Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge
Abstract
The SARS-CoV-2 pandemic continues to place a substantial burden on healthcare systems. Outpatient therapies for mild-to-moderate disease have reduced hospitalizations and deaths in clinical trials, but the real-world effectiveness of monoclonal antibodies and oral antiviral agents in solid organ transplant recipients (SOTR) with coronavirus disease-2019 (COVID-19) is largely uncharacterized. We conducted a single-center, retrospective review of 122 SOTR diagnosed with COVID-19 in the outpatient setting during the Omicron surge to address this knowledge gap. The mean age was 54 years, 57% were males, and 67% were kidney transplant recipients. The mean time from transplant to COVID-19 diagnosis was 75 months. Forty-nine (40%) received molnupiravir, 24 (20%) received sotrovimab, and 1 (0.8%) received nirmatrelvir/ritonavir. No outpatient therapy was administered in 48 (39%). All 122 SOTR had >30 days follow-up. Rates of hospitalization within 30 days of initiating therapy for molnupiravir, nirmatrelvir/ritonavir, and sotrovimab were 16% (8/49), 0% (0/1), and 8% (2/24), respectively, compared to 27% (13/48) in patients without outpatient therapy. There were no deaths in those who received any therapy versus 3 (6%) deaths in patients without outpatient therapy (p = .002). Overall, our experience suggests a role for monoclonal antibodies and oral antiviral agents in reducing COVID-19-related morbidity and mortality in SOTR.
Abbreviations
-
- ED
-
- emergency department
-
- ICU
-
- intensive care unit
-
- MMF
-
- mycophenolate mofetil
-
- RRR
-
- relative risk reduction
-
- SOTR
-
- solid organ transplant recipient
-
- YNHHS
-
- Yale New Haven Health System
1 INTRODUCTION
With over 980 000 deaths and 80 million cumulative cases in the United States as of April 24, 2022,1 the SARS-CoV-2 pandemic places a substantial burden on healthcare delivery systems, and the Omicron variant ensures the continued relevance of coronavirus disease-2019 (COVID-19).2 Vaccination, in combination with other mitigation efforts, is a key strategy to reduce infection rates. Nonetheless, persons with immune dysfunction are at heightened risk for breakthrough infections after vaccination,3 and vaccinated solid organ transplant recipients (SOTR) remain at risk for severe disease.4
In this setting, outpatient therapies for mild-to-moderate disease are vital for reducing the risk of disease progression and preventing both COVID-19-related hospitalizations and deaths.5 Monoclonal antibodies (e.g., sotrovimab6) and oral antiviral agents (e.g., molnupiravir7 and nirmatrelvir/ritonavir8) have proven efficacious in preventing hospitalization and death in at-risk adults in clinical trials, but the real-world effectiveness of these therapies for COVID-19 in SOTR during the Omicron era is largely uncharacterized. To address this knowledge gap, we conducted a retrospective review of our transplant center's experience with outpatient therapies in SOTR during the recent Omicron surge.
2 MATERIALS AND METHODS
2.1 Study population
Our study population consisted of all adult (age ≥ 18 years) SOTR in the Yale-New Haven Health System (YNHHS) with a positive SARS-CoV-2 antigen or polymerase chain reaction test on or after January 1, 2022. Because sequencing results are not uniformly available to confirm variants' identities, we selected this start date to ensure that the majority of cases in the present study were caused by Omicron (89.1% of national cases between December 26, 2021 and January 1, 20221 and estimated 98% of Connecticut cases on January 1, 20229). We excluded SOTR admitted or already hospitalized at the same timepoint as their COVID-19 diagnoses and those with documented graft failure at the time of diagnosis. Resultantly, the present sample represents mild-to-moderate COVID-19 in an outpatient population. Our center does not perform lung transplantation, and no lung transplant recipients were included in the present study. The Yale University Institutional Review Board reviewed and approved our study protocol (#2000027876).
2.2 Data collection and outcomes
For included patients, we recorded age, sex, race, ethnicity, transplant type, time since transplantation, maintenance immunosuppression, SARS-CoV-2 vaccination status, history of acute rejection ≤3 months before COVID-19 diagnosis, and history of prior SARS-CoV-2 infection ≥90 days before the current episode. We defined breakthrough infection as SARS-CoV-2 infection >14 days after completion of recommended vaccine series. For SOTR ≤12 months since transplantation, we also recorded the induction regimen. YNHHS created a clinical pathway for managing outpatient COVID-19 patients, which included prioritizing outpatient therapy for patients at risk for progression to severe COVID-19 (Figure S1). As of February 10, 2022, the clinical pathway favored the use of sotrovimab over molnupiravir for severely immunosuppressed patients. It is important to note that an earlier version of the clinical pathway (i.e., prior to February 10, 2022) was utilized during most of our study period and it did not explicitly specify the preference for sotrovimab over molnupiravir for severely immunosuppressed patients. If the date of symptom onset could not be confirmed with accuracy at our center, the first positive SARS-CoV-2 test was designated as day 1 of symptom onset, and the pathway was followed accordingly.
At our center, SOTR taking antimetabolites (i.e., mycophenolate mofetil [MMF], mycophenolate sodium, or azathioprine) are routinely instructed to hold the medication for 7–14 days at the time of COVID-19 diagnosis; however, individual providers may hold other medications or continue maintenance immunosuppression if clinically indicated (e.g., patient with multiple prior episodes of rejection and minimally symptomatic COVID-19). We recorded all changes to immunosuppressive medications for patients included in our study. After COVID-19 diagnoses, per the clinical pathway, we recorded whether patients received sotrovimab, molnupiravir, nirmatrelvir/ritonavir, or no outpatient therapy. When possible, adherence to the therapies' suggested administration schedules was recorded for patients who received a monoclonal antibody or oral antiviral agents. Adverse events were also recorded when documented by treating clinicians. For patients who did not receive outpatient therapy for COVID-19, we recorded the documented reason when available in the electronic medical record.
We recorded emergency department (ED) visits, hospital admissions, intensive care unit (ICU) admissions, mechanical ventilation, and all-cause mortality to evaluate clinical outcomes within 30 days of initiating therapy. For each case, we recorded whether ED visits, hospital admissions, and ICU admissions were attributable to COVID-19. For SOTR with COVID-19 who did not receive COVID-19-related outpatient therapy during our study period, these data were recorded within 30 days of diagnosis. Biopsy-proven or clinically significant graft rejection (i.e., requiring anti-rejection treatment without any biopsy) episodes within 30 days of either therapy initiation or COVID-19 diagnosis were also recorded when present.
2.3 Statistical analysis
Demographic and clinical data were summarized with descriptive statistics. Means and standard deviations were determined for continuous variables and counts and percentages for categorical variables. Relative risk reduction (RRR) calculations were calculated by comparing outcome rates in individual treatment groups versus outcome rates in the no outpatient therapy group. Chi-square tests were used to compare categorical variables and performed using Microsoft Excel. Kruskal-Wallis tests were performed to compare numerical and ordinal variables using R version 4.0.2. A p-value less than .05 was considered statistically significant.
3 RESULTS
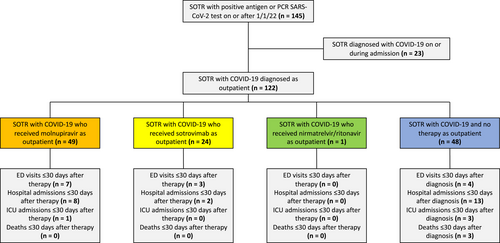
Between January 1, 2022, and February 16, 2022, we identified 145 cases of COVID-19 in SOTR who received care in our healthcare system (Figure 1). Twenty-three cases were diagnosed upon admission or hospitalization and were resultantly excluded. Table 1 summarizes the demographic and clinical characteristics of the 122 cases included in our study. The mean age was 54 years (standard deviation [SD] ± 14), the mean length of time since transplantation was 75 months (SD ± 69), 57% (70/122) were males, and 67% (82/122) were kidney transplant recipients. Twelve (12/122; 10%) patients had a history of SARS-CoV-2 infection ≥90 days prior, and 87% (106/122) had received ≥1 dose of a SARS-CoV-2 vaccine. Fifty-eight (58/122; 48%) patients had received ≥3 doses of an mRNA vaccine series or two doses of Ad26.COV2.S (Johnson and Johnson). Only one patient (1/122; 0.8%) had received 4 doses of an mRNA vaccine. In total, 56/122 (46%) were breakthrough infections. Demographic and clinical characteristics of the three groups (molnupiravir, sotrovimab, and no therapy) were compared; nirmatrelvir/ritonavir was excluded due to n = 1. The only significant variation was the proportion of SOTR who received ≥3 doses of an mRNA vaccine or two doses of Ad26.COV2.S (p = .04).
| Patient characteristics | Molnupiravir (n = 49) | Nirmatrelvir/ritonavir (n = 1) | Sotrovimab (n = 24) | No therapy (n = 48) | Total (n = 122) | p value |
|---|---|---|---|---|---|---|
| Age (years, mean ± SD) | 55 ± 14 | 51 | 57 ± 12 | 51 ± 15 | 54 ± 14 | .49a |
| Male (No., %) | 25 (51%) | 0 | 14 (58%) | 31 (65%) | 70 (57%) | .40b |
| Race | ||||||
| Black (No., %) | 6 (12%) | 0 | 6 (25%) | 14 (29%) | 26 (21%) | .11b |
| White (No., %) | 31 (63%) | 1 | 12 (50%) | 23 (48%) | 67 (55%) | .28b |
| Other (No., %) | 8 (16%) | 0 | 5 (21%) | 9 (19%) | 22 (18%) | .89b |
| Unknown (No., %) | 4 (8%) | 0 | 1 (4%) | 2 (4%) | 7 (6%) | .65b |
| Latinx (No., %) | 10 (20%) | 1 | 5 (21%) | 12 (2 5%) | 28 (23%) | .85b |
| Transplant type | ||||||
| Heart | 9 (18%) | 0 | 1 (4%) | 3 (6%) | 13 (11%) | .08b |
| Kidney | 32 (65%) | 1 | 14 (58%) | 35 (73%) | 82 (67%) | .44b |
| Kidney-heart | 0 | 0 | 1 (4%) | 0 | 1 (0.8%) | - |
| Kidney-liver | 1 (2%) | 0 | 2 (8%) | 1 (2%) | 4 (3%) | .31b |
| Kidney-pancreas | 0 | 0 | 0 | 1 (2%) | 1 (0.8%) | - |
| Liver | 7 (14%) | 0 | 6 (25%) | 8 (17%) | 21 (17%) | .52b |
| Time since transplantation (months, mean ± SD) | 62 ± 66 | 191 | 93 ± 71 | 76 ± 68 | 75 ± 69 | .09a |
| Maintenance immunosuppression | ||||||
| Azathioprine (No., %) | 1 (2%) | 0 | 2 (8%) | 4 (8%) | 7 (6%) | .35b |
| Belatacept (No., %) | 9 (18%) | 1 | 4 (17%) | 12 (25%) | 26 (21%) | .62b |
| Cyclosporine (No., %) | 3 (6%) | 0 | 1 (4%) | 1 (2%) | 5 (4%) | .61b |
| Everolimus (No., %) | 1 (2%) | 0 | 0 | 0 | 1 (0.8%) | - |
| Mycophenolate sodium or mycophenolate mofetil (No., %) | 38 (78%) | 1 | 14 (58%) | 36 (75%) | 89 (73%) | .20b |
| Prednisone (No., %) | 34 (69%) | 1 | 17 (71%) | 37 (77%) | 89 (73%) | .68b |
| Sirolimus (No., %) | 2 (4%) | 0 | 1 (4%) | 1 (2%) | 4 (3%) | .83b |
| Tacrolimus (No., %) | 35 (71%) | 0 | 18 (75%) | 34 (71%) | 87 (71%) | .93b |
| Receipt of ≥1 dose SARS-CoV-2 vaccine (No., %) | 45 (92%) | 1 | 21 (88%) | 39 (81%) | 106 (87%) | .30b |
| Receipt of ≥3 doses of an mRNA vaccine series or 2 doses of Ad26.COV2.S (Johnson and Johnson) (No., %) | 30 (61%) | 1 | 9 (38%) | 18 (38%) | 58 (48%) | .04b,c |
| Current episode is reinfection (No., %) | 4 (8%) | 0 | 0 | 8 (17%) | 12 (10%) | - |
- a Kruskal-Wallis test.
- b Chi-square test.
- c p value <.05 was considered statistically significant.
In total, 8% (10/122; 5 kidneys, 3 hearts, 2 livers) underwent transplantation ≤12 months before their current COVID-19 diagnoses. Alemtuzumab with methylprednisolone was the most common induction regimen (4/10; 40%), administered to 4 kidney transplant recipients. No patients were treated for an acute rejection episode ≤3 months before COVID-19 diagnosis. In total, 63/89 (71%) patients taking mycophenolate sodium or MMF were instructed to hold the medication. One kidney transplant recipient <1 year since transplantation had the MMF dose reduced by half. One kidney transplant recipient was instructed to hold everolimus. Of the 7 SOTR on azathioprine, 3 (43%) were instructed to hold the medication at the time of COVID-19 diagnosis.
In total, 49/122 (40%) patients received molnupiravir, 24/122 (20%) received sotrovimab, and 1/122 (0.8%) received nirmatrelvir/ritonavir. No outpatient therapy was administered in 48/122 cases (39%). For patients who did not receive outpatient therapy, 26/48 (54%) had no documentation of explicit reason why outpatient therapy was not administered, 14/48 (29%) had symptoms for >7 days by the time they were evaluated for therapy, 5/48 (10%) were asymptomatic, 2/48 (4%) were referred for therapy but did not receive it, and 1/48 (2%) filled the prescription for molnupiravir but declined to use it.
Among patients receiving molnupiravir, 17/49 (35%) reported taking the full intended course; however, 29/49 (59%) had no detailed documentation concerning adherence. Four patients who received molnupiravir experienced adverse events (rash, n = 2; gastrointestinal complaints, n = 2). The single patient who received nirmatrelvir/ritonavir reported full adherence, and all patients who received sotrovimab tolerated the infusion. No adverse events were documented for patients receiving nirmatrelvir/ritonavir or sotrovimab. The patient who received nirmatrelvir/ritonavir was on belatacept, MMF, and prednisone as maintenance immunosuppression and was instructed to hold MMF for 10 days in the setting of COVID-19.
At the time of review, >30 days of follow-up were available for all 122 patients. For patients who received molnupiravir, 7/49 (14%) had ED visits and 8/49 (16%) had hospital admissions ≤30 days after initiating therapy, with 1/7 visits and 7/8 admissions related to COVID-19. For sotrovimab, 3/24 (13%) patients had ED visits and 2/24 (8%) had hospital admissions, with 2/3 visits and 1/2 admissions related to COVID-19. No significant differences in ED visits or hospitalizations between patients receiving sotrovimab or molnupiravir were identified (Table S1). The single patient who received nirmatrelvir/ritonavir did not have an ED visit or hospital admission ≤30 days after initiating therapy. For the 48 patients who did not receive outpatient therapies, 4/48 (8%) had ED visits and 13/48 (27%) had hospital admissions ≤30 days after diagnosis, with 2/4 visits and 7/13 admissions attributable to COVID-19. The difference in the percentage of hospital admissions between patients who received therapy (n = 10/74, 14%) and those who did not (n = 13/48, 27%) trended toward significance (p = .06).
In total, 4/122 (3%) patients had ICU admissions ≤30 days after diagnosis or therapy initiation, and three of four required mechanical ventilation. Two of four ICU admissions were attributable to COVID-19. One of four patients admitted to the ICU received molnupiravir, and the remaining three had not received outpatient therapies. Overall, death occurred in 3/122 (2%) patients, and none had received outpatient therapy. The difference in the percentage of death within 30 days between patients who received therapy (n = 0/74, 0%) and those who did not (n = 3/48, 6%) was statistically significant (p = .002). Compared to no therapy, the RRRs for hospitalization or death within 30 days were 44% and 71% for molnupiravir and sotrovimab, respectively. The difference in the percentage of hospitalization or death within 30 days between sotrovimab (n = 2/24, 8%) and no therapy (n = 14/48, 29%) was statistically significant (p = .045). In terms of rejection episodes, one patient (0.8%) experienced clinically significant rejection <30 days after COVID-19 diagnosis and received thymoglobulin and pulse dose methylprednisolone.
4 DISCUSSION
Real-world data on monoclonal antibodies and oral antiviral agents in SOTR with mild-to-moderate COVID-19 are limited.10-12 We report 30-day hospitalization rates after initiation of molnupiravir and sotrovimab as 16% (8/49) and 8% (2/24), respectively, versus 27% (13/48) in patients without outpatient therapy. One patient received nirmatrelvir/ritonavir and did not experience hospitalization or death ≤30 days after initiating therapy. No deaths occurred in patients who received outpatient therapies, and three deaths occurred in patients without outpatient therapy. Overall, our single-center experience suggests the role of monoclonal antibodies and oral antiviral agents in reducing morbidity and mortality of COVID-19 in SOTR.
To our knowledge, we present the largest real-world dataset on molnupiravir use in SOTR. In the MOVe-OUT phase 3 trial, the all randomized, intention-to-treat population who had been hospitalized or died at day 29 was lower in the molnupiravir group versus placebo (6.8% vs. 9.7%; −3% difference, 95% C.I. -5.9 to −0.1).7 The same trial's time-to-event analysis demonstrated a roughly 30% lower rate of hospitalization or death at day 29 for patients who received molnupiravir versus placebo.7 In our experience, the percentage of patients with hospitalization ≤30 days after initiating molnupiravir was 16% (8/49) versus 27% (13/48) in those without an outpatient therapy, and no deaths occurred in the molnupiravir group. The observed outcomes may have been influenced by the higher proportion of patients in the molnupiravir group who had received ≥3 doses of an mRNA vaccine or two doses of Ad26.COV2.S. Patients in the current study tolerated molnupiravir with few short-term adverse events.
These data suggest a role for molnupiravir use in SOTR with mild-to-moderate COVID-19. However, it is important to note that nirmatrelvir/ritonavir performed better in a clinical trial involving at-risk adult patients, with an 89% relative risk reduction of hospitalization and all-cause mortality at day 28 compared to placebo.8 There is also concern that molnupiravir's use may lead to further mutagenesis in circulating variants.13, 14 Despite these drawbacks, it may be a viable option in SOTR with limited access to monoclonal antibodies and contraindications to nirmatrelvir/ritonavir due to significant drug–drug interactions with immunosuppressive agents.
It is important to note that 46% of our study population had a breakthrough infection, and these data suggest the continued utility of antiviral agents in hosts with breakthrough SARS-CoV-2 infection.
Similar to our experience with molnupiravir use, patients who received sotrovimab had a reduced percentage of hospitalizations and deaths relative to patients who did not receive outpatient therapy. Prior to the emergence of Omicron, other real-world studies have also demonstrated the effectiveness of monoclonal antibodies (e.g., bamlanivimab or casirivimab-imdevimab) in preventing COVID-19 disease progression in SOTR.10, 11 Our results are consistent with these observations10, 11 and the phase 3 trial showing a relative risk reduction of hospitalization or death for at-risk adults who received sotrovimab.6 Our limited number of patients who received sotrovimab (n = 24) precludes an in-depth comparison to molnupiravir; however, we did not identify significant differences in 30-day outcomes between patients who received molnupiravir versus sotrovimab. Larger datasets may confirm or overturn this observation, but it is important to note that sotrovimab is no longer recommended for the treatment of COVID-19.15 On April 5, 2022, its emergency use authorization was withdrawn by the United States Food and Drug Administration due to lack of efficacy against the circulating BA.2 sub-variant.15, 16
Due to limited use at our center, we are unable to comment on the safety or effectiveness of nirmatrelvir/ritonavir. There are data to support the feasibility of using nirmatrelvir/ritonavir in SOTR,12 but widespread adoption of nirmatrelvir/ritonavir in this population may prove challenging and will require systematic approaches to adjusting immunosuppression to avoid drug–drug interactions.17 During the Omicron surge, our center did not have a standardized protocol to ensure its safe administration in patients taking contraindicated medications (e.g., tacrolimus). Our center was also unable to operationalize the administration of outpatient remdesivir, and we instead offered sotrovimab. A 3-day course of remdesivir has been demonstrated to result in an 87% lower risk of hospitalization or death in the placebo-controlled, randomized PINETREE trial,18 and real-world data on its use for COVID-19 in outpatient SOTR are needed.
Despite its strengths, our study is subject to several limitations. First, our dataset's small size, retrospective approach, and single-center nature limit the ability to fully characterize differences in outcomes between outpatient therapies. Specifically, our outcomes do not account for differences in severity of underlying comorbidities between SOTR. We also cannot comment on the effectiveness of nirmatrelvir/ritonavir due to the low number of patients in our study. Additionally, our data may not be generalizable to lung transplant recipients, and our study population was largely composed of kidney transplant recipients (67%). Second, we report limited follow-up time, and morbidity and mortality of COVID-19 beyond 30 days are not captured in the present study. Third, we do not have complete information on patient adherence to molnupiravir, and the observed effectiveness may differ based on adherence to the intended 5-day course.
Our study is also subject to selection bias because treatment options for SOTR were contingent on comorbidities enumerated in our center's treatment algorithm, and certain treatments (e.g., nirmatrelvir/ritonavir) were not available for all SOTR. Additionally, we are unable to provide the number of SOTR who sought care at outside institutions, and these cases are not captured in the present study. Finally, the duration of symptoms was not available for all cases, and management decisions based on the YNHHS clinical pathway used the date of positive SARS-CoV-2 test when the date of symptom onset was unknown. As a result, it is conceivable that not all patients had symptom durations within the suggested time frames for treatment initiation.
Overall, our single-center experience suggests that outpatient therapies have a role in the management of mild-to-moderate COVID-19 in SOTR, a population at risk for severe disease.4 Although our data are limited in number and subject to selection bias, patients who received molnupiravir and sotrovimab had reduced hospitalization and death during the Omicron surge. Given the continued importance of the SARS-CoV-2 pandemic,1 wider accessibility of outpatient therapies is needed, as are well-powered, real-world studies in at-risk populations.
ACKNOWLEDGMENTS
No external funding source supported this study.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.