New biological treatments for asthma and skin allergies
[Correction Statement: Correction added on 25 October 2019 after first online publication: Apostolos Bossios' affiliation was previously incorrect and has been corrected in this version.]
Abstract
Allergies are typically endemic, complex and heterogeneous diseases with a high impact at quality of life. Mechanistically, type 2 immune responses involving eosinophil and basophil granulocytes, mast cells and humoral factors such as IgE are key drivers of allergic diseases. Fighting allergic diseases knows three strategies: prevention, symptomatic and causative therapy. While remarkable progress was made in understanding molecular events in allergies as a prerequisite for effective prevention and desensitization, this review article focuses on the most efficient symptomatic treatments—that is using more and more specific antibodies neutralizing particular immune pathways. We highlight and classify recent and upcoming developments in the three prototype chronic allergic diseases allergic asthma, chronic spontaneous urticaria and atopic eczema. In all three examples, biologics such as dupilumab or omalizumab become reliable and efficient therapeutic options. Finally, we give an outlook how a diagnostic and therapeutic workflow might look like in the near future for these three major burdens of society.
1 INTRODUCTION
The development of biological treatments that specifically block the cytokines either directly or via their receptor offers a broad spectrum of new and efficient treatment options for inflammatory diseases. Effective application of these new treatments demands an in-depth knowledge of disease pathology. During the last decades, profound research delivered comprehensive insights into the pathomechanisms of asthma and skin allergies. However, personalized treatment regimens are still hampered by the high disease heterogeneity. Within this review, we will give an overview on how mediators of type 2 inflammation derived from T helper (Th) 2 cells, type 2 innate lymphoid cells (ILC2) and B cells drive the pathology of asthma, chronic spontaneous urticaria (CSU) and atopic eczema (AE). We will discuss disease biomarkers and attempts to define disease endotypes, and we will summarize biological treatment options, approved or in development, targeting type 2 but also nontype 2 inflammation.
2 ASTHMA
2.1 Current state of the art of definition and epidemiology
Asthma is a common chronic and heterogeneous condition affecting more than 300 million people worldwide,1 with a varying prevalence (ie from 21% in Australia to 0.2% in China).2, 3 Variation also exists between genders; in children, boys are most affected but that changes at puberty to a higher prevalence in women (around 20%).4
Asthma has a high social impact, mainly in low- and middle-income countries, where years of life lost due to asthma are increasing.2 The economic burden of asthma is estimated to exceed the combined burden of tuberculosis and HIV/AIDS.5
2.2 Pathogenesis
Inflammation represents a key feature of asthma pathogenesis, with a variety of host/environment interactions that are diverse in time and tissue,2 leading to its complexity and its heterogeneity. In fact, our current understanding is that asthma represents more syndrome than a single disease,6 and a great unmet medical need is a better understanding of asthma endotypes7 to assign the most appropriate therapy to each patient.
While there is a substantial number of nonallergic asthma endotypes, this review focuses at type 2 immune-mediated (allergic) asthma.8-14
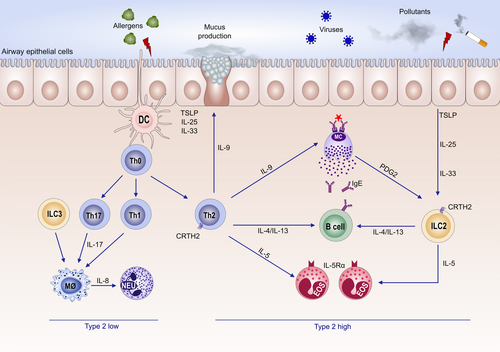
Type 2 is usually characterized by presence of serum immunoglobulin E (IgE) antibodies and/or a positive skin prick test to allergens. It is most frequently observed in children. In allergic individuals, the allergen uptake in the airways by dendritic cells drives expansion of Th2 cells that secrete pro-allergic cytokines such as interleukins (IL)-4, IL-5, IL-9 and IL-13. These cytokines are also produced by innate lymphoid cells type 2 (ILC2) cells15 that differentiate from progenitor cells in presence of so-called alarmins such as thymic stromal lymphopoietin (TSLP), IL-33 and IL-25.2 Both Th2 and ILC2 cells express the IL-33 receptor ST216 as well as the prostaglandin D2 receptor (DP2; also known as CRTH2). Furthermore, an impaired epithelial barrier induces further activation of ILC2 and Th2,17 leading to increased asthma severity.
IL-4 plays a key role in B cell isotype switching and IgE synthesis. IgE binds to its high-affinity receptor at mast cells and induces their activation and degranulation. Immediate release of preformed mediators such as histamine, tryptase and heparin, as well as de novo synthesis of several lipid mediators including prostaglandins and leukotrienes subsequently leads to bronchoconstriction.18 IL-5 is essential for maturation and survival of eosinophils.11, 19 IL-9 mediates mast cell and eosinophil accumulation. Eosinophilia correlates to airway remodelling and mucus production.11, 19-22 IL-13 plays an important role in airway bronchial hyper-reactivity, goblet-cell metaplasia and mucus production as well as in fibrosis23 (Figure 1).
2.3 Current indication for biologic therapy of asthma
Long-term treatment goals are to achieve symptom control and to minimize the risk of future exacerbation, fixed airflow limitation and side effects of treatment.24 A comprehensive approach includes nonpharmacological measures, that is avoidance of triggers and a stepwise approach (steps 1-5) with increasing doses of medications, primarily ICS, often in combination with a second controller, starting with a β2 agonist and eventually adding leukotriene receptor antagonists or theophylline (for adults) before the use of systemic corticosteroids.24 Inhalation technique control and assessments of comorbidities are also key factors in asthma treatment.
Around 5% of patients need escalation to step 5, the use of systemic steroids, and may even then remain uncontrolled which defines them as patients with severe asthma according to ERS/ATS criteria.25 For those patients, biologic therapy is indicated.26 Current targets for type 2 asthma are IgE (Omalizumab), IL-5 (mepolizumab and reslizumab), IL-5Ra (benralizumab) and IL-4Ra (dupilumab) (Table 1).
| Status and efficacy in | |||||
|---|---|---|---|---|---|
| Drug | Target | AE | Asthma | Urticaria | |
| Type 17 immunity | Ustekinumab |
IL-12p40 (IL-12 and IL-23) |
Off-label Not effective105 |
- | Off-label |
| Etanercept | TNF-α |
Off-label Contradictory/ not effective |
TNF-α inhibitors are no longer under development due to an increased rate of SAEs of golimumab in phase II |
Off-label Case reports81 |
|
| Adalimumab | TNF-α | - |
Off-label Case reports81 |
||
| Infliximab | TNF-α |
Off-label Potentially effective with TCS co-therapy104 |
- | ||
| Innate immunity | Anakinra | IL-1 | Off-label | - | Off-label |
| Tocilizumab | IL-6R |
Off-label Case reports106 |
- | off-label | |
| Bermekimab | IL-1a |
Phase II (NCT0349674) Dose-dependent effects |
|||
| Type 2 immunity | Dupilumab | IL-4Ra | approved | Approved | Phase II (NCT03749135) 80 |
| Mepolizumab | IL-5 |
Off-label No long-term studies available 107 |
Approved |
Off-label Case reports 82 |
|
| Reslizumab | IL-5 | - | ~50% improvement34, 36, 37 | - | |
| Benralizumab | IL-5Ra |
Phase II (NCT03563066) Recruiting |
~50% improvement39 | Conflicting results | |
| Omalizumab | IgE |
Off-label Conflicting results |
Approved | Approved | |
| Ligelizumab | IgE | - | - | Phase III (NCT03437278)74, 75 | |
| Quilizumab | IgE, membrane-bound | - | - | Failed primary end point77 | |
| Rituximab | CD20 |
Off-label Conflicting results |
- |
Off-label Case reports78 |
|
| Tralokinumab | IL-13 |
Phase II (NCT03526861) promising study results121 |
Discontinued | - | |
| Lebrikizumab | IL-13 |
Phase II (NCT03443024) studies without TCS use needed to evaluate efficacy as monotherapy120 |
Discontinued | - | |
| Tezepelumab | TSLP |
Phase II (NCT03809663) Studies without TCS use needed to evaluate efficacy as monotherapy |
Phase II Promising results Moved to phase III (NCT03347279)50 |
- | |
| ANB020 | IL-33 | Phase II (NCT03533751) | Phase II (NCT034699934) | - | |
| REGN3500 | IL-33 | Phase II (NCT03738423) | Phase I (NCT03112577) | - | |
| GSK3772847 | IL-33R | - | Phase II (NCT03207243) | - | |
| Fevipiprant | CRTh2 antagonist | Phase II (NCT017856029 | Phase III (NCT03215758) | - | |
| Others | Nemolizumab | IL-31a |
Phase II (NCT03100344) |
- | - |
| Fezakinumab | IL-22 |
Phase II (NCT01941537) Effective in a subset of patients129 |
- | - | |
| KHK4083 and KY1005 | OX40 |
Phase II (NCT03703102, NCT03754309) Recruiting |
- | - | |
| AK002 | Siglec-8 | - |
Phase II (NCT03436797) Active |
||
Omalizumab, a humanized, monoclonal antibody (mAb) directed against IgE, was the first biologic-based therapy, available in clinical settings in the early 2000s. It is licensed for moderate to severe allergic asthma in patients’ ≥6 years old with IgE higher than 30 IU/L. Omalizumab prevents IgE from binding to its high-affinity receptor (FcεRI), which is present on mast cells and basophils, blocking their allergic response. It also downregulates the expression of high-affinity IgE receptors on mast cells.27 Several randomized control trials (RCTs) and real-life studies28, 29 have shown that Omalizumab reduces asthma exacerbation (by about 25%) and hospital admissions in both children and adults.27 Omalizumab reduces also virus-associated exacerbations,30 possibly by increasing anti-viral response, IFN-α, from dendritic cells.31 Omalizumab is well tolerated, with a low risk (0.1-0.2) of anaphylaxis.32
Mepolizumab and reslizumab are both mAb that bind to IL-5, preventing it from binding to its receptor.33 They are licensed for patients with severe asthma and high blood eosinophils (≥ 150 cells/µL for mepolizumab, ≥ 400 cells/µL for reslizumab).
Mepolizumab has been shown to reduce asthma exacerbation by about 50%, with a small improvement in lung function (FEV1 increase in 110 mL) and QoL.34 In patients with OCS-dependent asthma, mepolizumab reduces its dosage by 50% in parallel with a reduction of exacerbation and with no loss of asthma control.28, 35 Mepolizumab has a safety profile similar to a placebo.
Reslizumab reduces asthma exacerbations similar to mepolizumab and improves FEV1 within 4 weeks when blood eosinophils are ≥400 cells/µL; it also results in an improved QoL.34, 36 Reslizumab is the only intravenous mAb, and its dose is weight based. Reslizumab is well tolerated, with adverse effects similar to a placebo, although three cases of anaphylaxis have been reported.37
Benralizumab is directed against IL-5Ra. Due to its afucosylation, benralizumab interacts with the FcγRIIIa receptor in natural killer (NK) cells to induce an antibody-dependent, cell-mediated cytotoxicity (ADCC), resulting in rapid depletion of eosinophils.38 It reduces significant asthma exacerbations at a level similar to other anti-IL-5 biologics, especially in blood eosinophils ≥300 cells/µL. Benralizumab has also an oral steroids-sparing effect together with exacerbation reduction by 70%.39 Benralizumab is well tolerated, but hypersensitivity reactions have been detected, including anaphylaxis, angioedema and urticaria.
Dupilumab targets the IL-4a receptor and blocks the signalling of both IL-4 and IL-13. It has been tested in moderate to severe asthmatics, reducing asthma exacerbations by approximately 50% and significantly improving lung function (FEV1) within 2 weeks in patients with elevated type 2 biomarkers (blood eosinophils ≥ 150 cells/µL and FeNO ≥ 25).40 In patients with steroid-dependent asthma, dupilumab reduced OCS use by 70%, accompanied by a 60% reduction in exacerbation and improved lung function.41 Dupilumab has a favourable safety profile, with side effects of injection site reaction and transient blood eosinophilia.
The main outcome of RCTs was reducing asthma exacerbations, and/or oral-steroid sparing effects as well as number of hospital admissions. In contrast, other important clinical outcomes such as lung function are not as conclusive. Omalizumab has shown minimal or equivocal improvement in lung function.42 In the anti-IL-5/5Ra antibody family, a recent Cochrane review found a small but significant improvement in mean pre-bronchodilator FEV1 of between 0.08 and 0.11/L.34 A dupilumab phase III study has shown an increase of FEV1 up to 0.32/L at 12 weeks.40 These studies highlight that future studies are needed to evaluate the effect of biologics in lung function decline.
All above-mentioned biologics have a good safety profile. However, as they all interfere with the immune system and patients would usually receive them for a long period of time, physicians need to be aware for any potential long-term immunomodulatory effects. Among biologics, best long-term evidence exists for anti-IgE treatment without any concerns until now. No long-term data exist for anti-IL-5/5Ra and for anti-IL-4Ra, yet. Here, biologic function of these targets should be kept in mind, eosinophils, for example, are considered diverse cells33 that do not only function as effector cells but are also involved in tissue homeostasis and, therefore, have a much broader role in allergic inflammation and helminth infections than assumed so far.33, 43
2.4 Selecting the biologic for severe uncontrolled asthma
Choosing the most appropriate biologic treatment is challenging.7, 42, 44 Confirming asthma severity, re-establishment of asthma diagnosis, comorbidities and patient adherence is essential before initiating a biologic therapy..
With the available biologics, the choice has to be made between anti-IgE, anti-IL-5/5Ra and anti-IL-4Ra. As there are no RCT studies directly comparing those biologics, the patient phenotype and endotype has to be assessed as best as possible to achieve the expected efficacy and safety of the treatment. Thus, the first step is to define the occurrence of type 2 or nontype 2 asthma and subsequently to characterize the underlying sub-endotype; allergic-predominant, eosinophilic-predominant or AHR (smooth muscle contraction and hyperresponsiveness) and mucus predominant.
In patients with an allergic-predominant phenotype; that is early-onset asthma, history of allergies and/or clinically significant SPT/RAST, IgE > 100 IU/mL, co-existence of allergic rhinitis, moderate high FeNO (ie up to 50 ppd) and low number of blood eosinophils (<300 cells/µL), omalizumab could be considered as the first choice due to its proven efficacy and safety. In patients with eosinophil-predominant asthma, that is late-onset asthma, no history of allergy or clinical significant SPT/RAST and normal IgE and high blood eosinophils ≥300/µL an anti-IL-5/5Ra should be the first choice.
In patients with characteristics from both sub-endotypes showing an allergic/eosinophilic overlap, either anti-IgE or an anti-IL-5/5Ra could be a possible choice. Anti-IgE has been shown efficient even in patients with blood eosinophils ≥ 300/µL at 16 weeks45 and has a documented long time safety profile, even during pregnancy46 There is a documented strategy for evaluating the effectiveness of anti-IgE therapy after 16 weeks, while responsive data for an anti-IL-5/5Ra treatment are still lacking.47 As anti-IL-5 treatment can be effective in patients that have been previously treated with anti-IgE, evaluation of therapeutic efficacy of anti-IgE after 16 weeks seems to be reasonable to decide if the patient should continue or switch to anti-IL-5/IL-5Ra.48 However, studies evaluating switching from anti-IgE or anti-IL-5/5Ra to anti-IL-4Ra are not available yet.
High blood eosinophils and a history of exacerbations predict an enhanced response to all three anti-IL-5 mAbs, which all show a similar reduction in asthma exacerbations. Thus, decision for therapy is made according to blood eosinophil levels, co-existence of nasal polyps49 and weight as predictors for treatment success.
Patients with broader clinical signs and symptoms which could be ascribed to IL-4 and IL-13 (goblet-cell hyperplasia, mucus secretion, smooth muscle contraction and hyperresponsiveness together with eosinophil recruitment) could especially benefit from dupilumab therapy.40, 42
2.5 Future targeted treatment
There is an increased interest in developing future targeted therapies, mainly for type 2 inflammation. Focus has been given to alarmins. Even if those epithelial-cell-derived cytokines can be induced by several stimuli, including environmental and microbial triggers, their key role in inducing Th2 and ILC2 cells has rendered them promising targets for the treatment of type 2 inflammation. Tezepelumab targeting TSLP decreased asthma exacerbations in patients with moderate asthma unrelated to blood eosinophils and FeNO.50 A treatment targeting IL-33, either directly (IL-33) or via its receptor (anti-ST2), is also in clinical development.
An interesting novel approach is to optimize airway delivery of mAbs. Currently, a nebulized biologic therapy approach targeting IL-13 is under development in animal models.51 Promising data have also emerged in the fields of small-molecule antagonists. Fevipiprant, a prostaglandin D2 (PGD2) type 2 receptor antagonist, has been shown to reduce eosinophilic inflammation52 and smooth muscle mass in moderate to severe asthmatics.53
Development of biomarkers to identify suitable patients and predict and monitor their response to biologics54 is crucial for the future.
3 DEFINITION OF SKIN ALLERGIES
Biologics are highly efficient and cost-intense therapies. Thus, they are generally only justified in severe and chronic diseases. Concerning skin diseases, occasionally self-limited skin allergies are treated with biologics. Namely, severe cases of drug-induced exanthema such as toxic epidermal necrolysis might be treated with a single injection of anti-TNF-α as early at onset as possible.55 However, allergic skin rashes such as allergic contact dermatitis (ACD) or drug exanthema are self-limited as soon as the trigger is removed and are usually not treated with biologics. Thus, skin allergies are defined here as chronic inflammatory conditions that are mediated by and/or associated with immediate and/or cytotoxic hypersensitivity reactions.
4 CHRONIC SPONTANEOUS URTICARIA
Chronic spontaneous urticaria (CSU) is a common disease with a prevalence of up to 1%.56 CSU is characterized by the recurrent spontaneous appearance of itchy wheals, angioedema or both for more than 6 weeks.57 Patients affected by CSU are often dramatically impaired in their quality of life, which is why consequent implementation of current treatment guidelines as well as development of new and better therapies is necessary.
4.1 Pathogenesis
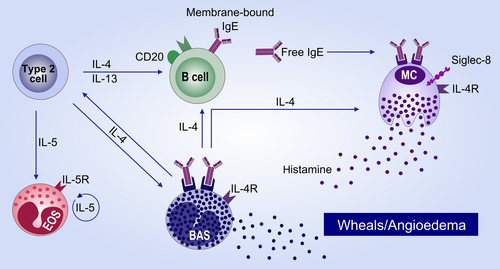
Signs and symptoms of urticaria are mainly caused by the activation of mast cells (MC) and the subsequent release of histamine. The exact mechanisms leading to activation of MC in chronic urticaria patients are, as of yet, not fully characterized. There is, however, strong indication that autoimmunity, either ‘autoallergic’ (type I, with IgE antibodies to local autoallergens) or ‘autoimmune’ (type IIb,with IgG autoantibodies to IgE or its receptor),58 is the most frequent cause of CSU59 (Figure 2). While in patients with autoallergic or autoimmune CSU, the respective autoantibodies are required for MC degranulation, and there are many co-factors that can be involved in modulating the activation status of MC, for example pseudoallergens, neuropeptides or bacterial products. Furthermore, in addition to MC, eosinophils, basophils and neutrophils are thought to contribute to the pathogenesis of CSU by migrating from the circulation into the skin at sites of MC degranulation, resulting in blood basopenia and cellular skin infiltration.60, 61 It is, as of yet, unclear how this mild leucocytic infiltrate contributes to CSU pathogenesis. Possibly, the inflammatory environment also modulates the activation threshold of MC.
4.2 Guideline-recommended treatment algorithm
An effective treatment for CSU patients should always aim for complete control of symptoms. To achieve this, urticarial symptoms and the burden of the patients need to be assessed continuously before and during treatment. To do so, validated scores and questionnaires are recommended, for example the Urticaria Activity Score (UAS), the Chronic Urticaria Questionnaire for the Quality of Life (CU-Q2oL) and the Urticaria Control Test (UCT).57 If CSU is not sufficiently controlled, for example if the patient has a UCT score of <12, treatment escalation should be performed as recommended by the current guidelines.57 The standard treatment of CSU is second-generation antihistamines in standard, that is once daily, dosing. In case of inadequate control, antihistamines dosing should be increased after 2-4 weeks or earlier, if symptoms are intolerable, to up to four times the standard dose. However, many patients still suffer from urticaria despite proper antihistamine treatment. In these patients, the guideline recommends as next step the addition of omalizumab. Omalizumab is currently the only licensed drug for the treatment of patients who are not controlled by a standard dosed antihistamine. For those patients who also fail to respond to omalizumab, the current guidelines recommend cyclosporine treatment after 6 months of omalizumab treatment weeks or earlier, if symptoms are intolerable.57
4.3 Proposed mechanism of action of Omalizumab
In autoallergic CSU, IgE against autoantigens is thought to be the relevant factor responsible for MC activation and thus for the elicitation of urticarial symptoms. Different groups have recently identified functional 'that is MC degranulating’ IgE against autoantigens such as thyroid peroxidase62 or IL-2463 and in a large proportion of CSU patients higher than normal levels of IgE are detected,64 with the majority of the total IgE being autoreactive.65 Furthermore, specific and functional IgE against staphylococcal enterotoxins has been identified in many CSU patients.66 In those patients where IgE is responsible for the degranulation of MC, the elimination of IgE by anti-IgE antibodies will result in cessation of symptoms, typically within the first days or weeks after the first injection.
There are, however, CSU patients who poorly respond to omalizumab or who show a late onset of symptom improvement, that is within months. These patients typically have low levels of total IgE, low basophil FcεRI expression and are positive in the basophil activation tests.67-69 In these patients, the effects of omalizumab are thought to be mediated via the downregulation of FcεRI on skin MC, which has been shown to occur within 3 months after start of Omalizumab treatment.61
While the above described mechanisms of action are likely to be the most relevant in most CSU patients, there may be subgroups of patients in which other mechanisms are relevant and where omalizumab is effective via different actions. For example, other proposed mechanisms of action of omalizumab include the ability of omalizumab to change mast cell releasability and to affect the coagulation cascade.70 Further ongoing research is aimed at fully characterizing all potential mechanisms of action of anti-IgE efficacy in CSU.
4.4 Biologics under investigation
In 2014, omalizumab has been licensed for the treatment of patients with antihistamine-refractory CSU. Since then, additional randomized controlled trials with omalizumab have been conducted in three forms of inducible urticaria, cholinergic urticaria,71 cold urticaria72 and symptomatic dermographism,73 all showing the potential of an effective anti-IgE treatment in inducible urticaria.
In CSU, first results of a randomized, double-blind, placebo- and comparator-controlled phase 2b trial with ligelizumab have been presented at the EAACI 2018. Ligelizumab is a humanized monoclonal IgG1 antibody that binds, similar to Omalizumab, to the Cε3 domain of IgE. The in vitro affinity of ligelizumab is about 50-fold higher than that of omalizumab, and allergen skin prick tests have shown much higher potency of ligelizumab in vivo as compared to Omalizumab.74, 75 In this study, more patients treated with ligelizumab 72 and 240mg achieved complete control of CSU symptoms as compared with to patients treated with omalizumab and placebo.76 Based on these positive results, there are ongoing phase 3 trials investigating the efficacy and safety of ligelizumab in CSU patients refractory to antihistamine treatment.
Based on the hypothesis that autoreactive antibodies are responsible for symptoms in CSU, a depletion of antibody-producing B cells could be beneficial in CSU patients. Quilizumab, a humanized monoclonal antibody that targets the M1 prime segment of membrane expressed IgE, has been investigated in a randomized, placebo-controlled phase 2 trial in CSU. The proposed mechanism of quilizumab is the specific reduction of IgE levels by causing the depletion of IgE-switched B cells and plasmablasts. The study, however, failed to reach the primary endpoint in comparison to placebo. This was most likely due to an only moderate reduction of IgE by ~30% until week 20.77
Rituximab, a chimeric monoclonal anti-CD20 antibody, depletes memory B cells that are necessary for autoantibody production. Overall, five individual case reports have been published, four of which have shown efficacy with a sustained response.78 So far, there is no published controlled trial on the efficacy of rituximab in CSU, and a trial registered on clinicaltrials.gov (NCT00216762) has been halted by the FDA due to safety concerns.
4.5 Future developments
There are currently two ongoing clinical trials with biologics assessing the proof of concept for the use in CSU. In a first pilot study, the efficacy of AK002, a humanized monoclonal antibody directed against Siglec-8, is assessed in patients with antihistamine-resistant CSU (NCT03436797). Siglec-8 is expressed by eosinophils and mast cells and activation of Siglec-8 is thought to induce inhibition or depletion of these cells, which would make it ideally suited for the treatment MC-related diseases such as CSU.79 As of yet, there are no published results of the trial available. In another multi-centre, randomized, placebo-controlled trial, dupilumab, a monoclonal anti-IL-4Rα antibody, is assessed for its efficacy and safety in patients with CSU (NCT03749135). While the trial is ongoing and results are not expected in the near future, a recently published case series of treatment-refractory CSU patients has shown efficacy of Dupilumab in six patients.80
Anti-TNF antibodies are widely used in dermatology, both in in-label indications such as psoriasis as well as in off-label indications. Regarding the efficacy of TNF-a antibodies in the treatment of CSU, there is only limited information available. A case series that retrospectively analysed 25 patients with CSU treated with etanercept or adalimumab reported a beneficial response in 15 (60%) of the patients.81
Similar to TNF, the potential pathogenetic mechanisms involving IL-5 in CSU are currently unclear. There are, however, two single case reports showing that anti-IL-5 treatment using mepolizumab82 or reslizumab83 can be beneficial in CSU. According to clinicaltrials.gov, a single-blind, nonrandomized trial is currently performed to assess the efficacy of Benralizumab in CSU (NCT03183024).
5 ATOPIC ECZEMA
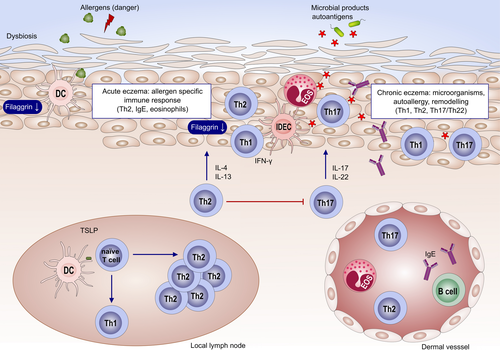
Atopic eczema (AE) or atopic dermatitis is the disease with the highest burden of all skin conditions throughout life.84 In fact, AE impacts the quality of life to a similar degree as epilepsy or diabetes in children or cancer in adults.85, 86 AE is very common, reaching a prevalence of up to 30% of all children and 3% of adults in the Western population.86 Its complex pathogenesis involves a genetic predisposition and environmental factors87 and leads to the triade of dry skin, itch and cutaneous inflammation88 (Figure 3).
5.1 Are allergies relevant for the pathogenesis of AE?
AE might develop independent of skin allergies and be mediated by nontype 2 inflammation,89 (Tables 2-4) but in 80% of the cases specific sensitizations to aeroallergens or food are identified. Especially in children, food allergens might be the major trigger of AE,90 while in later life usually sensitizations to aeroallergens such as birch (bet v 1) are common. These sensitizations might then cause cross-reactivity to food, for example apples and other fruits.91 However, the relevance of allergies for AE is not entirely clarified. Of particular importance, here is the role for specific immunotherapy. While some studies suggest a positive effect for AE, there is conflicting evidence whether desensitization might influence AE in a positive way.92 Ongoing and future efforts will need to determine which subgroups or endotypes of AE might benefit best.93, 94 Also in case of allergic contact dermatitis (ACD), evidence is conflictive regarding the impact on the course of AE.95 Depending on the eliciting hapten, ACD reactions might even be less frequent and attenuated in AD patients.96 This inconsistency is probably related to the fact that haptens drive distinct immune responses97 that might reinforce the type 2 immunity of AE or not—the first is the case for fragrances, the latter for Th1/Th17 skewing haptens such as nickel, imiquimod98 or DNCB. In line with this, AE patients were reported to generally develop a Th2-skewed ACD reaction.99 Thus, reactions to nickel might be less frequent or attenuated as compared to the general population, while ACD to fragrances might be more frequent in AE. Finally, the atopy patch test (APT) identifies AE patients that develop eczematous lesions to aeroallergens.100 Confirming the relevance for the APT, a subgroup of AE patients has been shown to react with skin exacerbation upon pollen challenge.101 Thus, skin allergies are relevant at least in a subgroup of AE.
5.2 Current biologic therapy of AE
European guidelines for the treatment of AE recommend a stepwise approach94, 100: avoiding triggers and basic treatment of the barrier is recommended in all stages of the disease. In moderate forms, AE should be treated early and hard with topical steroids and in remission with a pro-active therapy; severe forms might be treated with cyclosporine, methotrexate, azathioprine or mycophenolate mofetil. However, all these therapies are of limited effectiveness and have long-term side effects. Thus, identifying specific and effective biologics for the treatment of AE was and still is a great unmet medical need. As this review article focuses on biologics, promising small molecules such as JAK inhibitors102 will not be discussed. Studies investigating biologics in AE treatment follow three general strategies (Table 1): adapting biologics approved for other skin diseases such as psoriasis for AE, most of them targeting nontype 2 pathways such as type 3 (Th17) immunity; biologics dampening acute phase reactions, for example IL-6 or IL-1b; and finally, biologics neutralizing type 2 (Th2) immunity.
In line with the classification of inflammatory skin diseases according to their immune response patterns,103 biologics highly efficient for psoriasis (type 3 according to103) fail to proof efficacy in AE (type 2a according to103). TNF inhibitors have been investigated in several case series with no convincing overall efficacy.104 Investigation of ustekinumab in a placebo-controlled trial resulted in SCORAD50 response at 16 weeks in 31% of the patients receiving serum as opposed to 19% in the placebo group.105 Due to the cross-over design, long-term effects could hardly be assigned to ustekinumab. Thus, it cannot be excluded there is a subset if AE that might benefit from these substances, but overall psoriasis biologics are not suitable for treating AE.
Biologics neutralizing acute phase substances such as IL-6 have also been investigated in the past for AE. However, there is very limited evidence, for example a case series of three patients treated with tocilizumab with good response, but development of side effects.106 In summary, there is the trend to modify innate and acute phase responses in AE; however, this trend is currently proceeded rather by investigating small molecules than antibodies.
| Key publications establish asthma heterogeneity phenotypes and endotypes |
|
| Establishment of global guidelines in asthma treatment |
| Defining asthma severity |
| Key phase III trials of biologicals in asthma |
| Key publications in Urticaria pathogenesis |
| Establishment of guidelines for definition, classification, diagnosis and management of Urticaria, current version (2018)57 |
| Key developments in diagnosis of atopic eczema |
|
| Key phase III trials of biologicals in CSU |
|
| Key publications in atopic eczema pathogenesis |
|
|
| Key developments in diagnosis of atopic eczema |
|
|
| Establishment of guidelines for atopic eczema diagnosis and treatment, current version (2018)94, 100 |
| Key phase III trials of biologicals in AE |
|
Finally, a major breakthrough in treating AE was achieved by neutralizing type 2 immunity. An early small study investigated the IL-5 antibody mepolizumab. Here, 4 out of 20 patients showed a PGA reduction, but there was no significant difference between the active drug and placebo groups at 14 days regarding SCORAD or CCL17 serum levels.107 The study was underpowered and too short, but still leaves room for speculations that a subgroup of AE patients might respond to neutralizing IL-5. Similarly, conflicting and way too few evidence exists regarding humoral factors of type 2 immunity as targets for AE treatment. A case series of AE patients treated with rituximab reported a good outcome in all 6 investigated patients after 24 weeks, with a mean reduction on EASI from 29 to 8108 or in severe childhood AE109; however, there are also negative reports.110, 111 More evidence exists regarding omalizumab, where the initial study in 21 patients with co-existing asthma and AE reported a SCORAD50 response in all 21 patients112; follow-up studies showed a more heterogeneous picture, with a responder rate of 5%-30% of AE patients.113-116 The response to omalizumab was independent of circulating IgE levels; thus, biomarkers guiding therapeutic decision for rituximab or omalizumab are amiss.
The first breakthrough in AE therapy was achieved by the IL-4 receptor alpha antibody dupilumab. As a consequence of several phase III studies showing an EASI75 response in >50% as monotherapy117 and >65% in combination with topical steroids,118 dupilumab was approved for moderate to severe AE in the US and Europe in 2017. Dupilumab also efficiently reduces pruritus and improves quality of life. Its safety profile is very high, with the exception of conjunctivitis that occurs in roughly 10% of AE patients and that requires special attention in this population.119
5.3 Future developments: focussing on type 2 immunity and epithelial cytokines
Besides dupilumab, there are two more biologics interfering with the type 2 cytokine IL-13, namely lebrikizumab and tralokinumab (Figure 4). In a phase II study with 209 patients assessing lebrikizumab that allowed concomitant topical steroids, 82% achieved an EASI50 response with 62% placebo responders.120 Tralokinumab showed good efficacy in phase II, with a dose-dependent mean EASI improvement by 15 points.121 Neutralizing the type 2 cytokine IL-31 that is a central mediator of itch markedly reduced pruritus in two phase II studies, but had only moderate effects at EASI scores.122, 123
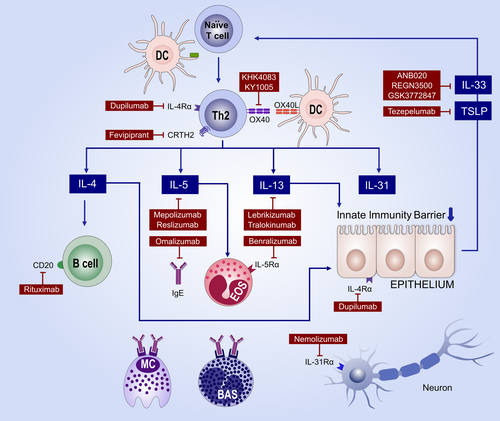
Targeting epithelial cytokines such as IL-17C and fezakinumab (IL-22 antibody) or IL-22R are at early stages of development. There is clear evidence that AE is a heterogeneous disease, probably comprising several endotypes.124 Comparisons of childhood versus adult AE or European versus Asian AE endotypes125 give evidence that the classification of AE is not precise enough for the currently available highly specific biologics. Molecular classifiers are at the step of clinical validation,126-128 but reliable biomarkers predicting clinical outcome of a therapy are very scarce. One recently suggested biomarker is the cutaneous level of IL-22 that predicts clinical response to fezakinumab, an antibody neutralizing IL-22 with an overall moderate efficacy.129 Thus, endotypes and biomarkers are prerequisites for the next breakthrough in AE therapy.89
6 SUMMARY AND OUTLOOK: A DIAGNOSTIC AND THERAPEUTIC WORKFLOW FOR ALLERGIC DISEASES
The pathogenesis of chronic inflammatory diseases usually involves the interaction of lymphocytes and epithelial cells. Depending on the dominating subtype of these lymphocytes and the epithelial immune response pattern, inflammatory skin diseases can be grouped.AE is assigned to type 2 immunity mediated via IL-4, IL-5, IL-9 and IL-13 that collectively induce an impaired epidermal barrier and insufficient innate immune response.103 Consequently, the most promising biologics to treat allergic asthma and skin allergies—either already licensed or in development—neutralize the type 2 immunity (Figure 4). However, asthma and skin allergy patients show a heterogeneous degree of response and there are a substantial number of nonresponders in all available or foreseen biologics.
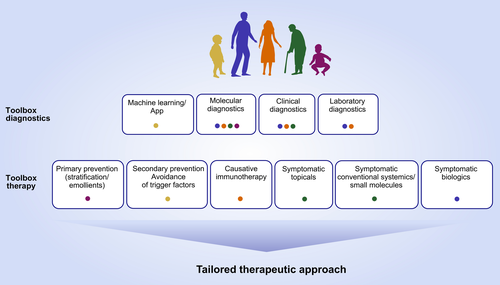
Remarkable advances defined endotypes of asthma7 and urticaria57 according to their pathogenesis as well as therapeutic response; in AE, initial progress has been made in understanding how distinct clinical entities and species might be linked to molecular events.89 The ideal future treatment algorithm of asthma and skin allergies needs to take into account these endotypes and would involve prevention, symptomatic and causative therapies (Figure 5). To achieve this aim, molecular diagnostics needs to improve. Currently, the greatest obstacle on the way to precision medicine in the field is the gap between advances in understanding pathogenesis and availability of specific therapies at the one hand side and missing predictive biomarkers and precise diagnostics at the other side.
ACKNOWLEDGMENTS
This study was funded by the German Research Foundation (EY97/3-2), Swedish Heart Lung Foundation (20180219), and the European Research Council (IMCIS 676858).
CONFLICTS OF INTEREST
Dr S. Eyerich has no conflicts to report; Dr Bossios reports personal fees (advisory and/or lecture honorarium) from TEVA, personal fees (advisory and/or lecture honorarium) from AZ, personal fees (advisory and/or lecture honorarium) from GSK, personal fees (advisory and/or lecture honorarium) from Novartis, outside the submitted work; Dr Metz Dr Metz reports personal fees from Bayer, personal fees from GlaxoSmithKline, personal fees from Beiersdorf, personal fees from Celgene, personal fees from Jenapharm, personal fees from Moxie GmbH, personal fees from Menlo Therapeutics, personal fees from Merz, personal fees from NeRRe, personal fees from Novartis, personal fees from Pierre Fabre, personal fees from Roche, personal fees from Sanofi, personal fees from Shire, outside the submitted work; Dr K. Eyerich reports personal fees from Abbvie, personal fees from Berlin Chemie, grants from Celgene, personal fees from Novartis, personal fees from Lilly, grants from Galapagos, grants and personal fees from Janssen, personal fees from Sanofi, outside the submitted work.




