Allergic reactions to the first COVID-19 vaccine: A potential role of polyethylene glycol?
The COVID-19 vaccine developed by Pfizer and BioNTech was approved by the Medicines and Healthcare Products Regulatory Agency (MHRA) in the United Kingdom (UK) on 2nd December 2020.1 MHRA is therefore the first regulator agency in the world to approve a vaccine to prevent coronavirus disease (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a virus that is responsible for a global pandemic.
The vaccine, named BNT162b2, is a messenger ribonucleic acid (mRNA) vaccine produced as a highly purified single-stranded, 5’-capped mRNA that has been generated through in vitro transcription in cell-free conditions from the corresponding DNA. The mRNA encodes the viral spike (S) from SARS-CoV-2.1 (Figure 1) The approval by MHRA was based on the results of a phase III trial involving 44,000 participants, which showed that BNT162b2 was 95% effective administrated in a two-dose regimen, which involves 30 µg per dose administrated 21 days apart.2 On 8th December 2020, the vaccine BNT162b2 was started to be administrated to the population at risk for COVID-19 in the UK. On the second day of the vaccination program, the National Health System (NHS) in England informed that two workers of the NHS experienced adverse allergic symptoms shortly after receiving the vaccine, which prompted MHRA to advise healthcare providers not to administrate the vaccine to subjects with a significant history of allergic reactions.3
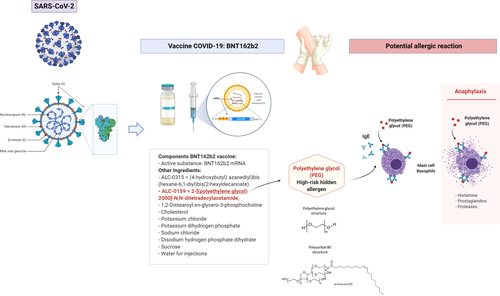
According to MHRA, vaccine BNT162b2 for COVID-19 prevention contains the following excipients: ALC-0315 = (4-hydroxybutyl) azanediyl)bis (hexane-6,1-diyl)bis(2-hexyldecanoate), ALC-0159 = 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3-phosphocholine, cholesterol, potassium chloride, potassium dihydrogen phosphate, sodium chloride, disodium hydrogen phosphate dihydrate, sucrose, and water for injections.1 From all the excipients officially declared one with the ability to cause allergic reactions is ALC-0159 since it contains polyethylene glycol (PEG) or macrogol (Figure 1). PEG is a hydrophilic polymer that is frequently used as an excipient in everyday products including medicines, cosmetics, or foods. Although anaphylactic reactions to PEG have not been frequently reported, in the last years an increased number of allergic reactions to PEG have been described in individuals due to the administration of certain drugs or due to the use of certain products for personal hygiene.4-6 Recently, the largest case series of allergic reactions to PEG was described in individuals that developed anaphylactic reactions to medications containing this compound. PEG was described as a high-risk hidden allergen in drug and food items that can induce allergic reactions difficult to detect by healthcare providers and might be therefore underdiagnosed.7 Cross-reactivity of PEG with Polysorbate 80 due to the shared chemical moiety: –(CH2CH2O)n has been described as well.8, 9 Skin prick testing and intradermal testing with different dilutions of PEG, basophil activation test, and oral provocation testing are recommended in suspected individuals. Since even severe anaphylactic reactions during skin testing have been described, testing of suspected individuals should be carefully done following published algorithms in specialized allergy centers.7 Recently, specific IgE to PEG has been detected with the help of a dual cytometric bead assay,10 such non-invasive methods would be helpful as additional diagnostic tools to screen for potential individuals at risk. Since even non-IgE-mediated hypersensitive reactions to PEG have been described in the literature, double-blind placebo-controlled oral challenges might be necessary in a subgroup of individuals.
For patients testing positive, avoidance of PEG and PEG analogues is strictly recommended.
Although the trigger of the adverse allergic reactions suffered by the two NHS workers after receiving the vaccine BNT162b2 against COVID-19 has yet to be determined, the potential role of the excipient ALC-0159 containing PEG as a high-risk hidden trigger of dangerous allergic reactions should be carefully considered before advising the administration of BNT162b2 vaccine. However, if hypersensitivity reactions to PEG and PEG analogous will be ruled out as a reason for the anaphylactic reactions observed, not all patients with food and drug allergies would have to be advised against BNT162b2 vaccination, but just those allergic to PEG, PEG analogues, or other excipients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




