf6 expression
Pam212 cells were treated with the UPR activator tunicamycin, the extreme sensitizer oxazolone, the strong sensitizer 2,4,6-trinitrochlorobenzene (TNCB), or the irritant sodium dodecyl sulfate (SDS). All chemicals increase spliced Xbp1 (sXbp1) expression in a dose (Figure S1a–d) and time (Figure 1A–E)-dependent manner. Splicing started 2–4 h after tunicamycin treatment (Figure 1A + B) while oxazolone (Figure 1C) and SDS (Figure 1E) treatment induced splicing already 1 h after stimulation with a peak after 6 h. For TNCB, significant splicing was observed after 16 h.
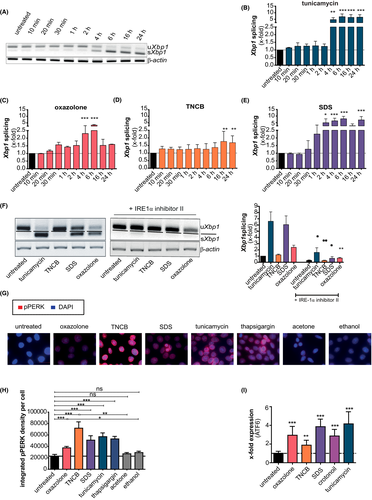
Additionally, IRE1α -mediated Xbp1 splicing is also activated in primary human keratinocytes (Figure S2a), indicating that the effect is not limited to murine keratinocytes.
To confirm that Xbp1 splicing was mediated by IRE1α activation, cells were pre-treated with the specific small molecule IRE1α inhibitor II. Blocking IRE1α activity led to complete inhibition of Xbp1 splicing 6 h after sensitizer/irritant treatment and a strong reduction of Xbp1 splicing after tunicamycin treatment (Figure 1F).
Next, we addressed the phosphorylation of PERK (Figure 1G + H). Treatment with tunicamycin and thapsigargin induced a strong PERK phosphorylation, while oxazolone showed only a faint phosphorylation compared to its solvent control ethanol. In contrast, TNCB and SDS (acetone/untreated as solvent control) induced strong phosphorylation.
Since we detected differences in the activation of IRE1α and PERK between oxazolone and TNCB, we controlled for ATF6 activation (Figure 1I). We observed a strong increase in gene expression levels after stimulation with tunicamycin (~4.16-fold) and SDS (~3.86-fold), while oxazolone showed a weaker (~2.94-fold) and TNCB just a weak (~1.89-fold) upregulation of Atf6 (Figure 1I), resembling the results observed for Xbp1 splicing (Figure 1A–F). To test whether the effect with the irritant was SDS-specific, we stimulated cells with the strong irritant crotonoil. As shown, also crotonoil stimulation resulted in an upregulation of Atf6 expression (~2.86-fold) (Figure 1I).
Taken together, sensitizers and irritants activate all three branches of the UPR. Interestingly, a difference in the strength of activation of the different UPR pathways between the extreme sensitizer oxazolone and the strong sensitizer TNCB is observed.
3.2 IRE1α and PERK inhibition blocks chemical-induced NF-κB nuclear translocation and activation
To address the influence of UPR inhibition on the activation of pro-inflammatory NF-κB signaling, we pre-treated Pam212 cells with IRE1α and PERK inhibitors (Figure 2A,B). Sensitizer and irritant treatment resulted in significant upregulation of p65 translocation and enhanced NF-κB activation. Pretreatment with IRE1α or PERK inhibitor led to significant reductions in upregulated NF-κB translocation and activation. The inhibitors significantly reduced even the extreme NF-κB activation by crotonoil treatment (containing PMA).
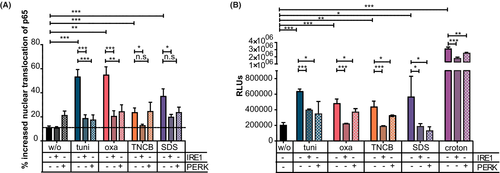
These results indicate that sensitizer- and irritant-induced activation of the UPR results in activation of the NF-κB signaling pathway.
3.3 Synergistic effects on IRE1α signaling and NF-κB activation by combination of different weak sensitizers or a weak sensitizer with the irritant SDS
Combination of different weak sensitizers or of weak sensitizers and irritants results in a CHS reaction that resembles one of a strong sensitizer.12 This led us to investigate the effect of the combination of weak sensitizers (resorcinol, cinnamic aldehyde (CA), DNTB, TNCB, and the irritant SDS on UPR activation and NF-κB translocation in Pam212 (Figure 3A,B). Sensitizers induced only weak Xbp1 splicing (Figure 3A, dark black bars). SDS treatment resulted in enhanced Xbp1 splicing. Next, we calculated the hypothetical splicing that would occur when the weak sensitizer combined with SDS would show an additive effect (Figure 3A, dark gray bars). However, for all tested combinations, experimental data (light gray bars) showed an increase in Xbp1 splicing that was higher than a purely additive effect (Figure 3A).
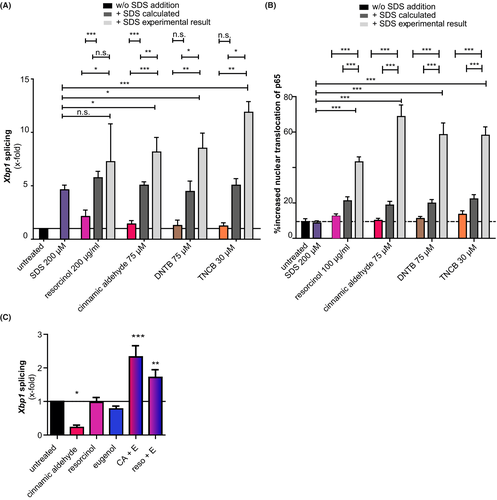
These results were reflected in the analysis of NF-κB translocation (Figure 3B), suggesting synergistic effects of combinations of weak sensitizers with irritants on the UPR activation.
Next, we assessed the effect of mixing different weak sensitizers on the activation of IRE1α. Neither CA, resorcinol, nor eugenol treatment resulted in an upregulated Xbp1 splicing, with CA leading to a reduction in Xbp1 splicing (Figure 3C). However, when CA or resorcinol are combined with eugenol, Xbp1 splicing is significantly enhanced (Figure 3C).
Taken together, combination of two weak sensitizers and also the combination of sensitizers with an irritant not only leads to a synergistic activation of the UPR but also leads to a synergistic activation of the pro-inflammatory NF-κB signaling pathway in keratinocytes.
3.4 UPR inhibitors reduce cytokine production in human keratinocytes and DC activation marker expression in a keratinocyte/dendritic cell co-culture assay
Cell culture supernatants of the human keratinocyte cell line HaCaT were analyzed for the release of the pro-inflammatory cytokine IL-633 (Figure 4A–C). Oxazolone, TNCB, or SDS dose dependently enhanced cytokine production, while treatment with either IRE1α or PERK inhibitor reduced cytokine production. The effect of the PERK inhibitor was comparable to the effect of the IRE1α inhibitor. This is in line with the activation of Xbp1 splicing (Figure 4D) and PERK phosphorylation in HaCaT cells (Figure 4E).
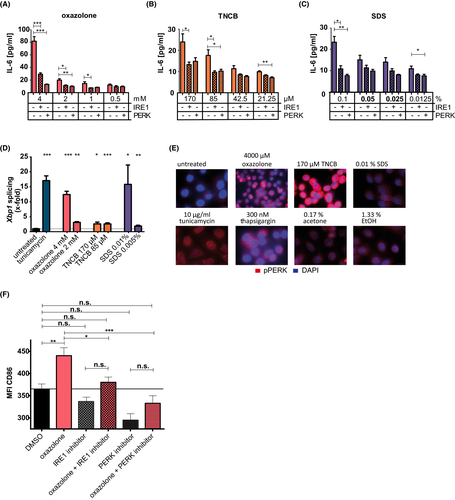
To analyze whether this reduced activation and cytokine production by keratinocytes would suffice to provide reduced maturation signals for immature DCs, we performed an adapted CoCaT-assay protocol.34 The expression of activation marker CD86 by the human monocytic DC-like cell line THP-1 was reduced in HaCaT cells pre-treated with UPR inhibitor (Figure 4F). This indicates that UPR inhibition can prevent the provision of a pro-inflammatory signal from keratinocytes to dendritic cells and thus prevents DC maturation.
Taken together, inhibition of UPR signaling results both in murine and in human keratinocytes in a reduced activation by sensitizers and irritants. This activation is necessary to produce pro-inflammatory cytokines like IL-6 and subsequently for the efficient activation of immature DCs.
3.5 Modulation of UPR influences sensitizer potency and CHS responses in vivo
Several animal studies have shown that the endogenous amphiphilic bile acid tauroursodeoxycholic acid (TUDCA) can act as a chemical chaperone, thereby inhibiting UPR dysfunction and ameliorating ER stress. Clinical trials were performed (eg, NCT 01855360, NCT 01857284, NCT 02218619, https://clinicaltrials.gov). Reduction of ER stress by TUDCA prior to sensitization significantly reduced TNCB-induced ear swelling responses upon challenge (Figure 5A). Responses obtained were like those in mice receiving vehicle control during sensitization, and thus most likely represent the irritative response to TNCB applied for elicitation. Interestingly, increasing UPR activity before sensitization by pretreating mice with the UPR activator tunicamycin resulted in a significant increase in ear thickness after elicitation compared to the group that received TNCB sensitization and elicitation only (Figure 5A).
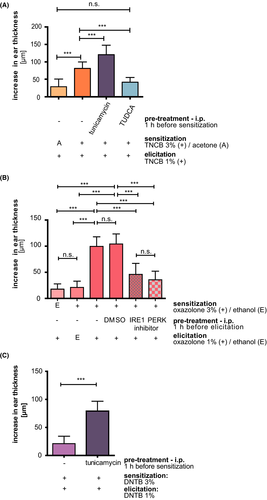
Next, we wanted to address the UPR influence on the CHS elicitation phase as a clinically more relevant setting. In addition, we decided to stress the system using the extreme sensitizer oxazolone. We also asked if specific inhibition of either the IRE1α or PERK pathway would suffice to modulate CHS responses. Therefore, we applied the inhibitors to already oxazolone-sensitized mice 1 h before elicitation. Sensitization and elicitation with oxazolone resulted in a significant increase in ear thickness compared to mice lacking either sensitization or elicitation (Figure 5B). However, pretreatment with either IRE1α– or PERK inhibitor resulted in significant reduction in ear swelling responses independent of the vehicle control (DMSO) (Figure 5B). This indicates that also interference with the UPR activation during the elicitation phase results in an inhibition of the inflammatory CHS response.
Taken together, reduction of the UPR results in a reduction of the CHS response, while enhanced UPR activation during sensitization resulted in an enhanced CHS response.
3.6 Induction of CHS to the weak sensitizer/tolerogen DNTB by UPR activation
Combination of SDS with DNTB was shown to turn the weak sensitizing/tolerogen DNTB into a strong sensitizer,35 potentially caused by enhancing DNTB penetration into the skin36 or increased tissue stress response and production of danger signals to the irritant.9, 19 To test our hypothesis, that enhanced UPR activation converts weak or non-sensitizers into potent ones, we treated mice with a known non-sensitizing dose of DNTB (3%), followed 5d later by an elicitation with 1% DNTB applied to the ears (Figure 5C). This setup resulted in very weak inflammation (21.1 ± 13.23 µm), while the irritation control, that is, mice treated with ethanol for sensitization and DNTB 1% for elicitation resulted just in background inflammation (5 ± 8.36 µm, data not shown). However, pretreatment of mice with tunicamycin before sensitization resulted in a significant increase in ear thickness after elicitation (78.89 ± 17.79 µm) with values resembling the response obtained after sensitization and challenge with similar concentrations of the strong sensitizer TNCB (Figure 5A + C).
These results show that modulation of the UPR response can modulate the sensitizer potency.
3.7 Topical inhibitor application inhibits IRE1α activation ex vivo and inhibits elicitation of oxazolone induced CHS responses
To address a more clinically relevant setting, we applied the inhibitors topically. Figure 6A + B shows that ears of ERAI (ER stress–activated indicator (ERAI) mice),32 exhibit background UPR activity owing to constant protein renewal, while activation of the UPR with tunicamycin results in a significant increase in Xbp1 splicing. The same effect is seen in oxazolone-treated ears. However, topical pretreatment with the IRE1α inhibitor in combination with oxazolone treatment reduced the fluorescence emission to the background level (Figure 6A,B).
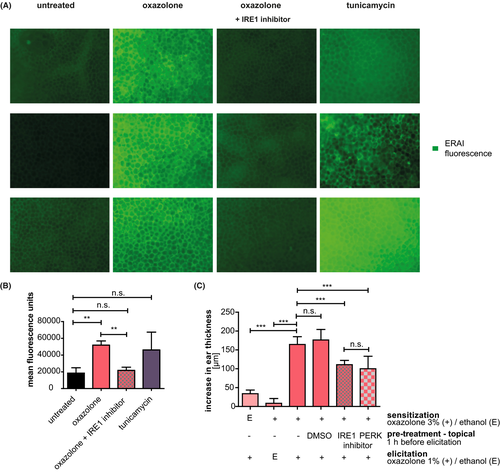
Importantly, Figure 6C shows that topical ear pretreatment of mice sensitized to oxazolone with either inhibitor results in a significant reduction of ear swelling responses.
This experiment indicates that topical application of the small molecule inhibitors is not only sufficient to block UPR activation but also to prevent the CHS response in a clinically relevant setting of already sensitized mice.
4 DISCUSSION
Chemical-induced skin inflammation is the key event of ACD and requires activation of the innate immune system and tissue stress and damage responses.19
Here, we show that oxazolone and TNCB activate all three UPR branches in keratinocytes. This is in line with previous results in the monocytic THP-1 cell line showing that the extreme sensitizer 2,4-dinitrofluorobenzene (DNFB) activated the PERK-eIF2α signaling pathway.31
Strikingly, our data show that TNCB treatment resulted in the weakest induction of the IRE1α pathway as compared to oxazolone but induced a strong phosphorylation of PERK, indicating that not all sensitizers activate the same UPR branches to the same extent. If this discrepancy between the strength of activation of the different UPR signaling pathways by different sensitizers reflects their intrinsic potential for sensitization in vivo or not remains to be shown, the same weak activation by TNCB was observed regarding ATF-6 expression. Whether or not this is due to a direct link between ATF-6 and IRE-1 activation remains to be shown. While Yoshida et al. propose that ATF-6 activation increases XBP-1 splicing37 the opposite effect was reported by Walter et al38, showing that silencing of ATF-6 results in increased XBP-1. Nevertheless, using a chemical chaperone-like TUDCA or small molecule inhibitors for IRE-1 or PERK in vivo, we were able to abrogate the sensitization to TNCB or elicitation of oxazolone CHS, respectively. This suggests that inhibition of either pathway is sufficient to reduce the tissue stress response and, most importantly, CHS.
HaCaT cells were used to address the question, whether or not the modulation of UPR activation might also play a role in the activation of human cells. Both inhibitors reduced the chemical-induced cytokine production. This is in line with the observation that the UPR enhances TLR signaling and promotes activation of pro-inflammatory signaling pathways.22, 39 Thus, as shown for the innate immune response to sensitizers, all UPR branches operate in a non-redundant manner and are all needed to overcome a critical activation threshold.40 Further studies will have to address whether the differential activation of the UPR branches by TNCB and oxazolone is due to a general difference between the two sensitizers.
Interestingly, we also observed a strong activation of the UPR with the irritants SDS and crotonoil, indicating that besides the direct protein binding and modification by sensitizers the so-called toxic effect of irritants and their intrinsic ability to modify protein structures plays an important role in the activation of the UPR. SDS denatures proteins thus most likely driving unfolded protein cargo accumulation. The active compound in crotonoil is phorbol 12-myristate 13-acetate (PMA), a potent activator of protein kinase C (PKC). While PKC-β plays a role in contact dermatitis, for example, in the activation of DCs,41 the exact mechanism of UPR induction by crotonoil remains to be determined.
Combinations of weak skin sensitizers or sensitizers with irritants result in synergistic or additive effects on the efficiency of sensitization.10, 42 Addition of SDS to nickel in the murine system43 compensated for the inability of nickel to bind to and activate murine TLR4. Direct binding of nickel to human TLR4 is essential for the innate inflammatory response.44 Likewise, the addition of the irritant SDS to the weak contact allergen/tolerogen DNTB resulted in a full sensitization to DNTB.35 To address the ability of the UPR to provide such heterologous pro-inflammatory signals,9, 40 we used 2,4-dinitrothiocyanobenzene (DNTB). Both DNTB and the structurally related 2,4-dinitrochlorobenzene (DNCB) form dinitrophenyl group coupled proteins. However, while DNCB is a strong sensitizer, DNTB is only a weak sensitizer or even tolerogenic. Remarkably, as described for SDS, UPR activation also enabled a sensitization to DNTB and enhanced the CHS response to the strong sensitizer TNCB. In combination with our observation that SDS rapidly activated the UPR branches and induced cytokine production in a UPR-dependent manner, this enhanced sensitization as a result of UPR activation by tunicamycin might explain the described effects of SDS treatment on the weak sensitizing potencies of sensitizers such as DNTB. Whether or not the efficiency to activate the UPR correlates with the sensitizing potency of sensitizers remains to be addressed. Interestingly, we have already shown that mice deficient for nuclear-related factor2 (Nrf2), which is involved in internal redox homeostasis, show an increased sensitization potential toward weak sensitizers.45
Our data firmly establish the UPR as an important mechanism involved in the orchestration of sensitizer-induced skin inflammation. This tissue stress response in concert with the innate immune response is needed to drive CHS responses. The importance of the UPR in immune responses is being recognized, and mechanistic parallels to pro-inflammatory signaling such as NF-κB activation have been shown.22, 39 The cross-talk between the UPR and the innate immune response will be investigated in future studies also including potential cross-talks between other innate immune cells playing essential roles in the CHS induction like neutrophils,46 basophils, and mast cells.47 It remains to be shown, whether the modulation of the UPR activation can be also observed in lesional vs nonlesional skin from patients. Ideally, control subjects or patients with a known allergy to a specific substance would be recruited, the substance applied, 6 h later punch biopsies taken and the epidermis analyzed for the influence of existing sensitization on the strength of UPR activation.
Importantly, as shown here, the small molecule UPR inhibitors can also be applied topically, potentially allowing for the formulation of a cream as basis for a localized topical CHS treatment. However, the reduction in ear swelling response after topical application of the inhibitors was lower than after i.p. injection, necessitating further work to address the optimal dose. Interestingly, the chemical chaperone TUDCA has already been included in several human phase 1–3 trials for the treatment of different diseases. While ACD is usually not a life-threatening disease and would not warrant severe side effects as they might occur due to a prolonged systemic UPR inhibition, localized and topical application might be beneficial compared to corticosteroid treatment.
In addition, for therapeutic applications, the time kinetic allowing for an effective treatment in patients remains to be addressed in further studies as well as potential benefits or increases in unwanted side effects from blocking different branches of the UPR at the same time remain to be shown. Last but not least, if the inhibitors can indeed be applied in a formulation for topical application in a cream that is stable enough for storage and can be used to prevent CHS in mice and eventually reduce ACD reactions in sensitized humans remains an interesting question.
In conclusion, our work shows the ability of sensitizers and irritants to activate all three branches of the UPR and indicates that modulation of the UPR results in a modulation of the CHS response. This not only provides new mechanistic insight into the processes underlying ACD but also provides novel therapeutic targets for the treatment of ACD.
ACKNOWLEDGEMENTS
This work was supported by a DSD/ADF stipend and a DFG grant (ES 431/1-4) to Esser P.R. and a DFG grant (MA 1567/14-1) to Martin S.F. The CLS Cell Lines Service GmbH is acknowledged as donor and the BIOSS Toolbox as source of the THP-1 cell line. Graphical abstract was generated using BioRender.com
CONFLICT OF INTEREST
All authors declare no conflict of interest directly related to the work. Dr. Apostolova reports personal fees from Novartis Pharma, non-financial support from Takeda, outside the submitted work. Dr. Zeiser reports personal fees from Novartis, personal fees from Incyte, personal fees from Mallinckrodt, outside the submitted work. T. Jakob reports grants, personal fees, and non-financial support from Novartis, grants, personal fees, and non-financial support from ALK-Abello, personal fees and non-financial support from Allergy Therapeutics/ Bencard, personal fees from Allergopharma, personal fees from Thermo Fisher, outside the submitted work. All other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
PRE and SFM developed the overall concept and designed research. FG, LV, MF, and PRE conducted the experiments, FG and PRE analyzed data. PA, RZ, and TJ provided reagents and/or conceptual input. FG and PRE drafted the manuscript. PRE and SFM provided funding and supervision. All authors discussed the results and contributed to the final manuscript.