Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts
Abstract
Interleukin-6 (IL-6) is a cytokine critical for innate and adaptive immune responses. However, persistent expression of high levels of IL-6 are associated with a number of pathologic conditions including autoimmune diseases and capillary leak syndrome. Importantly, in kidney transplant patients, IL-6 may play a role in mediation of cell-mediated rejection (CMR) and antibody-mediated rejection (AMR). This is likely due to the importance of IL-6 in stimulating B cell responses with pathogenic donor-specific antibody (DSA) generation and stimulation of T effector cell responses while inhibiting T regulatory cells. Data from preliminary clinical trials and clinical observations show that tocilizumab (anti–IL-6R) and clazakizumab (anti–IL-6) may have promise in treatment of CMR, AMR and chronic (cAMR). This has led to a phase 3 placebo, randomized clinical trial of clazakizumab for treatment of cAMR, a condition for which there is currently no treatment. The identification of IL-6 production in vascular endothelia cells after alloimmune activation reveals another potential pathway for vasculitis as endothelia cell IL-6 may stimulate immune cell responses that are potentially inhibitable with anti–IL-6/IL-6R treatment. Importantly, anti–IL-6/IL-6R treatments have shown the ability to induce Treg and Breg cells in vivo which may have potential importance for prevention and treatment of DSA development and allograft rejection.
1 THE ANTIBODY THEORY OF TRANSPLANTATION
One of the most important advances in kidney transplantation occurred in 1969 with the publication of an article by Drs Patel and Terasaki in the New England Journal of Medicine pointing out the significance of a positive crossmatch test in kidney transplantation.1 This seminal paper showed that antibodies present in the sera of patients that reacted with surrogate targets on donor lymphocytes were highly predictive of graft rejection and loss. Although the nature of those antibodies was not clear at the time, subsequent advancements showed that pathogenic antibodies were specific for HLA class I and II antigens. The crossmatch test was subsequently implemented by transplant centers as a method for allocating kidneys. Here, any crossmatch positivity was used to decline the kidney for transplantation and for decades creating groups of immunologically disadvantaged patients who had limited or no access to life-saving transplantation.
Despite the passage of more than 50 years, alloantibodies remain a persistent and often impenetrable barrier to successful transplantation. Allosensitization remains one of the most difficult and as yet unsolved problems in transplant medicine. Desensitization therapies have emerged to address the allosensitization issue, but, to date, no specific therapy can address all facets of the alloimmune response.
2 THE EMERGENCE OF ANTIBODY-MEDIATED REJECTION (AMR)
The advent of T cell-centric immunosuppressive regimens resulted in excellent short-term graft survival. However, despite this, long-term survival was not improved. Here, the identification of donor-specific alloantibody (DSA) responses was shown to be primarily responsible for ongoing kidney injury and graft loss.2-5 Unfortunately, this suggest that current immunosuppressive regimens are effective in preventing but not treating established alloantibody responses. The prime movers for this response are polymorphic HLA antigens that stimulate graft-directed immunity usually when subtherapeutic immunosuppression is present. While the impact of HLA antibodies on kidney allograft survival were recognized, true clarification awaited the advent of sensitive luminex solid-phase assays to detect DSAs and the development and application of the Banff diagnostic criteria for AMR. It is estimated that 25% of all kidney transplant recipients have developed de novo DSA (dnDSA) by 10 years after transplant.6, 7 What we know now is that chronic AMR (cAMR) represents the most common cause for allograft failure worldwide.6, 7 As previously mentioned, regimens aimed at treating AMR and cAMR have evolved from desensitization protocols, all of which have significant limitations. Complicating this problem is the lack of robust clinical trials in AMR/cAMR and as yet, there are no Federal Drug Administration (FDA)-approved therapies for the prevention and treatment of AMR/cAMR.7
In 2019, the Transplantation Society convened a group of experts from around the world to address the treatment of AMR. After 2 days of deliberation, the conclusions of this expert group of physicians and scientists were summarized as follows: “While it was agreed that the aims of treatment are to preserve renal function, reduce histological injury, and reduce the titer of donor-specific antibody, there was no conclusive evidence to support any specific therapy. As a result, the treatment recommendations are largely based on expert opinion. It is acknowledged that properly conducted and powered clinical trials of biologically plausible agents are urgently needed to improve patient outcomes”.7
Despite this discouraging assessment of potential treatments for AMR/cAMR, there are encouraging advancements that have occurred since those assessments were published. Here, we will discuss data regarding inhibition of interleukin-6/interleukin-6 receptor (IL-6/IL-6R) signaling as potential therapies for treatment of AMR/cAMR. However, first, it is important to review the immunologic capacities of IL-6 and its potential as a pathologic agent in transplant rejection.
3 IL-6: A CYTOKINE IMPORTANT IN IMMUNE CELL ACTIVATION
IL-6/IL-6R signaling is an essential component of host defenses, tissue homeostasis, and immune regulation. However, excessive IL-6 production can occur from chronic inflammatory events or excessive transduction of IL-6/IL-6R due to gain of function mutations in the IL-6R. These activation events result in excessive IL-6 production, stimulating autoimmunity, alloimmuity and tissue injury. Monoclonal antibodies aimed at IL-6/IL-6R have evolved and are used to treat a number of autoimmune and inflammatory disorders.8-11 Here, we will discuss the rational of applying anti–IL-6/IL-6R therapy to the problem of AMR/cAMR. There are several excellent articles that discuss the immunobiology of IL-6/IL-6R signaling, effector functions, pathogenicity, and relevance to human diseases as these will not be discussed in this review.8-13
One of the first observations regarding IL-6 was its role in promoting the development and maturation of B cells- > plasmablast- > plasma cells with an important role in stimulating and maintaining antibody production.12-14 Further elucidation of this pathway implicates IL-6 as a potent stimulator of T follicular helper cells (Tfh) induction, germinal center (GC) formation that drives progression of naive B cells to plasma cells, and high-affinity antibody production.14, 15 In studies using IL-6−/− mice, hypogammaglobulinemia and deficient responses to T-dependent antigens were noted. A recent article also expanded our knowledge of how IL-6 promotes Tfh cell development in the GC. Using a model of influenza infection to stimulate GC formation in mice, investigators showed that sustained T cell receptor (TCR) stimulation is required for maintaining germinal center T follicular helper (GC-Tfh) cells. To down regulate T cell responses, Tfh cells produce copious amounts of IL-2 and induces interleukin-2 receptor (IL-2R) expression, thus initiating a negative regulatory pathway of IL-2 signaling that normally inhibits Tfh cells. However, the investigators noted this did not happen and despite the GC-Tfh cells receiving prolonged TCR stimulation, they did not respond to the inhibitory effects of IL-2 production. The important question is how do GC-Tfh cells maintain their low response rates allowing the GC activity of stimulating B cells to occur? Here, they identified intrinsic IL-6 signaling as the major contributor to this process. The pathways responsible for this effect relied on IL-6 inhibition of IL-2Rβ (CD122) activation by preventing STAT5 association with the Il2rb locus. This IL-6–induced inhibition of IL-2Rβ expression allows GC-Tfh cells to receive sustained TCR signaling, driving inflammatory, and immune activation pathways without initiation of IL-2-dependent regulatory T cell (Treg) pathways.16
These observations may shed light on important regulatory pathways induced by anti–IL-6/IL-6R. In this regard, an important study examined the role of Tfh cells and plasmablast in patients with active rheumatoid arthritis (RA).14 These investigators showed that patients with active RA had elevated Tfh cells, IL-21–producing Tfh cells, and plasmablasts in peripheral blood. The investigators also assessed IL-6 production by various cell subsets. The authors showed that plasmablasts(CD19+, CD38high, CD27high) were the primary source for IL-6 production. Importantly, the authors postulate the plasmablasts were responsible for Tfh cell stimulation and GC formation. Here, tocilizumab (anti–IL-6R) therapy significantly reduced numbers of Tfh (CD4+, CXCR5+, ICOS+) cells, suggesting IL-6 inhibition decreased inflammation and immune activation. A key question here is does inhibition of Tfh cells by anti–IL-6/IL-6R treatment extinguish the process by allowing STAT-5 dependent expression of IL-2Rβ, resulting in stimulation of Treg cells? We will discuss this later.
IL-6 is important in regulation of CD4+ cell differentiation and proliferation of Th1 or Th2 cells. IL-6 is also critical for T naive cell transformation to Th17 cells, which mediate autoimmunity and inflammation.9, 14, 17 As previously mentioned, IL-6 is critical for inhibition of T regulatory (Treg) (CD4+, CD25+, FoxP3+) cell development, by preventing conversion of Th17 and Tfh to Treg cells. This is a critical element of immune regulation since excessive IL-6 production increases Th17 cells important in mediation of allograft rejection. We now know that Th17 cells contribute to allograft injury, suppress Treg cell development and stimulate profibrotic cytokines resulting in progressive declines in renal function and graft loss.18
4 IL-6 REGULATION OF B CELL FUNCTION
We now know that B cells and plasmablasts are key sources of steady-state IL-6 production which increase significantly in autoimmunity and inflammatory conditions.10, 13, 14 B cells express IL-6Rs in late stages of development, which are critical for classic IL-6/IL-6R signaling. Gp-130 is also highly expressed on B cells and plasma cells allowing them to be activated by the trans-signaling pathway mediated by circulating IL-6/IL-6R complexes.10, 14 Plasma cells express high levels of IL-6Rs, which is likely important for maturation and IgG production. B cell development also relies on BLIMP-1 expression, induced by IL-6.19 IL-6 is also required for class switch from IgM->IgG.
In vitro data shows that anti–IL-6/anti–IL-6R treatment exerts a potent inhibition of IgG production in activated B cells.10, 14 See Figure 1A for the importance of IL-6 in B cell development/maturation and the relationship to donor-specific HLA antibody (DSA) development and immune injury to allograft posttransplant.
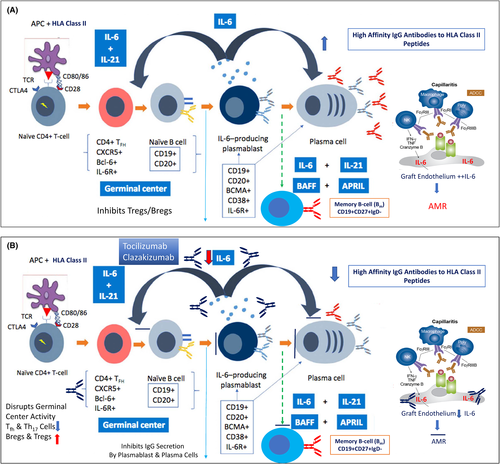
5 IMPORTANCE OF ENDOTHELIAL CELL IL-6 PRODUCTION IN VASCULAR INJURY OF ALLOGRAFTS
A seminal paper by Fogal et al.20 reported on the role of IL-6 in mediating human coronary allograft injury using a SCID mouse model. Here, the investigators showed that perioperative injury (likely ischemic) is known to exacerbate allograft rejection which is primarily mediated by cellular memory in humans. The authors showed that when human coronary arteries are transplanted into an immunodeficient mouse there were significant increases in IL-6 production in the endothelial cells. On day 2 after transplant, the investigators transfused alloreactive human immune cells and noted that by 1 month there was significant T cell expansion (Th17+) and severe intimal proliferation. Importantly, if the investigators treated the animals with anti–human IL-6 after transplantation, and before transfusion of alloreactive immune cells, they showed significant reductions in endothelial cell IL-6 expression, limited T cell infiltration, and significant reductions in intimal proliferation. Also, the investigators showed that anti–IL-6 treatment reduced the Th17+ cell population while increasing the FoxP3 T cells (Treg) that were felt responsible for the lack of progression of the intimal cellulitis and obliterative vasculitis. This paper is important since it shows that IL-6 expression occurs in vascular tissue and is likely responsible for initiation of inflammatory T cell and B cell activation. Inhibiting this expression early limits vascular injury and deviates T cells from Th1+->Treg profiles.
In a recent report, Lion et al.21 reported on the impact of clazakizumab (anti–IL-6, CSL Behring) using an in vitro model of HLA-antibody–mediated endothelial injury. The investigators showed that endothelial cell cultures incubated with PBMCs from unrelated individuals stimulated expansion of Th17 and Th1 cell populations and increased expression of the chemokine CCL2. In addition, evidence for complement activation on the endothelium was detected by measuring C5b-C9 MAC. However, in cultures treated with clazakizumab, the endothelial cells did not activate T effector functions as there was a decrease in Th17 and Th1 cell populations. This was also associated with a decrease in complement activation as reduced C5b-C9 MACs were decreased compared to controls.
Since these two studies were performed in in vitro models and animal models of vascular injury, firm conclusions cannot yet be drawn regarding application to human transplantation. However, the data are exciting and drive our thinking in a different direction away from only looking at immune cell production and modification by IL-6. Here, treatment of the endothelial cells producing IL-6 significantly inhibited endothelial injury by blocking the recruitment of inflammatory T cells and potentially inhibiting complement activation. This and other observations of immunomodulatory actions of anti–IL-6/IL-6R agents has led to a large NIH-sponsored multicenter randomized control trial of tocilizumab in heart transplant recipients aimed at determining if treatment will reduce the incidence of coronary artery vasculopathy and improve outcomes (NCT03644667).
6 EVIDENCE FOR IL-6 MEDIATION OF ALLOGRAFT INJURY
IL-6 has long been recognized as an important cytokine responsible for some aspects of allograft rejection in kidney transplant recipients. In early reports, IL-6 mRNA transcripts were identified in renal allograft biopsies from patients with acute rejection.22 In another report, serum IL-6 levels from 90 kidney allograft recipients, were determined and compared to normal individuals. Here, patients with normal allograft function had low or normal (0–5 pg/ml) levels of IL-6, comparable to normal non-transplant patients. However, patients with allograft rejection had significant elevations compared to normals.23 These studies were performed well before the advent of anti–IL-6/IL-6R therapies, thus were not actionable.
We also evaluated serum IL-6 levels in highly HLA-sensitized patients on chronic dialysis compared to normal individuals using Luminex methodology. (Figure 2A).9 Each circle represents a single patient. Here, IL-6 and CRP levels were significantly elevated in highly HLA-sensitized patients on dialysis compared to normals. Our findings suggested that chronic dialysis is associated with a persistent inflammatory state likely mediated by elevations of IL-6 and CRP that contributes to increased risk for sensitization, ongoing immune stimulation, and potential risk for rejection posttransplant. We also analyzed IL-6 levels in highly HLA-sensitized patients posttransplant comparing patients who had stable renal function to those who experienced AMR, cell-mediated rejection (CMR), and ischemic injury (Figure 2B). Here, we saw elevations of IL-6 in AMR, CMR, AMR+CMR, and in those undergoing biopsy for ischemic injury. We also showed that levels tended to increase prior to biopsy confirmation of disease. Thus, our data did not discriminate between inflammatory or ischemic events, limiting predictive value.
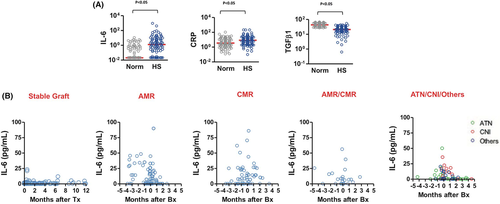
In a recent article, Van Loon et al.24 reported on immune activation events associated with the presence of DSAs and AMR of kidney allografts. The investigators examined a panel of 28 cytokines, chemokines and growth factors and associated them with DSA presence and immune events including AMR in 293 kidney transplant patients. In this study, the presence of elevated levels of IL-6 was the best predictor of DSA+ versus DSA− and AMR+ versus ARM−.
In a report by Chung et al,25 cytokine levels and T cell subsets were analyzed in subjects with chronic allograft dysfunction, stable kidney function and normal individuals. Kidney transplant patients with chronic allograft dysfunction demonstrated high circulating IL-6 and IL-17 levels as well as higher levels of Th17 T cells. The investigators also noted increases in profibrotic gene expression suggesting IL-17 was mediating renal tubular cell injury and chronic fibrosis. The investigators also indicated that treatments aimed at inhibiting the IL-6/Th17 pathway could potentially impact the course of chronic allograft injury.
7 TARGETING IL-6/IL-6R FOR PREVENTION AND TREATMENT OF AMR
As mentioned previously, there are currently no approved therapies for treatment of AMR/cAMR.7 In fact, there is no good evidence to support any approach with existing therapeutics for the treatment of AMR/cAMR. From our own experience,26 in evaluating 80 patients with AMR/cAMR who received standard of care treatment (SOC) including PLEX, IVIg, and rituximab and were followed for 5 years, we found that Banff scores reflecting extensive transplant glomerulopathy (Tg), chronic interstitial fibrosis, and tubular atrophy were associated with higher risk for graft loss. Importantly, our data also indicated a failure of therapeutic efforts to significantly reduce DSA levels was also associated with poor graft outcomes. This data indicated the importance of modulating DSAs for preserving graft function. However, proper evaluation of a recommended therapeutic agent for AMR/cAMR requires a complete assessment of the natural history of patients with ARM/cAMR who received no treatment. In this regard, Irish et al.27 made a significant contribution to our understanding of the magnitude of the problem and what relevant endpoints for clinical trials would look like.
The investigators assessed the relationship between change in estimated glomerular filtration rate (eGFR) following AMR diagnosis and the risk of subsequent death-censored graft failure using a joint modeling framework. A total of 91 patients from 3 participating centers were included in the analysis. Here, 54 patients (59%) met criteria for death-censored graft failure and 62 patients (68%) met all-cause graft failure. Analysis of death-censored graft survival rates at 12, 36, and 60 months after diagnosis of AMR from pooled data of three centers were 88.9%, 58.9%, and 36.4%, respectively. Further analysis indicated a linear trend in eGFR decline. This occurred primarily in the first 12 months post-diagnosis of active AMR (a delta of −9.084 ml/min/1.73 m2 at 1 year). Importantly, the authors showed that an extrapolated 30% improvement in the eGFR slope during the first year after AMR diagnosis would result in a 10% improvement in death-censored graft failure at 5 years. The authors conclude that results from this study would be helpful in informing the design and implementation of clinical trials for therapeutics aimed at treating AMR/cAMR. Indeed, the FDA accepted this data and allowed use of eGFR comparisons as a primary end point in the first multicenter controlled clinical trial of clazakizumab for treatment of cAMR. This is the first trial of an agent for transplantation that was allowed to use a surrogate end point as a primary end point (IMAGINE, NCT03744910).
8 TOCILIZUMAB (ANTI–IL-6R) FOR TREATMENT OF AMR
Certainly, this type of information would be of direct benefit in the generation of trials to assess the efficacy of anti–IL-6/ILR therapies in treatment of AMR/cAMR. In this regard, based on animal data and our initial trial of tocilizumab for desensitization28, 29 we embarked on a single-center, open-label clinical case study of tocilizumab for treatment of cAMR in 2011.
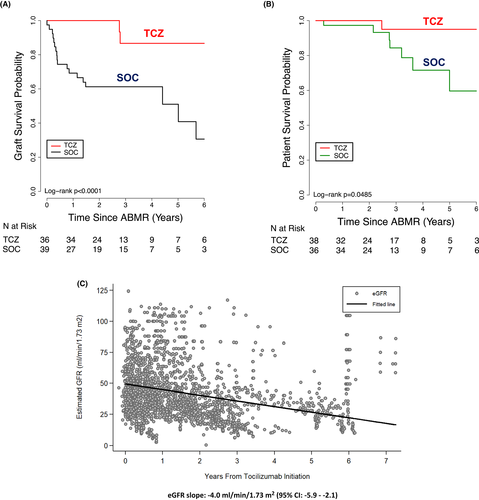
We compared N = 37 patients with cAMR + TG receiving 6–12 months of tocilizumab treatment to a historical cohort of patients treated with PLEX, IVIg, and rituximab (N = 39). To our knowledge, this was the first trial of anti–IL-6/IL-6R for treatment of cAMR. In this long-term case study, we saw a significant benefit in improved graft survival, DSA reduction, and glomerular filtration rate stabilization when data accrued over a 6-year observation period were compared to rituximab + IVIG ± PLEX treatment30 (Figure 3A,B). Analysis of pathologic features of AMR/cAMR also showed improvements from pretreatment biopsies that included, reduced C4d+ scores and reduced glomerulitis and peritubular capillaritis. To put this in context of the expectations outlined in the paper by Irish et al,27 we recently analyzed the individual slope change in eGFR in tocilizumab-treated patients with cAMR using a linear mixed effects model by individual's follow-up time (Figure 3C). Over a 7-year follow-up time, we demonstrated a yearly rate of eGFR decline at ~4 cc/min/1.73 m2. This compares to a 9 cc/min/1.73m2 observed in untreated patients with AMR/cAMR. Importantly, Irish et al.27 demonstrated that a 30% improvement in eGFR projected to a 10% improvement in 5-year graft survival. Although encouraging, it is important to wait for data from the randomized controlled trials for proof of efficacy. Subsequently, there have been many other reports demonstrating benefits in treatment of AMR, cAMR, and CMR with tocilizumab in adult and pediatric kidney transplant patients. Treatment of AMR/cAMR with long-term administration of tocilizumab had been overall safe and without significant side effects in our hands.30-36 A recent report by Cabezas et al.35 evaluated the reported an evaluation of published literature from case studies regarding the efficacy of tocilizumab in treating cAMR. They evaluated 117 cases of cAMR and their conclusion showed that in most cases there was a significant reduction in levels of DSAs and reduced inflammation and microvascular lesions on biopsies after tocilizumab treatment. In addition, stabilization of renal function was observed. Importantly, observed adverse events were “light to moderate,” and mortality was not linked to tocilizumab treatment. The authors also reported that the primary side effects were infection but did not occur more frequently in patients receiving tocilizumab when compared to those receiving standard of care therapy. It is also important to report that a recent article by the same group showed that tocilizumab given as a desensitization agent in 13 HLA-sensitized patients did not alter DSAs and did not result in significant changes in T cell or B cell subsets. However, the authors found that there was a significant reduction in plasmablast after tocilizumab therapy. There was also no impact on circulating IgG, IgM, and IgA levels.37
9 CLAZAKIZUMAB (ANTI–IL-6) FOR PREVENTION AND TREATMENT OF AMR
Clazakizumab (anti–IL-6) (CSL, LLC, King of Prussia, PA) is a humanized IgG1 antibody that binds with high potency and neutralizes human IL-6. Clazakizumab was extensively studied in normal individuals and in patients with rheumatoid arthritis, psoriatic arthritis, Crohn's disease, graft-versus-host disease, and oncology indications. Clazakizumab is not yet FDA approved for any indication. In 2016, we began talks with the manufacturers of clazakizumab to entertain studies in treatment of cAMR (NCT03744910). This study is now complete and initial results have been published.38 For treatment of cAMR, we enrolled 10 patients with established cAMR who received monthly treatments of clazikizumab 25 mg SQ. Importantly, we determined eGFR values at month −24, 0, +12, and +24 of therapy initiation. Findings from this study included demonstrating a stabilization of eGFR after initiation of clazakizumab therapy (eGFR −24 months [52.8 ± 14.8 cc/min], 0 month [38.1 ± 12.2 cc/min], +12 months [41.6 ± 14.2 cc/min], and +24 months [38.1 ± 20.3 cc/min]). We also saw reductions in DSA levels and Banff scores for C4d and g + ptc scores. Two grafts were lost during the study, both in patients who stopped clazakizumab at 6 months and 12 months post-study initiation. Adverse events were minimal during the 2.5-year study period. We also saw a trend to reductions in total IgG levels and an increase in Treg cells at the +24 months evaluation point.
Another important trial of clazakizumab for treatment of cAMR was carried out in Vienna, Austria, and Berlin, Germany (NCT03444103).39 This was a randomized, blinded trial of clazakizumab examining clinical, safety, and biopsy parameters at 1-year posttreatment. Twenty patients were entered and randomized to clazakizumab versus placebo treatment. Important findings from this study can be summarized as follows: As safety was the primary objective of this study, it is important to note that five patients (25%) with clazakizumab treatment developed serious infectious events, and two (10%) developed diverticular disease resulting in trial withdrawal. When compared to placebo, patients receiving clazakizumab showed significant reductions in DSAs and rejection-related gene-expression patterns. Eighteen patients had allograft biopsies after 51 weeks with 7 (38.9%) showing a negative AMR molecular score. For Banff analysis, 27.8% showed complete resolution of C4d staining and 22.2% showed complete resolution of all features of AMR. eGFR decline was significantly slower with clazakizumab compared with placebo (P = .04). Importantly, the investigators allowed patients treated with placebo in the randomized portion of the study to receive clazakizumab. Here the slope of eGFR decline for patients who were switched from placebo to clazakizumab improved and no longer differed significantly from patients initially allocated to clazakizumab.
This study is very important since it showed in a short-term randomized trial that clazakizumab was effective in reducing DSAs and improving eGFRs. The robustness of these observations are supported by the benefits in patients initially treated with placebo who stabilized their eGFRs. Of course, a major concern with this study relates to infection in five patients and bowel perforations in two. Our study did not show this and likely because we reduced standard immunosuppression with MMF after initiation of clazakizumab and entry criteria in our study precluded patients with history of diverticular disease and inflammatory bowel disease to be entered. This, we think, will reduce or eliminate many of these complications. It is also an exclusion criteria for the IMAGINE study.
The final conclusions of the two studies were that data from both would support the further investigation of clazakizumab as an agent to treat cAMR. In this regard, an FDA phase 3 labeling study is now underway (IMAGINE, NCT03744910) to evaluate the efficacy of clazakizumab for treatment of cAMR in kidney transplant patients. The primary end point will be eGFR compared to placebo at 2 years after 200 patients have been entered. Longer term end points will also be assessed. There are currently 105 centers in this study world-wide.
Another study performed by our group involved assessing the efficacy of clazakizumab as a desensitization agent in of 20 highly HLA-sensitized end-stage renal disease patients awaiting kidney transplantation (NCT03380962).40 This was a phase 2 open-label study of clazakizumab for desensitization of highly HLA-sensitized patients awaiting HLAi kidney transplantation. The protocol design included initial treatment of patients with PLEX × 5 sessions followed by IVIg 2 gm/kg (maximum dose: 140 g) at the last PLEX. The patients then received clazakizumab 25 mg SQ monthly up to month 6 and were followed up for up to 9 months to determine if transplantation occurred. Those that received transplants were maintained on clazakizumab posttransplant (25 mg SQ monthly). All transplanted patients were followed up for up to 1-year posttransplant where assessments were done. Patients beyond 1-year posttransplant continued on clazakizumab as well. Outcomes of the protocol showed that most patients show significant reductions in HLA antibodies (both class I and II) at 6 months. This also allowed all 20 patients to be transplanted, some with DSAs at transplant, but at acceptable MFIs. Importantly, those patients who were DSA + at transplant showed abatement over time and at 1 year only one patient had a DSA at 5000 MFI. Five patients experienced rejection episodes that were generally mild to moderate. Importantly, meant eGFR at 1-year posttransplant was 58cc/min/1.73 m2. The overall safety profile was acceptable as we followed the entry criteria and posttransplant immunosuppressive protocols similar to the clazakizumab AMR trial. Of great interest to us was the observation that at 6–12 months posttransplant, we saw significant increases in peripheral blood Treg and Breg cells. Suggesting that long-term treatment with clazakizumab alters the T cell/B cell profiles from inflammatory to regulatory. There was one graft loss during the study. This was from a technical complication at time of surgery. This patient stayed in the study and subsequently was transplanted successfully within the study period.
10 ANTI–IL-6/IL-6R THERAPY: INDUCERS OF TREG AND BREG CELLS?
Is there a role for anti–IL-6/IL-6R therapy in inducing Treg and Breg cells for modification of AMR/cAMR? This, of course, is an important and as yet unanswered question. However, some recent data can shed light on this possibility.
Granofszky et al.41 examined the role of IL-6 inhibition on a mouse model of allogenic bone marrow transplantation. Here, infusion of recipient Tregs induces mixed chimerism and tolerance in an irradiation-free BMT model. Ex vivo generation of donor-specific Tregs (ds-Tregs) is gaining great interest as a possible strategy to limit current immunosuppressive burdens and possibly establish tolerance. However, these protocols are expensive and limited in their ability to generate enough ds-Tregs to provide sustained immune regulation. However, boosting endogenous Tregs with immunomodulatory therapies in vivo is an attractive alternative which would eliminate current limitations associated with performing adoptive cell therapy and could be widely clinical applicable. We and others have shown IL-6 inhibits Treg differentiation and its inhibition with anti–IL-6/IL-6R treatment increases Treg numbers in animal models and human studies in vivo.28, 32, 35, 38 Granofszky et al.41 evaluated treatment with anti–IL-6 instead of Treg transfer to determine if this would lead to multi-lineage chimerism in recipients of fully mismatched BMT. The addition of anti–IL-6 to the treatment regime significantly prolonged donor skin and heart graft survival. Endogenous Fox3+ Tregs expanded in anti–IL-6-treated BMT recipients, while dendritic cell (DC) activation and memory CD8+ T cell development were inhibited. The authors conclude that IL-6 inhibition promotes BM engraftment, Treg expansion and donor graft survival and could provide an alternative to adoptive Treg cell therapy in the clinical setting.
Do Tregs and Breg have a role in prevention of AMR/cAMR? This question was recently addressed by Louis et al.42 The authors addressed our lack of focus on cellular regulatory mechanisms in assessing the pathogenesis of AMR. They indicated that there has been considerable advances in understanding the cellular effector mechanisms responsible for DSA generation leading to AMR; however, no assessments have been made in identification of cellular regulators of such immune responses. To address this, the authors used high dimensional flow cytometry to assess Treg and transitional B cells in 96 kidney transplant recipients. They also used a co-culture assay to address the ability of Treg and Breg cells to suppress antibody responses in vitro. The authors demonstrated that Treg and Breg cells were strong inhibitors of Tfh cell–mediated B cell differentiation to plasmablast and antibody producing plasma cells. Importantly, when assessing patient groups posttransplant, they found that patients with no evidence of DSAs posttransplant had durably expanded Tregs and Bregs. However, patients who developed DSAs and AMR showed profound reductions in Treg and Breg numbers. Of great interest was the loss of T-bet+CXCR5+Treg+ and T-bet+CD21− transitional B cell clusters in patients with AMR which correlated with inflammatory DSA responses, extensive microvascular inflammation and higher rates of kidney allograft loss. The authors identified Treg and Breg subsets that appear to be important in prevention and/or control of allosensitization and evolution to AMR/cAMR.
Taken together, the data seen in human kidney transplant patients treated with tocilizumab or clazakizumab, with one exception,37 demonstrate increased circulating Treg cells with reduction in Tfh and Th17 effectors and improvements in biopsy findings for patients with CMR, AMR, and cAMR. Our data from the study of clazakizumab in highly HLA sensitized patients where the drug was given after transplant for up to 2 years showed significant expansion of Treg and Breg cells with low inflammation scores on protocol biopsies and significant reductions in existing DSAs and no dn-DSAs in 20 patients followed up for 1-year posttransplant. Here, there may be a consideration of use of anti–IL-6 therapies as a standard posttransplant protocol, especially in highly HLA-sensitized patients. However, this should be evaluated in controlled trials, especially for the ability to expand Treg/Breg cells in vivo. The potential sites of immunomodulator actions of anti–IL-6/IL-6R are summarized in Figure 1B.
SUMMARY
IL-6 is a pleiotropic cytokine important in both innate and adaptive immune responses. The unique signaling pathways (classic, trans-signaling and trans-activation) allow IL-6 to rapidly activate any cell expressing gp-130 and induce inflammatory events. The recognition of the importance of IL-6 expression in endothelial cells and their role in stimulating inflammatory T cell/B cell activation and intimal proliferation of vasculature open and new vista in explaining the pathogenesis of vascular injury due to antibodies. Importantly, new studies of anti–IL-6 therapy (clazakizumab, IMAGINE) are now underway to determine efficacy in treatment of cAMR. The rational for this study is supported by many smaller clinical case studies and observations in kidney transplant patients with AMR/cAMR who received treatment with tocilizumab. Finally, the observations regarding the importance of maintaining healthy Treg/Breg profiles for prevention of dn-DSA and AMR/cAMR development are intriguing, especially with data showing that anti–IL-6/IL-6R treatment can induce Treg and Breg cells in vivo. Ongoing studies of IL-6 biology in transplantation will continue to contribute to a better understand the pathogenesis of AMR/cAMR and develop rational therapeutic approaches. Finally, it is critical for the transplant community to finish the IMAGINE study to determine safety and efficacy in a large and rigorous randomized controlled trial.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Jordan discloses he has received grants and consultation fees from CSL Behring. He also has stock options and patents licensed by CSL Behring.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.