Management of patients with atopic dermatitis undergoing systemic therapy during COVID-19 pandemic in Italy: Data from the DA-COVID-19 registry
Andrea Chiricozzi, Marina Talamonti and Marco Galluzzo are the authors contributed equally to this work.
Abstract
Background
Few and small studies have described the management of immunomodulant/immunosuppressive therapies or phototherapy in atopic dermatitis (AD) patients during coronavirus disease 2019 (COVID-19) pandemic.
Methods
A national registry, named DA-COVID-19 and involving 35 Italian dermatology units, was established in order to evaluate the impact of COVID-19 pandemic on the management of adult AD patients treated with systemic immunomodulant/immunosuppressive medications or phototherapy. Demographic and clinical data were obtained at different timepoints by teledermatology during COVID-19 pandemic, when regular visits were not allowed due to sanitary restrictions. Disease severity was assessed by both physician- and patient-reported assessment scores evaluating itch intensity, sleep disturbances, and AD severity.
Results
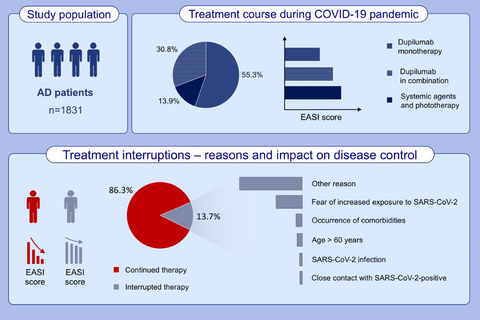
A total of 1831 patients were included, with 1580/1831 (86.3%) continuing therapy during pandemic. Most patients were treated with dupilumab (86.1%, 1576/1831) that was interrupted in only 9.9% (156/1576) of cases, while systemic immunosuppressive compounds were more frequently withdrawn. Treatment interruption was due to decision of the patient, general practitioner, or dermatologist in 39.9% (114/286), 5.6% (16/286), and 30.1% (86/286) of cases, respectively. Fear of increased susceptibility to SARS-CoV-2 infection (24.8%, 71/286) was one of the main causes of interruption. Sixteen patients (0.9%) resulted positive to SARS-CoV-2 infection; 3 of them (0.2%) were hospitalized but no cases of COVID-related death occurred.
Conclusions
Most AD patients continued systemic treatments during COVID pandemic and lockdown period, without high impact on disease control, particularly dupilumab-treated patients.
Graphical Abstract
Among 1831 studied AD patients, 86.1% were treated with dupilumab. Patients continuing therapy experienced a marked reduction of disease severity during pandemic. The causes of treatment interruption included: fear of increased susceptibility to SARS-CoV-2 infection (24.8%), occurrence of comorbidities (5.9%), age above 60 years (5.2%), SARS-CoV-2 infection (2.8%), close contact with SARS-CoV-2-positive subject (2.4%), other reasons, for example, inability to maintain drug supply, non-medical/unspecified causes (58.7%).
Abbreviations: AD, atopic dermatitis; COVID-19: coronavirus disease 2019; EASI, eczema area severity index; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
CONFLICTS OF INTEREST
A. Chiricozzi served as advisory board member and consultant and has received fees and speaker's honoraria or has participated in clinical trials for AbbVie, Almirall, Biogen, Fresenius Kabi, Leo Pharma, Lilly, Janssen, Novartis, Sanofi Genzyme, and UCB Pharma. G. Fabbrocini acted as speaker and consultant for AbbVie and Leo Pharma. G. Girolomoni has been principal investigator in clinical trials sponsored by and/or and has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Celgene, Celltrion, Eli-Lilly, Genzyme, Leo Pharma, Menlo therapeutics, Novartis, OM Pharma, Pfizer, Regeneron, Samsung, Sandoz, and UCB Pharma. A. Offidani has been a scientific consultant/speaker/clinical study investigator for AbbVie, Celgene, Janssen, LEO Pharma, Eli-Lilly, MSD, Novartis, Pfizer, Sanofi, Alfasigma, and Almirall. M.T. Rossi has received personal fee for advisory board meeting from Sanofi, Abbvie, Novartis, and Cantabria. L. Bianchi reports personal fees from speaker and as consultant for AbbVie, Novartis, Janssen-Cilag, Pfizer, UCB, and Leo Pharma, outside the submitted work. L. Stingeni has been principal investigator in clinical trials sponsored by and/or received personal fees from AbbVie, Almirall, Celgene, Eli-Lilly, Janssen, Novartis, and Sanofi-Genzyme. G. Pellacani has been principal investigator in clinical trials sponsored by and/or received personal fees from AbbVie, Almirall, Eli-Lilly, Leo Pharma, Novartis, and Sanofi. A. Patrizi has served as a speaker and received honoraria from Sanofi-Genzyme for lectures, research grants and as an advisory board member. C. Foti has been speaker for Sanofi and Abbvie. M.C. Fargnoli has served on advisory boards, received honoraria for lectures and research grants from Almirall, AbbVie, Galderma, Leo Pharma, Mylan, Medac Pharma, Celgene, Pierre Fabre, UCB, Eli-Lilly, Pfizer, Janssen, Novartis, Sanofi Genzyme, Roche, Sun Pharma, and MSD. F. Rongioletti has served on advisory board, received honoraria for lectures and research grants from Novartis, AbbVie, Janssen-Cilag, Eli-Lilly, Leo Pharma, and Sanofi-Genzyme. P. Amerio has received speaker honoraria from Sanofi, AbbVie, Janssen, Celgene, Novartis, and Sandoz. G. Micali has been a scientific consultant/clinical study investigator for Abbvie, Eli-Lilly, Janssen- Cilag, LEO Pharma, and Novartis. C. Patruno has been a consultant and held sponsored conferences for AbbVie, Novartis, Pfizer, and Sanofi. I. Zalaudek has been a consultant and/or speaker for Novartis, Celgene, and Amgen. K. Peris reports grants and personal fees for advisory board meeting from Almirall, AbbVie, Biogen, Lilly, Celgene, Galderma, Leo Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, Sun Pharma, and Janssen. The other authors reported no conflicts of interests.