Performance of basophil activation test and specific IgG4 as diagnostic tools in nonspecific lipid transfer protein allergy: Antwerp-Barcelona comparison
Ine I. Decuyper
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Pediatric Department, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorMariona Pascal
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Search for more papers by this authorAthina L. Van Gasse
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Pediatric Department, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorChristel Mertens
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorAraceli Díaz-Perales
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Plant Biotechnology Institute (UPM-INIA), Madrid, Spain
Search for more papers by this authorGiovanna Araujo
Allergy Section, Pneumology Department, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
Search for more papers by this authorMaria Torradeflot
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Search for more papers by this authorJosefina Rius
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Search for more papers by this authorSara Balsells
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Search for more papers by this authorRosa M. Muñoz-Cano
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Allergy Section, Pneumology Department, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
Search for more papers by this authorJoan Bartra
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Allergy Section, Pneumology Department, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
Search for more papers by this authorLynne Li
Department of Medicine, University of British Columbia, Vancouver, BC, Canada
Search for more papers by this authorVito Sabato
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorMargo M. Hagendorens
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Pediatric Department, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorChris H. Bridts
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorLuc S. De Clerck
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorCorresponding Author
Didier G. Ebo
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Correspondence
Didier G. Ebo, Department of Immunology-Allergology-Rheumatology, Faculty of Medicine and Health Sciences, Antwerp University, Campus Drie Eiken, Universiteitsplein 1, D.T.594, 2610 Antwerp, Belgium.
Email: [email protected]
Search for more papers by this authorMargaretha A. Faber
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorIne I. Decuyper
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Pediatric Department, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorMariona Pascal
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Search for more papers by this authorAthina L. Van Gasse
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Pediatric Department, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorChristel Mertens
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorAraceli Díaz-Perales
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Plant Biotechnology Institute (UPM-INIA), Madrid, Spain
Search for more papers by this authorGiovanna Araujo
Allergy Section, Pneumology Department, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
Search for more papers by this authorMaria Torradeflot
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Search for more papers by this authorJosefina Rius
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Search for more papers by this authorSara Balsells
Immunology Department, Centre de Diagnòstic Biomèdic (CDB), Hospital Clínic de Barcelona, Barcelona, Spain
Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Universitat de Barcelona, Barcelona, Spain
Search for more papers by this authorRosa M. Muñoz-Cano
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Allergy Section, Pneumology Department, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
Search for more papers by this authorJoan Bartra
Spanish Network for Allergy – RETIC de Asma, Reacciones adversas y Alérgicas (ARADYAL), Madrid, Spain
Allergy Section, Pneumology Department, Institut Clínic Respiratori (ICR), Hospital Clínic de Barcelona, Barcelona, Spain
Search for more papers by this authorLynne Li
Department of Medicine, University of British Columbia, Vancouver, BC, Canada
Search for more papers by this authorVito Sabato
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorMargo M. Hagendorens
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Pediatric Department, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorChris H. Bridts
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorLuc S. De Clerck
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorCorresponding Author
Didier G. Ebo
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Correspondence
Didier G. Ebo, Department of Immunology-Allergology-Rheumatology, Faculty of Medicine and Health Sciences, Antwerp University, Campus Drie Eiken, Universiteitsplein 1, D.T.594, 2610 Antwerp, Belgium.
Email: [email protected]
Search for more papers by this authorMargaretha A. Faber
Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorAbstract
Background
Recent studies show that nsLTP sensitization is not limited to the Mediterranean basin and can present diverse clinical phenotypes. It remains challenging to predict clinical outcome when specific IgE antibodies (sIgE) to nsLTPs are present. This study compares both clinical and in vitro allergy characteristics but also diagnostic performance of a basophil activation test (BAT) and sIgG4 in nsLTP-sensitized patients from Antwerp (ANT, Belgium) and Barcelona (BCN, Spain).
Methods
Adult subjects with positive sIgE rPru p 3 and/or rMal d 3 ≥ 0.10 kUA/L (n = 182) and healthy controls (n = 37) were included. NsLTP-sensitized individuals were stratified according to clinical symptoms with peach/apple, respectively. BAT rPru p 3 and rMal d 3 were performed and sIgG4 antibodies to both components quantified.
Results
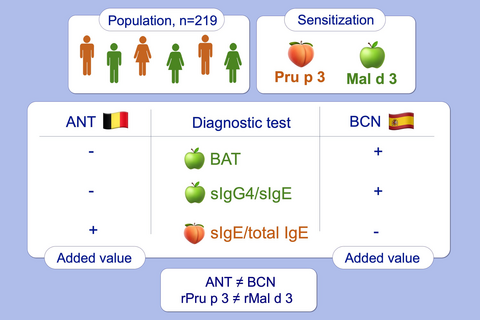
In BCN, only ratios of sIgG4/sIgE rMal d 3 and BAT rMal d 3 (0.001 µg/mL) can identify clinically relevant Mal d 3 sensitization (sensitivity of 60%-63% and a specificity of 75%-67%, respectively). In ANT, only the sIgE/total IgE rPru p 3 ratio shows added value (sensitivity 60% and specificity 83%). Finally, it appears that symptomatic patients in BCN are more sensitive to lower allergen concentrations compared to ANT. In addition, it was shown that ANT patients were more often sensitized to pollen and that specific pollen sources differed between regions.
Conclusions
NsLTP-related allergy profiles and diagnostic performance differ significantly between regions and are component-specific, which makes extrapolation of data difficult to do. In addition, it seems that basophil sensitivity might show geographical differences. Additional research is needed to confirm these findings.
Graphical Abstract
This study explores the performance of BAT, sIgE/total IgE, sIgG4 and sIgG4/sIgE ratio in Pru p 3- and Mal d 3-sensitized populations from Antwerp (ANT) and Barcelona (BCN). In BCN, only ratios of sIgG4/sIgE rMal d 3 and BAT rMal d 3 can identify clinically relevant Mal d 3 sensitization, whereas in ANT, only the sIgE/total IgE rPru p 3 ratio shows added value. NsLTP sensitization and diagnostic performance cannot be extrapolated from one population to another. BAT, basophil activation test; nsLTP, nonspecific lipid transfer protein.
CONFLICTS OF INTEREST
Proposal: Prof De Clerck reports funding from the company Roche, during the conduct of the study; grants from Roche, outside the submitted work. The remaining authors declare that they have no conflicts of interest.
Supporting Information
| Filename | Description |
|---|---|
| all14040-sup-0001-Supinfo.docxapplication/docx, 216.2 KB | |
| all14040-sup-0002-Repositorytables.docxapplication/docx, 20.5 KB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Pastorello EA, Robino AM. Clinical role of lipid transfer proteins in food allergy. Mol Nutr Food Res. 2004; 48(5): 356-362.
- 2Egger M, Hauser M, Mari A, Ferreira F, Gadermaier G. The role of lipid transfer proteins in allergic diseases. Curr Allergy Asthma Rep. 2010; 10(5): 326-335.
- 3Salcedo G, Sanchez-Monge R, Diaz-Perales A, Garcia-Casado G, Barber D. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin Exp Allergy. 2004; 34(9): 1336-1341.
- 4Beezhold DH, Hickey VL, Kostyal DA, et al. Lipid transfer protein from Hevea brasiliensis (Hev b 12), a cross-reactive latex protein. Ann Allergy Asthma Immunol. 2003; 90(4): 439-445.
- 5Decuyper II, Faber MA, Lapeere H, et al. Cannabis allergy: a diagnostic challenge. Allergy. 2018; 73(9): 1911-1914.
- 6Fernandez-Rivas M, Bolhaar S, Gonzalez-Mancebo E, et al. Apple allergy across Europe: how allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006; 118(2): 481-488.
- 7Gomez F, Aranda A, Campo P, et al. High prevalence of lipid transfer protein sensitization in apple allergic patients with systemic symptoms. PLoS ONE. 2014; 9(9):e107304.
- 8Schocker F, Luttkopf D, Scheurer S, et al. Recombinant lipid transfer protein Cor a 8 from hazelnut: a new tool for in vitro diagnosis of potentially severe hazelnut allergy. J Allergy Clin Immunol. 2004; 113(1): 141-147.
- 9Mothes-Luksch N, Raith M, Stingl G, et al. Pru p 3, a marker allergen for lipid transfer protein sensitization also in Central Europe. Allergy. 2017; 72(9): 1415-1418.
- 10Gaier S, Oberhuber C, Hemmer W, et al. Pru p 3 as a marker for symptom severity for patients with peach allergy in a birch pollen environment. J Allergy Clin Immunol. 2009; 124(1): 166-167.
- 11Gao ZS, Yang ZW, Wu SD, et al. Peach allergy in China: a dominant role for mugwort pollen lipid transfer protein as a primary sensitizer. J Allergy Clin Immunol. 2013; 131(1): 224-226 e1-3.
- 12Faber MA, Van Gasse AL, Decuyper II, et al. IgE-reactivity profiles to nonspecific lipid transfer proteins in a northwestern European country. J Allergy Clin Immunol. 2017; 139(2): 679-682.e5.
- 13Skypala IJ, Cecchi L, Shamji MH, Scala E, Till S. Lipid Transfer Protein allergy in the United Kingdom: Characterization and comparison with a matched Italian cohort. Allergy. 2019; 74(7): 1340-1351.
- 14Pascal M, Vazquez-Ortiz M, Folque MM, et al. Asymptomatic LTP sensitisation is common in plant-food allergic children from the Northeast of Spain. Allergol Immunopathol (Madr). 2016; 44(4): 351-358.
- 15Gonzalez-Mancebo E, Gonzalez-de-Olano D, Trujillo MJ, et al. Prevalence of sensitization to lipid transfer proteins and profilins in a population of 430 patients in the south of Madrid. J Investig Allergol Clin Immunol. 2011; 21(4): 278-282.
- 16Christensen MJ, Eller E, Mortz CG, Brockow K, Bindslev-Jensen C. Exercise lowers threshold and increases severity, but wheat-dependent, exercise-induced anaphylaxis can be elicited at rest. J Allergy Clin Immunol Pract. 2018; 6(2): 514-520.
- 17Pascal M, Munoz-Cano R, Reina Z, et al. Lipid transfer protein syndrome: clinical pattern, cofactor effect and profile of molecular sensitization to plant-foods and pollens. Clin Exp Allergy. 2012; 42(10): 1529-1539.
- 18Scala E, Till SJ, Asero R, et al. Lipid transfer protein sensitization: reactivity profiles and clinical risk assessment in an Italian cohort. Allergy. 2015; 70(8): 933-943.
- 19Sanchez-Lopez J, Tordesillas L, Pascal M, et al. Role of Art v 3 in pollinosis of patients allergic to Pru p 3. J Allergy Clin Immunol. 2014; 133(4): 1018-1025.
- 20Faber MA, Gasse AL, Decuyper II, et al. Cross-reactive aeroallergens: which need to cross our mind in food allergy diagnosis? J Allergy Clin Immunol: Pract 2018; 6(6): 1813-1823.
- 21Asero R, Pravettoni V. Anaphylaxis to plant-foods and pollen allergens in patients with lipid transfer protein syndrome. Curr Opin Allergy Clin Immunol. 2013; 13(4): 379-385.
- 22Scala E, Abeni D, Guerra EC, et al. Co-sensitization to Profilin is associated with less severe reactions to Foods in nsLTPs- and Storage Proteins-Reactors and with less severe respiratory allergy. Allergy. 2018; 73(9): 1921-1923.
- 23Wangorsch A, Larsson H, Messmer M, et al. Molecular cloning of plane pollen allergen Pla a 3 and its utility as diagnostic marker for peach associated plane pollen allergy. Clin Exp Allergy. 2016; 46(5): 764-774.
- 24Santos AF, James LK, Bahnson HT, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015; 135(5): 1249-1256.
- 25Fernandez-Rivas M, Garrido Fernandez S, Nadal JA, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009; 64(6): 876-883.
- 26Gomez-Casado C, Garrido-Arandia M, Gamboa P, et al. Allergenic characterization of new mutant forms of Pru p 3 as new immunotherapy vaccines. Clin Dev Immunol. 2013; 2013: 385615.
- 27Palomares F, Gomez F, Bogas G, et al. Immunological changes induced in peach allergy patients with systemic reactions by Pru p 3 sublingual immunotherapy. Mol Nutr Food Res. 2018; 62(3): 1700669.
- 28Garrido-Fernandez S, Garcia BE, Sanz ML, Echechipia S, Lizaso MT, Tabar AI. Are basophil activation and sulphidoleukotriene determination useful tests for monitoring patients with peach allergy receiving sublingual immunotherapy with a Pru p 3-enriched peach extract? J Investig Allergol Clin Immunol. 2014; 24(2): 106-113.
- 29Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008; 74(4): 201-210.
- 30Santos AF, Douiri A, Becares N, et al. Basophil activation test discriminates between allergy and tolerance in peanut-sensitized children. J Allergy Clin Immunol. 2014; 134(3): 645-652.
- 31Mayorga C, Gomez F, Aranda A, et al. Basophil response to peanut allergens in Mediterranean peanut-allergic patients. Allergy. 2014; 69(7): 964-968.
- 32Simons FE, Ardusso LR, Dimov V, et al. World Allergy Organization Anaphylaxis Guidelines: 2013 update of the evidence base. Int Arch Allergy Immunol. 2013; 162(3): 193-204.
- 33Diaz-Perales A, Garcia-Casado G, Sanchez-Monge R, Garcia-Selles FJ, Barber D, Salcedo G. cDNA cloning and heterologous expression of the major allergens from peach and apple belonging to the lipid-transfer protein family. Clin Exp Allergy. 2002; 32(1): 87-92.
- 34Hoffmann HJ, Santos AF, Mayorga C, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015; 70(11): 1393-1405.
- 35Fernández-Rivas M, Bolhaar S, González-Mancebo E, et al. Apple allergy across Europe: How allergen sensitization profiles determine the clinical expression of allergies to plant foods. J Allergy Clin Immunol. 2006; 118(2): 481-488.
- 36Brockow K, Kneissl D, Valentini L, et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol. 2015; 135(4): 977-984.e4.
- 37Ballmer-Weber BK, Beyer K. Food challenges. J Allergy Clin Immunol. 2018; 141.