MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation
Edited by: Hans-Uwe Simon
Abstract
Several unmet needs have been identified in allergic rhinitis: identification of the time of onset of the pollen season, optimal control of rhinitis and comorbidities, patient stratification, multidisciplinary team for integrated care pathways, innovation in clinical trials and, above all, patient empowerment. MASK-rhinitis (MACVIA-ARIA Sentinel NetworK for allergic rhinitis) is a simple system centred around the patient which was devised to fill many of these gaps using Information and Communications Technology (ICT) tools and a clinical decision support system (CDSS) based on the most widely used guideline in allergic rhinitis and its asthma comorbidity (ARIA 2015 revision). It is one of the implementation systems of Action Plan B3 of the European Innovation Partnership on Active and Healthy Ageing (EIP on AHA). Three tools are used for the electronic monitoring of allergic diseases: a cell phone-based daily visual analogue scale (VAS) assessment of disease control, CARAT (Control of Allergic Rhinitis and Asthma Test) and e-Allergy screening (premedical system of early diagnosis of allergy and asthma based on online tools). These tools are combined with a clinical decision support system (CDSS) and are available in many languages. An e-CRF and an e-learning tool complete MASK. MASK is flexible and other tools can be added. It appears to be an advanced, global and integrated ICT answer for many unmet needs in allergic diseases which will improve policies and standards.
Abbreviations
-
- AHA
-
- Active and Healthy Ageing
-
- AIRWAYS ICPs
-
- Integrated Care Pathways for Airway diseases
-
- AR
-
- Allergic rhinitis
-
- ARIA
-
- Allergic Rhinitis and its Impact on Asthma
-
- CARAT
-
- Control of Allergic Rhinitis and Asthma Test
-
- CDSS
-
- Clinical decision support system
-
- EIP
-
- European Innovation Partnership
-
- ICP
-
- Integrated care pathway
-
- ICT
-
- Information and communications technology
-
- MACVIA-LR
-
- Contre les MAladies Chroniques pour un VIeillissement Actif en Languedoc-Roussillon
-
- MASK
-
- MACVIA-ARIA Sentinel NetworK
-
- MeDALL
-
- Mechanisms of the Development of Allergy (FP7)
-
- QOL
-
- Quality of life
-
- RAPP
-
- RhinAsthma Patient Perspective
-
- RCT
-
- Randomized control trial
-
- RQLQ
-
- Rhinoconjunctivitis Quality of Life Questionnaire
-
- SCUAD
-
- Severe Chronic Upper Airway Disease
-
- U-BIOPRED
-
- (IMI)
-
- VAS
-
- Visual analogue scale
Allergic rhinitis (AR) is among the most common diseases globally 1 and ranks first in Europe (over 25% of the European population). It exists in all age groups, and it often starts early in life 2 and persists across the life cycle 3, 4. The burden and costs are substantial 5. It often impairs social life, work and school performance 6-8 and has a major impact on healthy ageing 9.
Several unmet needs have been identified. MASK-rhinitis is a simple system centred around the patient. It has been devised to fill many of the gaps using Information and Communications Technology (ICT) tools and a clinical decision support system (CDSS) based on the most widely used guideline in AR (ARIA) 10. It is a product of the European Innovation Partnership on Active and Healthy Ageing 11 and is currently being launched in 15 countries. Patient empowerment is essential to the project. MASK-rhinitis represents a novel tool to diagnose, stratify and manage patients with AR and to assess treatment efficacy. It has the potential to have major impact on health policies and planning. In the future, the combination with biomarkers will further improve the impact of MASK-rhinitis.
MACVIA-LR (Fighting chronic diseases for active and healthy ageing, http://macvia.cr-languedocroussillon.fr) is a reference site of the European Innovation Partnership on Active and Healthy Ageing 12. It initiated the project AIRWAYS ICPs, an integrated care pathway (ICP) for airway diseases 13.
Unmet needs in allergic rhinitis
Early diagnosis and management of patients with respiratory allergic diseases
Although AR is common in all age groups, it is very often overlooked and under-diagnosed, especially in preschool children and the elderly. The Polish Presidency of the EU Council (2011) targeted chronic respiratory diseases in children to promote their early recognition, prevention and management and, ultimately, to promote AHA 9.
Clinical diagnosis is difficult, and symptoms may relate to allergic and nonallergic rhinitis as well as rhinosinusitis 14. There is a need for a simple diagnostic tool.
Patient stratification
The treatment of AR is now well established. Although the majority of patients present with controlled symptoms during pharmacologic treatment, 10–20% are still uncontrolled and should be characterized as suffering from severe chronic upper airway disease (SCUAD) 15. Patients with SCUAD have impaired quality of life, sleep, school and/or work performance 16, 17.
Many AR patients are over 65 years of age. The presentation of the disease, as well as the efficacy and safety of treatments, may differ in older adults. However, data are not yet available from RCTs.
Time of onset of the allergy season
For patients allergic to pollen, knowledge of the onset of the pollen season is of vital importance in order to start treatment as early as possible for the control of symptoms. When travelling, patients are often concerned about potential symptoms and/or bothered by symptoms outside their usual symptom ‘window’. It is therefore of importance to forecast the onset of the pollen season and to characterize seasons in different places.
Pollen counts are currently proposed to assess the exposure of pollen-allergic patients. However, counts often correlate imperfectly with symptoms 18-22 as (i) they do not represent allergen exposure alone 19, 23, 24, (ii) the number of pollen grains needed to elicit symptoms is not well defined and differs depending on the pollen species, (iii) there is a nonlinear relationship between pollen and allergic symptoms 25, 26 and (iv) interactions between pollens and atmospheric conditions or air pollution may exist 27, 28. Furthermore, for large geographical areas, pollen samplers are sparsely located. Patients may live at a distance from the sampler, and the levels of allergens in their environment may differ quite extensively from the levels detected by the sampler. Individualized pollen counts would be preferable 29 but are not feasible on a large scale. Finally, pollen counts are only available several days after onset of the season.
The assessment of allergen content in the air is feasible using antibody-based methods 18, 19, 30 or the biomolecular identification of pollen genomes 31. However, sophisticated methods are required which may not account for all of the pollen species in the ambient air, and individual measurements are not feasible.
Meteorological data may, in the future, be of interest to predict the onset of the season, but more information is needed 32-35. Combining several data sources using advanced data engineering may offer advances, but this method is still complex and not available for all pollen species in many different areas 36, 37.
Internet-based surveillance systems using search engine queries 38 and social media 39 are recent techniques with the potential to extend or even substitute the more costly disease surveillance systems 40. A few studies analysing online searches of pollens, rhinitis symptoms and allergies have shown associations with pollen counts 41. The analysis of online searches, in particular using Google trends, has shown potential in predicting changes in flu infections 42 and in other areas of medicine 38. Nevertheless, this type of big data analysis is just beginning 38 and more research is needed to prove its value in predicting the onset of allergic rhinitis symptoms due to the pollen season 43. Moreover, the onset of the pollen season cannot be predicted using these models.
In the meantime, other novel approaches such as a personalized pollen-related forecast 44, 45 and an ICT sentinel network based on patients’ symptoms should be developed. However, these approaches need to be simple and user friendly.
Continuous management of symptoms during allergen exposure
Allergen exposure varies daily, and patients with respiratory allergic symptoms need regular monitoring of symptoms in order to optimize treatment. A clinical decision support system (CDSS) may be beneficial to optimize treatment and assess disease control after commencement of the allergy season. Moreover, such a system has the potential to improve patients’ compliance to treatment. Guided management of allergic diseases including asthma was found to be effective 46, 47 with clear evidence provided by the Finnish Asthma Programme 48 and the Allergy Programme 49, 50.
Comorbidity assessment
Conjunctivitis, chronic rhinosinusitis and asthma are frequent AR comorbidities that need to be identified and treated to achieve good AR control 51. ICPs that include asthma screening and assessment, as recommended by ARIA (Allergic Rhinitis and its Impact on Asthma) 6, 7, may result in improved outcomes and should be tested. In addition, optimal AR control may facilitate the control of concomitant asthma.
Needs for a multidisciplinary team for an ICP
Integrated care pathways (ICPs) are structured multidisciplinary care plans which detail essential steps in the care of patients with a specific clinical problem 52. They promote the translation of guideline recommendations into local protocols and their subsequent application to clinical practice. An ICP forms all or part of the clinical record, documents the care given and facilitates the evaluation of outcomes for continuous quality improvement 53. ICPs can help empower patients and their care providers (health and social). They differ from clinical practice guidelines as they focus on the quality and co-ordination of care. ICPs need to have a mechanism for recording variations/deviations from planned care. Variation between recommendations and practice identified within an ICP should be noted as a variance 54, 55. In AR, there is a need for ICPs to combine the views of patients, pharmacists, primary care physicians, specialists, and other healthcare professionals.
Biomarkers in respiratory allergic diseases
Biomarkers are of great importance in respiratory allergic diseases and asthma, and a large body of research is focusing on their identification and validation. Biomarker identification can be based on systems medicine approaches combining transcriptomics, proteomics, epigenetics and metabolomics in large patient cohorts. One recently completed EU project, MultiMod, resulted in a generally applicable strategy to integrate such data for diagnostic purposes using systems medicine principles 56. Two EU-funded projects are currently ongoing: U-BIOPRED (IMI) in severe asthma 57 and MeDALL (FP7) in allergy 58, 59. MeDALL has already made critical observations concerning IgE biomarkers for the diagnosis and prognosis of allergic diseases 2, 60. It is hoped that these projects will help identify biomarkers to enhance personalized medicine 61, 62 and to improve patient stratification and clinical trials. Another ongoing EU project, CASyM, has generated a road map for the implementation of systems medicine in clinical research and practice ( https://www.casym.eu/).
Innovation in clinical trials
In randomized controlled trials (RCTs), it is essential to have clarity with regard to the definitions of disease severity and control as well as comorbidities and risk factors (e.g. smoking). RCT outcomes should be validated and standardized, so that meaningful comparisons between RCTs can be made 63. Several gaps exist in RCTs in respiratory allergy. Among them the importance of the placebo effect and the evaluation of efficacy using a single assessment tool combining symptoms, medications and quality of life 64. Novel tools for the evaluation of RCTs on AR and its common comorbidities are needed, if possible using ICT.
Climate change effects on allergic diseases
Allergy prevalence continues to grow due to novel interactions between known allergens and other environmental factors. An increase in the prevalence and severity of allergy and asthma is anticipated due to climate changes 65. Worsening ambient air pollution and altered local and regional allergen production 66 and reduction in biodiversity may play a significant role 67. This anticipated higher allergic disease burden will affect clinical practice as well as policies and public health planning.
Patient empowerment
To satisfy patient expectations, asthma and AR should be appropriately diagnosed and controlled. Patients need to be motivated to become educated and to actively increase their own health literacy to be able to take over the responsibility of their own specific condition. Patient organizations have been involved in the design, dissemination and implementation of ARIA. ICT can empower patients and thus enable them to define specific goals and to monitor disease status and control. It can also support the patient's decisions.
Tools
ARIA
ARIA was initiated during a WHO workshop in 1999 (published in 2001) 6, 7. The ultimate aim of ARIA is to achieve control of AR globally. ARIA has reclassified AR as mild/moderate–severe and intermittent/persistent. This classification closely reflects patients’ needs and underlines the close relationship between rhinitis and asthma. A module devoted to the pharmacist exists 68. In its 2010 Revision, ARIA developed clinical practice guidelines for the management of AR and asthma comorbidities based on GRADE (Grading of Recommendation, Assessment, Development and Evaluation) 69. ARIA is disseminated and implemented in over 60 countries of the world 10. ARIA has been endorsed by several ministries of health.
Variance has been tested and it was found that the ARIA classification of mild vs moderate–severe and intermittent vs persistent rhinitis is valid. A modified ARIA severity classification has also recently been validated as mild, moderate and severe, both in adults 70 and children 71, although its impact on treatment stratification remains an unmet need.
The 2015 ARIA revision leading to ICPs was finalized and presented at the AIRWAYS ICPs meeting in Lisbon 1–2 July 2015 (Figure 1).
Measures of allergic rhinitis control
Concepts of disease severity, activity, control and responsiveness to treatment are linked but constitute different domains 72. Control and severity are not well delineated in AR 72. Severity is the loss of function in the target organ(s) induced by disease 73. It is important to highlight that severity may vary over time and needs to be regularly re-evaluated 74. Control is the degree to which therapy goals are currently met 74 such as glycemic control in diabetes 75, and can be assessed in patients before or during treatment to guide therapy. However, for AR, the patients’ view of severity relates to the negative impact that rhinitis has upon life; control is a measure by which their symptoms are alleviated.
Measures of AR control include (i) symptom scores, (ii) patient self-administered visual analogue scales (VAS) 16, 76-81, (iii) objective measures of nasal obstruction (such as peak nasal inspiratory flow, acoustic rhinometry and rhinomanometry) 82, (iv) a recent modification of the ARIA severity classification 83, (v) patients' reported outcomes such as quality of life (QOL) 7, 63 scores with several items 80, 84 or composite symptom-medication scores 85. However, it is important to make the score for clinical use simple and responsive to change.
VAS is a psychometric response scale for subjective characteristics or attitudes used in a large variety of diseases. The continuous (or ‘analogue’) aspect of VAS differentiates it from discrete scales such as the Likert scale. The sensitivity and reproducibility of VAS results are very similar, although the VAS may outperform the other scales in some cases 86, 87.
In AR, VAS for all nasal symptoms appears to be sufficient to appreciate disease control 88 and is particularly relevant to primary 89 or pharmacy care 68. VAS can be used in all age groups including preschool children (guardian evaluation) 90 and the elderly 91. Furthermore, it can be used in a wide variety of languages 81, 91-97. VAS levels vary with the ARIA classification in many languages 76, 79, 81, 98. A VAS level of 50 mm is suggestive of moderate–severe AR 99-101 although in some studies the cut-off was of over 60 mm 94. VAS was used to define SCUAD 16, and patients with a low VAS level after treatment had a considerably improved Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) or work productivity (WPAI-AS). However, those with a level of over 50 mm had no improvement. VAS has been validated in cell phones (Sarah Acaster, personal communication).
VAS was found to be responsive to change in real-life cluster-randomized trials 102, 103. The minimal clinically relevant difference was set for a VAS level of 23 mm during treatment, whatever the baseline VAS level 104. A level of over 23 mm appears to be a relevant cut-off. VAS changes appear to encompass both symptoms and disease-specific QOL 88, 104.
VAS was highly responsive to change in double-blind, placebo-controlled RCTs 92, 93, 102, 105-108. These multicentre studies in Europe and Canada showed that patients easily cope with VAS in different languages.
These studies combine to indicate that VAS may be a simple and useful tool for the assessment of AR control and to follow the efficacy of treatment.
Electronic monitoring of allergic diseases
e-Allergy screening: Premedical system of early diagnosis of allergy and asthma based on online tools
The late diagnosis of allergic diseases and asthma is a serious problem. Patients with first symptoms of respiratory allergies are often misclassified in primary care. As a result, patients are either untreated or treated symptomatically, generally for a long period of time. This behaviour is detrimental to the patient, the healthcare system and the society, as it impacts on indirect costs 5.
One solution to this problem was presented in 2011 at a conference of experts during the Polish Presidency of the EU Council 109. It is an e-Allergy – premedical system capable of providing an early diagnosis of allergy and asthma on the basis of online tools. The concept is based on a screening questionnaire with built-in algorithms. It assesses individual risk of allergic diseases and includes 24 questions. The process takes about 5 minutes. The questions are selected depending on previous responses. The result is displayed in the form of risk calculation for the selected allergic diseases (asthma, allergic rhinitis, atopic dermatitis and allergy to Hymenoptera venom).
To develop the algorithm, data from the Epidemiology of Allergy in Poland (ECAP) ( www.ecap.pl) were used 110. Over 20 000 people responded to the study questionnaire, and almost 5000 were subjected to additional allergological tests. Various advanced methods of statistical analysis, including an artificial neural network, have been used to develop the algorithm. The system is calibrated to maximize the effectiveness of a group of people suffering from allergic diseases.
E-allergy screening can be used both by the public with suspected allergies and physicians. The initial diagnosis can lead to an evaluation. It is performed by a primary healthcare professional and, if needed, confirmed by a specialist. The role of e-allergy is to support, not replace, the physicians and also to speed up the process of unrecognized allergic diseases by proper management.
Daily tool based on VAS using cell phones
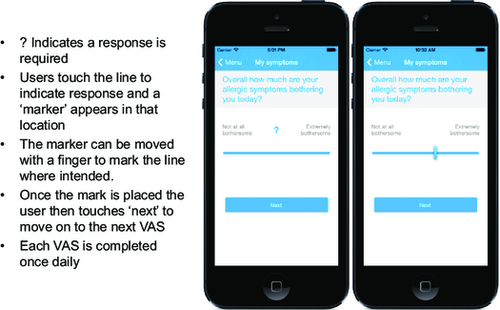
MASK aerobiology, approved by AIRWAYS-ICPs, is a very simple IOS/Android App. It is already available and is being expanded to other systems with interoperability. Patients selected by physicians trained in allergy represent the sentinels for the onset of the season. The VAS represents a reliable and valid measure of rhinitis control 10, 72 (Figure 2). It can be used across the life cycle 90, 91, 111.
CARAT
Asthma frequently occurs in association with allergic rhinitis, and a combined management approach has been suggested. The Control of Allergic Rhinitis and Asthma Test (CARAT) is the first questionnaire to assess the control of both diseases concurrently 112-115. An overall score of more than 24 indicates good control of asthma and rhinitis, while a change of four points between two occasions indicates a clinically relevant change 115. In addition, answers to individual questions may be used to identify the specific problems of a patient (e.g. night symptoms or overuse of reliever medication). However, to have an impact on healthcare, it needs to be disseminated and adopted. At present, the adaptation of CARAT for use in different languages and cultures is being led by volunteer researchers and clinicians in 15 countries. Website and smartphone applications have been developed, and a free open model of distribution has been adopted to contribute to the dissemination of CARAT. CARAT can be used in a range of settings and circumstances in primary and secondary care for clinical, research and audit purposes, and also in ambulatory pharmacies 116. It can be used both in adults and children 117, 118 and strengthens the partnership between patients and doctors in the management of asthma and rhinitis. CARAT can be administered every 2–4 weeks both in paper and electronic forms 119 and represents an additional tool for daily assessment.
RhinAsthma Patient Perspective
‘RhinAsthma Patient Perspective (RAPP)’ is the first valid questionnaire to assess the individual health-related QoL of patients with asthma and rhinitis in clinical practice. It is a simple eight-question tool with good measurement properties and sensitivity to health changes. RAPP is easy to complete and score, and the results enable immediate interpretation both for the physician and for the patient. The score, calculated by summing the responses of each item, ranges from 8 (no impact on QoL) to 40 (the worst possible QoL). A cut-off point of 15 has demonstrated the best sensitivity and specificity in discriminating the achievement of an optimal health-related QoL. A change of two points in the RAPP score was found to be the minimal clinical difference that patients perceived as important, either ‘beneficial or harmful.’ A new tool for the smartphone has been developed 120.
Clinical decision support system
Identifying the most suitable patients for whom an intervention is appropriate is critical for the delivery of a cost-effective health system. In many diseases, the management of patients uses ICT tools including integrated care pathways, e-health and CDSS. This has made a significant improvement and has sometimes led to a change of management in health systems. A CDSS 121, 122 immediately proposes advice for (standardized) pharmacologic treatment defined by the physician during a consultation before the pollen season. Care pathways based on AIRWAYS ICPs 13 will guide the healthcare professional. Patients with SCUAD are defined as those resistant to treatment despite optimal treatment (VAS level > 50%). Moreover, individual complaints of rhinitis, conjunctivitis or asthma are monitored by the system 123. Computer-analysed VAS responses may be measured using discrete values due to the discrete nature of computer displays, and VAS can be used in internet-based questionnaires 124.
Bias reduction, patient empowerment and identification of new markers through a Living Lab approach
Systematically collecting and mining/analysing data from patients’ mobile phones where they can enter quantitative and qualitative information is indeed a promising use of innovative health technologies 125. This should allow, on an almost continuous mode, a long-term close monitoring of and connection to the patient. To our knowledge, this has not been addressed before by any other technology. However, a major requirement for the implementation of such clinical protocols is the validity of data 125, 126. In validating such protocols, bias by the degree of usage of devices by the patients and bias in information input due to the context or human factors need to be identified and eradicated; such factors are very difficult to control. The overall bias will normally be balanced by the long-term use of the application by the patient, as patients’ data are always compared to their previous declarations. However, it is possible and desirable to improve the results and reduce the time necessary to obtain them. Contrary to drugs, where the administration of medications to patients may be appropriately controlled during clinical trials, the usage of mobile phones, especially at home, is known to depend heavily on (i) the usability of such devices and supported applications, (ii) their context of use (including ongoing activities, social environment, presence of third parties), and (iii) the constraints they impose on patients, with a strong probability of weak compliance, hazardous on/off usage or even rejection and abandon by the patients 127, 128. Similarly, the adoption of these new practices, including participation and interactions from family members or professionals, is an issue 129.
Inappropriate and/or irregular use of the system – a social and behavioural bias – cannot be identified in the data analysis. This can compromise the scientific validity of the entire results. Furthermore, opportunities to address behavioural or psychological markers are not seized, even when they are already identified as possible candidates by practitioners. It is therefore both mandatory and potentially highly valuable to properly address usage problems at the patients’ end and to ensure the usability of a selected mobile application.
The involvement and commitment of the patients and of the healthcare and social professionals involved from the start and during all phases of the project is the only way to address the problem. It is highly recommended to adopt a co-design/co-evaluation and user-centred design approach to the project 130. This will be a lever to gain long-term adherence of both patients and health care providers. The participation of Living Labs for Health and Autonomy will secure the many tasks to be carried out throughout the project with the users and all participants. It will ensure a proper usage validity of the collected data right from the first phases:
- Analysis of the context of use.
- Co-design of the protocol with patients and physicians.
- Evaluation/optimization of device usability, human machine interface and adoption potential.
- Follow-up of device usage during the collecting phase.
Additional tools
An e-CRF and an e-learning tool will be added to the MACVIA-ARIA suite of instruments.
MASK: the global and integrated ICT answer for unmet needs in allergic rhinitis : empowerment of patients
Electronic monitoring of the pollen season
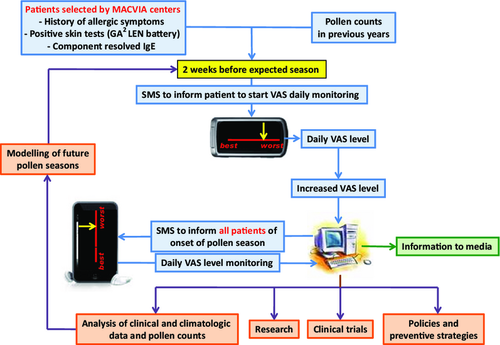
Mobile phone messaging facilitates the management of AR 131 and chronic diseases 132, 133. Using cell phones with a touch screen, patients are geolocalized and can evaluate their symptoms daily by VAS (Figure 2). At the predicted time of the pollen season, based on local calendars and/or forecast models where available, patients receive an SMS and an E-mail indicating that they should monitor VAS daily for global symptoms on the dedicated mobile device. This information is coded and sent to a central database. Daily, 4 VAS assessments (global evaluation, nasal, ocular and bronchial symptoms) are completed by the patient on a cell phone. The information is sent to a clinical decision support system (CDSS) for an optimal management of all the patients using the system. The system is initially being deployed in 13 countries with 14 languages (translation and back-translation, cultural adaptation and legal issues).
MASK-aerobiology is monitored daily and will be completed with CARAT at the onset of the pollen season and thereafter every 2 weeks (Figure 3).

Applications include information to patients and to the media with regard to the pollen season, optimal management of the patients with allergic symptoms, clinical trials, research and climate evaluation (Figure 4).
CDSS based on ARIA 2015 to optimize control during allergen exposure and stratification of patients
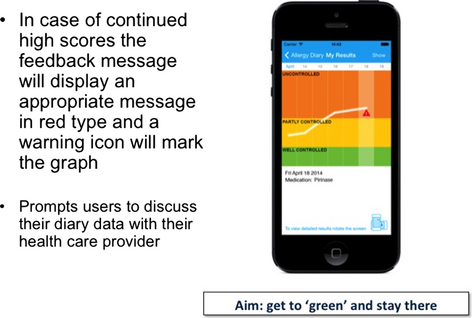
The chronic respiratory diseases CDSS (AIRWAYS-CDSS) is based on the ARIA 2015 revision (in preparation) and will enable the standardization of patient management. Patients with uncontrolled disease based on VAS e-health despite optimal treatment according to guidelines will be considered as SCUAD (severe chronic upper airway diseases) 15 (Figures 4-6). However, the physicians will determine the strategy to be used for their individual patients. All medications available in the given country are listed in the App according to the IMS list of drugs. The CDSS will be available at the end of 2015.
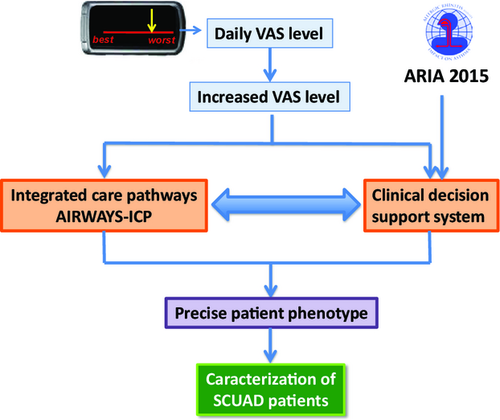
These two innovative tools (allergy sentinel network and AIRWAYS-CDSS) will be combined in MASK-rhinitis and will make it possible to assess some of the unmet needs of clinical trials in allergic diseases. They will allow optimal management of the patients, assessment of control, compliance to treatment as well as patient stratification.
Validation of ARIA guidelines
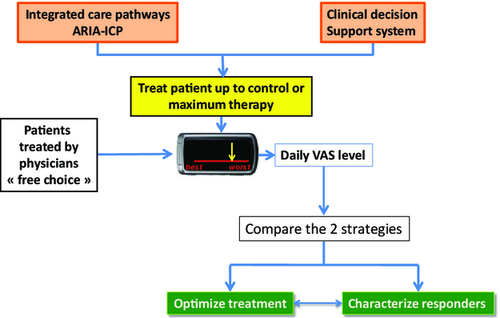
There is a need to validate guidelines using cluster-randomized trials to define whether the new strategy is more effective than a free treatment choice. The International Consensus of Rhinitis 102 and ARIA 2001 103 were both validated. MASK will also be validated using the same methodology (Figure 7).
MASK-rhinitis, a single tool for the ICP
An ICP has a focus on an interactive and multidisciplinary pathway (Figure 8). MASK can be used by:
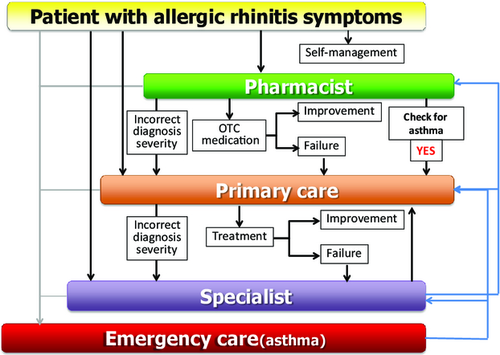
- Patients, to screen for allergic diseases (in a later stage, biomarkers will help to confirm the allergic origin of the symptoms).
- Pharmacists, to guide them in the prescription of OTC medications and direct the uncontrolled patients to physicians.
- The primary care physician, to prescribe appropriate treatment and to follow up with the patient according to the physician's instructions (CDSS) and assessment of control.
- The specialist, if there is failure to gain control by the primary physician.
These tools should be customized to be applicable globally.
Clinical trials
These three innovative tools (CARAT, allergy sentinel network and AIRWAYS-CDSS) are combined in MASK-rhinitis and will make it possible to perform innovative clinical trials in AR (Figure 9) including trials of allergen-specific immunotherapy 64, 134.
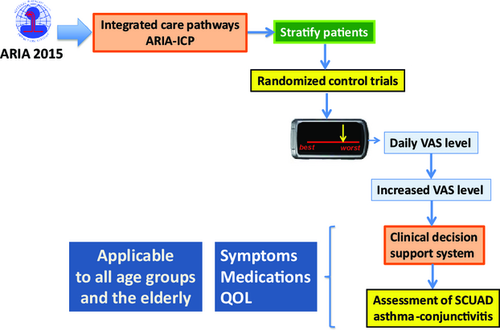
- Phenotypic characterization of allergic patients with stratification of patient severity, characterization of patients with SCUAD and characterization of patients to be treated.
- Randomized controlled trials (placebo-controlled or real-life cluster-randomized trials).
- Follow-up of patients in clinical settings during treatment.
- Follow-up of patients in clinical settings after treatment has been stopped (persistent effects).
- Assessment of side-effects due to treatment.
Implementation and application of MASK-rhinitis
Promotion of active and healthy ageing
The developmental origin of ageing is on the EU political agenda. The Polish Priority of the EU Council (2011) promoted the recognition, prevention and management of CRDs in children to ultimately impact AHA (1095). The developmental determinants of chronic diseases in ageing were reinforced during the Cyprus Presidency of the EU Council (2012), which proposed to fight against NCDs across the life cycle 135. A meeting at the European Parliament organized by the Region Languedoc Roussillon under the auspices of the Cyprus EU Priority (November 2012) was focused on CRDs 136. MASK-rhinitis will help to detect symptomatic patients early, to improve management, to increase school and work productivity and, ultimately, to promote AHA.
Early detection of symptomatic patients
One of the major problems of patients suffering from pollen allergy is the identification of the onset of the pollen season at home as well as alertness when pollen peaks are to be expected. Another problem is when travelling to regions where the seasons of pollens eliciting symptoms may differ compared to home (Table 1). As patients will be geolocalized, they will be informed about the level of the pollen season and will also be able to determine the season when travelling using MASK-rhinitis.
| Early premedical diagnosis |
| Optimal treatment proposed to control symptoms and prevent severe disease (e.g. asthma exacerbations) |
| Optimal duration of the treatment |
| Reduction in costs incurred by pollen allergy |
Stratification of patients with severe allergic diseases
Patient stratification is needed to identify patients with SCUAD, those for whom specific immunotherapy or other interventions are appropriate. This is critical for the delivery of a cost-effective health system. Although all studies are not consistent, in many diseases, ICT tools, ICPs, e-health and CDSS are likely to define the phenotypes of allergic patients. The main challenge for allergic diseases in the 21st century is to understand their complexity. The vast majority of AR patients can be treated using a simple algorithm. However, a substantial number of these patients are uncontrolled despite treatment 16 and require a personalized (tailored) approach.
Clinical trials
In specific immunotherapy RCTs, it is recommended to monitor pollen counts in order to determine the onset of the season and to correlate counts with symptoms. As discussed earlier, pollen counts alone may misrepresent exposure, especially if performed at a locality that is remote to that of a particular patient. As a result of such potential confounders, unconvincing data have been produced and a placebo-based method was found to be more effective 137. Moreover, there is a need to define the peak pollen season. MASK-rhinitis is suitable for this approach 64.
Scientific studies
Not all patients respond to pharmacologic treatment and/or immunotherapy. Research is needed in well-phenotyped patients to find novel therapeutic approaches. MASK-rhinitis can help characterize patients so that they can be stratified in further analyses. Global partnerships and platforms should ensure the application of standard methodology and protocols in the collection and sharing of samples and data 138.
Assessment of the effects of climate change and land use
Climate change impacts aeroallergens, particularly pollen 139 and moulds 140. The potential effect of land-use changes on pollen release may interact with climate change 141. Allergenic pollens are well known in Europe 66, but climate change can exert a range of effects on pollen 142-146. Pollination may start earlier in the future due to climate change 147, 148. The duration of the pollen season is extended in some species. Some plants produce a greater quantity of pollen 149-151 or pollen with stronger allergenicity 152-155 under modified climatic conditions. New allergenic pollen types can appear and result in patients developing new allergies (e.g. ragweed pollen). The pattern of change will vary regionally depending on latitude, altitude, rainfall and storms, land-use patterns, urbanization, transportation and energy production 156.
An integrated approach is needed to anticipate a higher allergic disease burden that will affect clinical practice and public health planning. A number of practical prevention strategies need to be proposed to meet this unprecedented public health challenge and to combat inequities. Both adaption and mitigation will be needed to counteract the effects of climate change in allergy (Table 2).
| To detect new sensitizations using pollen counts or derived methods |
| To detect changes in pollen seasons |
| To develop policies for prevention |
Implementation of the European Environment and Health
Continued support will be provided to research addressing the aims of the major policy initiatives such as the European Environment and Health Action Plan (2004–2010), the Fifth Ministerial Conference on Environment and Health and the EU Sustainable Development Strategy with its environment and public health components. MASK-rhinitis also includes strong socio-economic perspectives. In the medium term, it will ensure the engagement of relevant stakeholders (e.g., user groups, civil society organizations, policymakers) and it will cultivate a multidisciplinary approach (including researchers from social sciences and humanities).
Policies and public health planning
In clinical epidemiology and public health, a uniform definition of AR and severity is needed to identify prevalence, burden and costs, to improve quality of care and to optimize healthcare planning and policies.
MASK: from the ARIA 2015 guideline to an integrated health system for allergic rhinitis and its asthma comorbidity
There is an urgent need to propose an innovative health system for one of the most common global diseases. Around 20% of the EU population suffers from AR and the costs are very high, particularly the indirect costs. Although most patients can self-manage their symptoms, many need OTC drugs from the pharmacists and a few (but still in millions of subjects) need medical advice. Fewer, but still in millions, will need specialist advice. It is very important that a common language is used between patients and pharmacists, primary care and specialists. MASK is able to provide this common language using e-health and a very simple tool (VAS). Moreover, the CDSS will help patients to self-manage under the control of their physicians. Adding CARAT or other tools, an economic evaluation can be provided to assess the benefits and cost savings (indirect and direct costs) of interventions 5. A warning on asthma is in place in MASK allowing the assessment of this important comorbidity in AR patients. Reimbursement patterns can also be monitored and health system stratification made possible 157. MASK based on ARIA 2015 appears to be in a unique position to make the links between all stakeholders.
Conflicts of interest
Elisabeth Bel: reports grants from Chiesi and GSK outside the submitted work, and personal fees from Cipla, Sanofi/Regeneron, GSK, and Novartis, outside the submitted work; Jean Bousquet: has received honoraria for: Scientific and advisory boards – Almirall, Meda, Merck, MSD, Novartis, Sanofi-Aventis, Takeda, Teva, Uriach. Lectures during meetings – Almirall, AstraZeneca, Chiesi, GSK, Meda, Menarini, Merck, MSD, Novartis, Sanofi-Aventis, Takeda, Teva, Uriach. Board of Directors – Stallergènes; Warner Carr: Clinical Research, Merck, Mylan, Regeneron, Genentech, Novartis, Oriel, Afferent, Teva- Consultant, Teva, Meda, AstraZeneca, Alcon, Allergan, Merck, Boehringer Ingelheim – Honorarium and Speakers Bureau, Teva, Meda, AstraZeneca, Mylan, Alcon, Allergan; Thomas Casale: reports personal fees from Circassia, grants from Merck, grants from Stallergenes, outside the submitted work; Adnan Custovic: reports grants from Medical Research Council, grants from The JP Moulton Charitable Foundation, grants from North West Lung Research Centre Charity, grants from European Union 7th Framework Programme, grants from National Institute of Health Research, personal fees from Novartis, personal fees from Thermo Fisher, personal fees from AstraZeneca, personal fees from ALK, personal fees from GlaxoSmithKline, outside the submitted work; Ronald Dahl: Consulting, given lectures for: Boehringer-Ingelheim, Novartis, TEVA, MEDA, Pfizer, ALK-Abello, Vectura, AZ, GSK, CIPLA; Ulf Darsow: has been speaker, investigator and ⁄ or been a member of advisory boards for Allergopharma, ALK Abello′, Bencard, GSK, Hermal, MEDA, Novartis Pharma, Stallergenes, Stiefel; Pascal Demoly: consultant (and speaker) for Stallergenes, ALK, Circassia and Chiesi and a speaker for Allergopharma, Merck, AstraZeneca, Menarini and GlaxoSmithKline. Investigator for Menarini, Pierre Fabre Médicaments, Stallergenes and ALK; Judah Denburg: is CEO and Scientific Advisor of the Allergy, Genes and Environment Network of Centres of Excellence (AllerGen NCE Inc); Alain Didier: have received honorarium for talks from Astra Zeneca, GSK, MSD, Novartis, Stallergènes and has consulting arrangement with GSK, Novartis, Stallergènes and Allerbio (ALK); Anh Tuan Dinh Xuan: has received honoraria from Aerocrine, Chiesi and Stallergenes for invited lectures during satellite scientific symposia of national and international meetings during the last 3 years; Stephen Durham: consultancy fees from ALK Abello, Circassia, Merck USA, Biomay and Leti, manufacturers of allergy vaccines. Research funding via Imperial College from Merck, ALK Abello and Biotech Tools; Mark Dykewicz: Merck (Consultant) and Novartis (research funding); Joao Fonseca: a member of advisory boards for Boehringer Ingelheim and Novartis, has received payment for lectures and services from A. Menarini, Aerocrine, AstraZeneca, Merck Sharp Dohme, Novartis, Teva; Carlos Ivancevich: Chief Editor of the WAO website, Interasma website and the Latin American Society of Allergy, Asthma and Immunology website. Collaborate with the laboratory Faes Pharma of Spain as scientific advisor on social media and speaker at symposiums of Sanofi-Aventis; Tari Haatela: reports personal fees from Boehringer Ingelheim, MSD and OrionPharma, outside the submitted work; Marc Humbert: has relationships with drug companies including Astrazeneca, Chiesi, GSK, Merck, Novartis, Pfizer, Roche, Sanofi and TEVA. In addition to being an investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards; Michael Hyland: reports grants and personal fees from Novartis, during the conduct of the study; personal fees from GSK, outside the submitted work; Sebastian Johnston: reports grants and personal fees from Centocor, grants and personal fees from Sanofi Pasteur, grants and personal fees from GSK, grants and personal fees from Chiesi, grants and personal fees from Boehringer Ingelheim, personal fees from Grünenthal, grants and personal fees from Novartis, grants, personal fees and Shareholding from Synairgen, outside the submitted work; In addition, Dr. Johnston has a patent Blair ED, Killington RA, Rowlands DJ, Clarke NJ, Johnston SL. Transgenic animal models of HRV with human ICAM-1 sequences. UK patent application No. 02 167 29.4, 18 July 2002 and International patent application No. PCT/EP2003/007939, 17 July 2003. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Anti-virus therapy for respiratory diseases. UK patent application No. GB 0405634.7, 12 March 2004. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Interferon-Beta for Anti-Virus Therapy for Respiratory Diseases. International Patent Application No. PCT/GB05/50031, 12 March 2004. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. The use of Interferon Lambda for the treatment and prevention of virally-induced exacerbation in asthma and chronic pulmonary obstructive disease. UK patent application No. 0518425.4, 9 September 2005. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Anti-Virus Therapy for Respiratory Diseases. US Patent Application – 11/517,763, Patent No. 7569216, National Phase of PCT/GB2005/050031, 04 August 2009. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Interferon-beta for Anti-Virus Therapy for Respiratory Diseases. European Patent Number 1734987, 5 May 2010. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Anti-Virus Therapy for Respiratory Diseases (IFNb therapy) Hong Kong Patent Number 1097181, 31 August 2010. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Anti-Virus Therapy for Respiratory Diseases (IFNb therapy). Japanese Patent Number 4807526, 26 August 2011. licensed, a patent Wark PA, Johnston SL, Holgate ST, Davies DE. Interferon-beta for Anti-Virus Therapy for Respiratory Diseases. New Hong Kong – Divisional Patent Application No. 11100187.0, 10 January 2011. licensed, and a patent Burdin N, Almond J, Lecouturieir, V, Girerd-Chambaz Y, Guy, B, Bartlett N, Walton R, McLean G, Glanville N, Johnston SL. Induction of cross-reactive cellular response against rhinovirus antigens European Patent Number 13305152, 4 April 2013. Pending; Jocelyne Just: on the advisory board for Novartis, ALK, and Thermofischer; is a speaker for AstraZeneca, Novartis, ALK, Stallergens, Teva; and has received grants from Novartis and Stallergens; Ludger Klimek: has received research grants for his institution from ALK Abelló (Germany/Denmark), Allergopharma (Germany), Stallergenes (Germany/ France), HAL Allergy (Germany/the Netherlands), Artu Biologicals (the Netherlands), Allergy Therapeutics/Bencard (UK/Germany), Hartington (Spain), Lofarma (Italy), Novartis/Leti (Germany/Spain), GlaxoSmithKline (UK/Germany), Essex Pharma (Germany), Cytos (Switzerland), Curalogic (Denmark), Roxall (Germany), Biomay (Austria), Thermo Fisher (Germany), Circassia (UK), Biotech Tools s.a. (Belgium), and Meda Pharma GmbH (Germany); and/or he has served as an advisor and on speakers’ bureaus for some of the aforementioned companies. LK has received travel grants from HAL Allergy (the Netherlands/Germany), Meda (Germany/Sweden) and Allergopharma (Germany), and he is a consultant for Bencard (Germany), Novartis/Leti (Germany), Meda (Germany), ALK Abelló (Germany/Denmark), Allergopharma (Germany) and Boehringer Ingelheim (Germany). LK is Board Member of the ENT Section of the European Academy of Allergy and Clinical Immunology (EAACI), Vice-President of the German Academy of Allergology and Clinical Immunology, Vice-President German Union of Allergologists, Member of the Board of Directors of the German Society for Otorhinolaryngology HNS. He is co-editor and author of different chapters of the textbook ‘Allergien bei Kindern und Jugendlichen’ (publisher: Schattauer-Verlag, Germany), author of one chapter in ‘Allergologie’ (publisher: Springer, Germany) and author of different chapters in ‘Allergologie’ (publisher: Schattauer-Verlag); Gerard Koppelman: grants outside this work from Dutch Lung Foundation, Ubbo Emmius Foundation and Stichting Astma Bestrijding; Piotr Kuna: reports personal fees from Adamed, personal fees from Allergopharma, personal fees from Almirall, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Celon Pharma, personal fees from Chiesi, personal fees from FAES, personal fees from GSK, personal fees from HAL, personal fees from Meda, personal fees from MSD, personal fees from Novartis, personal fees from Pfizer, personal fees from Polfarmex, personal fees from Polpharma, personal fees from Stallergen, personal fees from Teva, personal fees from Lekam, outside the submitted work; Désirée Larenas: Speaker para: Astrazeneca, Pfizer, MIT, Glenmark, MEDA, MSD, Novartis, UCB. Advisory board: Boerhinger-ingelheim, Novartis, Astrazeneca, MEDA, Mit, Glenmark, MSD, Pfizer. Grants from development of guidelines: TEVA, Pfizer, Novartis, UCB, Sanofi, GSK, Carnot, Senosiain, MEDA, MSD, Astrazeneca; Brian Lipworth: unrestricted grant support from Meda, Teva, Chiesi, Almirral. Multi centre grants from Jansen, Pearl, Roche, AZ, Teva. Consulting for Meda, Chiesi, Neopharma, Cipla, Sandoz. Ad boards for Teva, Meda, Chiesi. Support to attend educational meetings from Boerhinger, Teva, Chiesi; Renaud Louis: research grants from GSK, Novartis, Chiesi over the last 2 years – in national Aboard of GSK, AstraZeneca and Mundipharma; Le Ltt: honorarium for lectures, funding for investigation, support to attend symposium of Astra Zeneca, Boehringer- Ingelheim, Glaxo Smith kline, Novartis, Pfizer and MSD. Consultant of Astra -Zeneca and Boehringer- Ingelheim; Antoine Magnan: Investigator: GSK Novartis Astra Zeneca Roche SANOFI Amgen Boehringer. Consultant: Novartis, MSD, Astra-Zeneca, ALK, TEVA, Mundipharma, Takeda, GSK, Boehringer. Symposia: ALK, Stallergènes, Novartis, MSD, Chiesi, GSK, Astra-Zeneca, Roche. Research Grants: MSD, Astellas, Sanofi, Novartis, Stallergènes; P Manning: Personal Benefits <$10 000: A. Menarrini, Shares: none, Non-Personal Interests/benefits >$10 000: none; Marcus Maurer: Grant/Research/Clinical Trial Support: Novartis; Genentech; Uriach; Abbott Laboratories; FAES; UCB; Moxie. Consultant/Advisory Boards: Novartis Genentech; Uriach; Abbott Laboratories; FAES; MSD; Almirall; UCB; Moxie; Sanofi; Ralf Mosges: reports personal fees from ALK-Abello, Ohropax, Meda, Servier, Stada, Menarini, Allergy Therapeutics, Novartis, Leti, Allergopharma, Bayer, Faes, GSK, Johnson+Johnson, MSD, grants and personal fees from Arthrocare, Bencard, Stallergènes, BiotechTools, Lofarma, grants from Ursapharm, Bitop, HAL, AIPreven, Optima, non-financial support from Greer, Roxall, personal fees and non-financial support from UCB, non-financial support from Atmos, outside the submitted work; member of the guidelines task force of the German Academy of Otorhinolaryngology, chairman of the International Standardisation Committee of the European Rhinologic Society (ERS) and chairman of the ENT-Section of the European Academy of Allergy, Asthma and Clinical Immunology (EAACI); Robert Naclerio: Advisory Board: GSK, Merck, Sanofi, Teva – Speaker: Merck, Teva; Ken Ohta: honoraria for lectures and advisory meetings from Kyorin, GSK, Boehringer Ingelheim, AstraZeneca and Astellas; Yoshitaka Okamoto: Research grant from Torii Co. Ltd., Shionogi Co. Ltd., – Lecture fee from Torii Co. LTD., MSD Co. LtD; Kimihio Okubo: Lecture Fee:GSK, MSD, Tanabe-Mitsubishi, Sanofi, Ono, Torii, Kyowa-Kirin – Consultancy: MSD, Tanabe-Mitsubishi, Sanofi, Ono, Torii, Taiho, Astellas, Teikoku; Pier Luigi Paggiaro: personal support for education and research from: AstraZeneca, Almirall, Boehringer Ingelheim, Chiesi, Guidotti-Malesci, GSK, Menarini, MSD, Mundipharma, Novartis, Takeda, Zambon; Nikos Papadopoulos: grant from GSK, Nestlé, Merck – Fees from: Abbvie, Sanofi, Meda, GSK, Novartis, Menarini, ALK-Abello, Allergopharma, Uriach, Stallergènes, MSD; Alberto Papi: has received grants, personal fees, and non-financial support from AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Boehringer Ingelheim, Merck Sharp & Dohme, Menarini, Novartis, Zambon, TEVA, Pfizer, Takeda, and Mundipharma; O Pfaar: has received research grants for his institution from ALK Abelló (Germany/Denmark), Allergopharma (Germany), Stallergenes (Germany/ France), HAL Allergy (Germany/the Netherlands), Artu Biologicals (the Netherlands), Allergy Therapeutics/Bencard (UK/Germany), Hartington (Spain), Lofarma (Italy), Novartis/Leti (Germany/Spain), GlaxoSmithKline (UK/Germany), Essex Pharma (Germany), Cytos (Switzerland), Curalogic (Denmark), Roxall (Germany), Biomay (Austria), Thermo Fisher (Germany), Circassia (UK), European Union (FP-7 Health-2013 Innovation 1), Biotech Tools s.a. (Belgium), and Meda Pharma GmbH (Germany); and/or he has served as an advisor and on speakers’ bureaus for some of the aforementioned companies. OP has received travel grants from HAL Allergy (the Netherlands/Germany) and Allergopharma (Germany), and he is a consultant for Bencard (Germany), HAL Allergy (the Netherlands), Novartis/Leti (Germany), Meda (Germany), ALK Abelló (Germany/Denmark), Allergopharma (Germany), Biotech Tools s.a. (Belgium), GfK Bridgehead (UK), Navigant Consulting (USA), Sanofi (USA), Guidepoint Global Advisors (USA), Thermo Fisher (Germany) and Stallergenes (Germany/France); he is Scientific Board Member of Mobile Chamber Experts (MCX), a GA2LEN Partner. OP is the current chairman of the Immunotherapy Interest Group (IT IG) of the European Academy of Allergy and Clinical Immunology (EAACI) and is the secretary of the ENT section of the German Society for Allergology and Clinical Immunology (DGAKI). He has received grants for the ‘Spezifische Immuntherapie’-award 2014 and the ‘Nachwuchsförderpreis’- award 2010 of the DGAKI. He is co-editor and an author of the textbook ‘Allergien bei Kindern und Jugendlichen’ (publisher: Schattauer-Verlag, Germany), ‘Allergologie’ (publisher: Schattauer-Verlag) and author of different chapters of ‘Allergologie- Handbuch’ (publisher: Schattauer-Verlag, Germany) and has received payment for development of educational presentations from GlaxoSmithKline (Germany), Bencard (Germany), and Novartis (Germany); Davor Plavek: reports grants from Ministry of Science, Education and Sports of Republic of Croatia, grants and personal fees from GlaxoSmithKline, grants and personal fees from MSD, personal fees from Sandoz, personal fees from Salveo, grants from Schering-Plough, outside the submitted work; Dirkje Postma: The University of Groningen has received money for Professor Postma regarding an unrestricted educational grant for research from Astra Zeneca. Travel to ERS and/or ATS has been partially funded by Astra Zeneca, Chiesi, GSK, Takeda. Fees for consultancies were given to the University of Groningen by Astra Zeneca, Boehringer Ingelheim, Chiesi, GSK, Takeda and TEVA. Travel and lectures in China paid by Chiesi; David Price: Board Membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva. Consultancy: A Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, and Teva; Grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, and Zentiva; Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda, and Teva; Payment for manuscript preparation: Mundipharma and Teva; Patents (planned, pending or issued): AKL Ltd.; Payment for the development of educational materials: GlaxoSmithKline, Novartis; Stock/Stock options: Shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care; received Payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva; Funding for patient enrolment or completion of research: Almirral, Chiesi, Teva, and Zentiva; and Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014); Dermot Ryan: Board member URIACH and Stllargenes. received payments from GSK and MEDA to deliver lectures on their behalf. Chair of the Primary Care Interest Group of EAACI; Miguel Roman-Rodriguez: has provided consultancy to or lectured on behalf of AstraZeneca, GlaxoSmithKline, Novartis, Almirall, Chiesi, Mundipharma, Boehringer-Ingelheim, Rovi and Teva; Glenis Scadding: Research grants from GSK, ALK. Honoraria for articles, consulting, lectures/ chairing and/or advisory boards: ALK, Astra Zeneca, Brittania Pharmaceuticals, Capnia, Church & Dwight, Circassia, GSK, Groupo Uriach, Meda, Merck, MSD, Ono Pharmaceuticals, Oxford Therapeutics, Sanofi-Aventis, Shionogi, UCB. Travel funding from Bayer, GSK; Estelle Simons: Uriach Medical Advisory Board, UpToDate, The Medical Letter; Rafael Stelmach: AstraZeneca; Boehringer Ingelheim; Bayer; Chiesi; Eurofarma; Glaxo Smith Kline; Mantecorp-Farmasa; Novartis; MSD; Nycomed; Reckitt Bekinser, related to sponsorship for achievement/participation of clinical trials, conferences or consultancy activities; Ana Todo Bom: Fee for speaking, reimbursement for attending or organising a symposium) from Novartis Farma, Faes Farma, Astrazeneca, Bial Aristegui, Thermo Fisher, Boehringer; Rudolf Valenta: has received research grants from Biomay AG, Vienna, Austria, Thermo Fisher, Uppsala, Sweden. He serves as a consultant for Biomay AG, Vienna, austria, Thermofisher, Uppsala, Sweden and Fresenius Medical Care, Bad Homburg, Germany; Ulrich Wahn: received fees for lectures and consultation within the last 5 years from: Stallergenes, Allergopharma, ALK, Novartis, Merck, and MEDA; All the other authors declare that they have no conflicts of interest.




