House dust mite drives proinflammatory eicosanoid reprogramming and macrophage effector functions
Funding information
This study was supported by the German Research Foundation (DFG) (grants ES 471/2-1 and ES 471/3-1) and by the Else Kröner-Fresenius-Stiftung (grant 2015_A195). CSW receives grant support by the German Center for Lung Research (DZL; 82DZL00302). MH was supported by Innovative Medicines Initiative Joint Undertaking under Grant agreement number 115317 (DIRECT): “The work leading to this publication has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no 115317 (DIRECT), resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution.”
Abstract
Background
Eicosanoid lipid mediators play key roles in type 2 immune responses, for example in allergy and asthma. Macrophages represent major producers of eicosanoids and they are key effector cells of type 2 immunity. We aimed to comprehensively track eicosanoid profiles during type 2 immune responses to house dust mite (HDM) or helminth infection and to identify mechanisms and functions of eicosanoid reprogramming in human macrophages.
Methods
We established an LC-MS/MS workflow for the quantification of 52 oxylipins to analyze mediator profiles in human monocyte-derived macrophages (MDM) stimulated with HDM and during allergic airway inflammation (AAI) or nematode infection in mice. Expression of eicosanoid enzymes was studied by qPCR and western blot and cytokine production was assessed by multiplex assays.
Results
Short (24 h) exposure of alveolar-like MDM (aMDM) to HDM suppressed 5-LOX expression and product formation, while triggering prostanoid (thromboxane and prostaglandin D2 and E2) production. This eicosanoid reprogramming was p38-dependent, but dectin-2-independent. HDM also induced proinflammatory cytokine production, but reduced granulocyte recruitment by aMDM. In contrast, high levels of cysteinyl leukotrienes (cysLTs) and 12-/15-LOX metabolites were produced in the airways during AAI or nematode infection in mice.
Conclusion
Our findings show that a short exposure to allergens as well as ongoing type 2 immune responses are characterized by a fundamental reprogramming of the lipid mediator metabolism with macrophages representing particularly plastic responder cells. Targeting mediator reprogramming in airway macrophages may represent a viable approach to prevent pathogenic lipid mediator profiles in allergy or asthma.
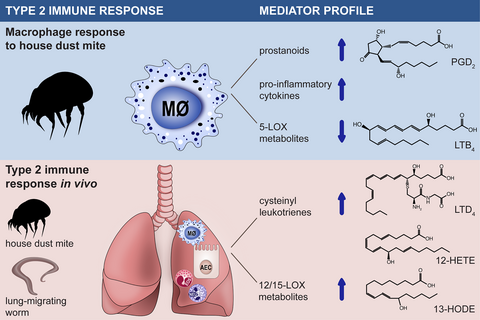
Graphical Abstract
House dust mite triggers the production of cyclooxygenase metabolites (prostaglandin D2, prostaglandin E2 and thromboxane) as well as of proinflammatory cytokines in human macrophages. House dust mite-exposed macrophages produce low levels of 5-lipoxygenase metabolites (e.g. leukotriene B4) and suppress neutrophil recruitment.
High levels of cysteinyl leukotrienes and 12-/15-lipoxygenase metabolites are produced during the type 2 immune response to house dust mite or nematode parasites in the airways.
AEC: Airway epithelial cell; COX: Cyclooxygenase; cysLT: Cysteinyl leukotriene; HDM: House dust mite; HETE: Hydroxyeicosatetraenoic acid; HODE: Hydroxyoctadecadienoic acid; LOX: Lipoxygenase
CONFLICTS OF INTEREST
CSW received grant support from Allergopharma, PLS Design as well as Zeller AG and received speaker honoraria from Allergopharma. All authors have no conflict of interest in relation to this work.