Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation
Abstract
Cancer is the leading cause of death after liver transplantation (LT). This multicenter case–control nested study aimed to evaluate the effect of maintenance immunosuppression on post-LT malignancy. The eligible cohort included 2495 LT patients who received tacrolimus-based immunosuppression. After 13 922 person/years follow-up, 425 patients (19.7%) developed malignancy (cases) and were matched with 425 controls by propensity score based on age, gender, smoking habit, etiology of liver disease, and hepatocellular carcinoma (HCC) before LT. The independent predictors of post-LT malignancy were older age (HR = 1.06 [95% CI 1.05–1.07]; p < .001), male sex (HR = 1.50 [95% CI 1.14–1.99]), smoking habit (HR = 1.96 [95% CI 1.42–2.66]), and alcoholic liver disease (HR = 1.53 [95% CI 1.19–1.97]). In selected cases and controls (n = 850), the immunosuppression protocol was similar (p = .51). An increased cumulative exposure to tacrolimus (CET), calculated by the area under curve of trough concentrations, was the only immunosuppression-related predictor of post-LT malignancy after controlling for clinical features and baseline HCC (CET at 3 months p = .001 and CET at 12 months p = .004). This effect was consistent for de novo malignancy (after excluding HCC recurrence) and for internal neoplasms (after excluding non-melanoma skin cancer). Therefore, tacrolimus minimization, as monitored by CET, is the key to modulate immunosuppression in order to prevent cancer after LT.
Abbreviations
-
- CET
-
- cumulative exposure to tacrolimus
-
- CI
-
- confidence interval
-
- HCC
-
- hepatocellular carcinoma
-
- HR
-
- hazard ratio
-
- IQR
-
- interquartile range
-
- LT
-
- liver transplantation
-
- mTOR
-
- mammalian target of rapamycin.
-
- OR
-
- odds ratio
1 INTRODUCTION
Long-term survival after liver transplantation (LT) has remained stagnant over the last decades despite the advances in surgical technique and perioperative care.1 Posttransplant malignancy, either de novo or recurred, is the leading cause of mortality after LT and accounts for 41% of premature deaths in this population.2 Indeed, the risk of cancer is two- to threefold higher in LT patients compared to age- and gender-matched general population.3-6 After LT, de novo tumors may be also biologically more aggressive and they are associated with a shorter survival than that observed in the general population.3
There is a growing evidence suggesting a potential link between chronic exposure to immunosuppressive drugs and oncogenesis. First, the evasion of the immune system is one of the critical hallmarks of cancer.7 The activation of the immune system is paramount to detect and destroy nascent tumors, a mechanism known as immune surveillance. Second, the tumors with the highest standardized incidence ratio among LT patients are those associated with viral infections (Kaposi sarcoma, nasopharyngeal carcinoma, cervical, and vulvar cancer), located in exposed areas (skin cancer), or originated in the immune system itself (lymphoproliferative disorders).8 Noteworthy, this particular tumor profile is identical between solid organ transplant and patients infected with the human immunodeficiency virus,9 being both populations characterized by an impairment of T cell–mediated immune response.
Despite these evidences, there is uncertainty regarding the potential pro-oncogenic effect of a particular immunosuppressive drug, or regarding the dose threshold beyond which the risk of cancer increases, or concerning the theoretical synergistic effect when combinations of several immunosuppressive drugs are used. Therefore, tailoring immunosuppression protocols to prevent cancer after LT is particularly challenging. The present study aimed to determine the risk factors of cancer after LT with a special focus on the impact of chronic exposure to immunosuppressive drugs.
2 MATERIALS AND METHODS
This is a multicenter case–control nested study with a quasi-cohort approach involving 16 LT institutions in Spain, which accounts for 67% of the Spanish LT activity, and with a reference population of approximately 31 million people. The quasi-cohort design was opted due to its efficiency to control bias in pharmacoepidemiology where risk exposures and outcomes are time dependent.10 The study complies with the principles contained in the Declaration of Helsinki and the European Union regulation 2016/679, and it was approved by the research ethics committee of Córdoba, Spain (code OBS-IMCA_2018, ref. 4093).
2.1 Study population
A consecutive cohort of patients who underwent LT from January 2010 to December 2015 and received tacrolimus-based immunosuppression at 16 transplant institutions composed the eligible cohort. Exclusion criteria were as follows: age <18 years old, re-transplantation, combined organ transplantation, donor with incidental malignancy, human immunodeficiency virus infection, and death within the first year after LT. Patients were followed until December, 2019. Patients developing malignancy after LT, either de novo or recurred, were identified in the eligible cohort and formed the group of cases. Controls were selected among patients who, after an identical follow-up period as cases, had not developed malignancy. The quasi-cohort approach ensured that person-years were comparable between the groups of cases and controls and allowed for computing hazard ratios (HRs).10 Cases and controls were matched according to a center-specific propensity score based on potential clinical risks factors of cancer (age, gender, smoking habit, alcoholic liver disease, hepatitis B status, hepatitis C status, and hepatocellular carcinoma [HCC] before LT). For each case, one control was selected within the same LT center (ratio 1:1) according to the nearest neighbor approach. This strategy aimed for a homogeneous distribution of potential clinical risk factors of cancer other than immunosuppression between cases and controls.
2.2 Evaluation of immunosuppression and outcomes
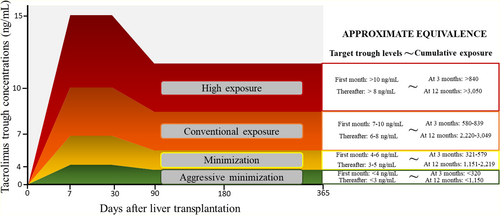
The exposure to immunosuppressive drugs within the first 12 months after LT was recorded in selected cases and controls. All available determinations of trough concentrations of tacrolimus were retrieved for each patient. Cumulative exposure to tacrolimus (CET) was calculated by the area under curve of trough concentrations, which has been previously associated with renal impairment after LT.11 Briefly, all measurements of trough concentrations of tacrolimus for a certain patient were plotted in a time-dependent graph. Each determination was joined with the next delineating a curve. Finally, the area under curve of trough concentrations was calculated by the Wagner–Nelson equation. An online calculator of CET is available at Web CET (imibic.org). Categorization of CET was performed according to previously defined thresholds11 in aggressive minimization, minimization, conventional exposure, and high exposure. Thresholds and approximate equivalence between target trough concentrations and CET are shown in Figure 1. The combination of tacrolimus with other immunosuppressive drugs was recorded, including the length of exposure within the first year. All patients received tapering corticosteroids, which were withdrawn between the third and the sixth month after LT, except for patients with autoimmune liver disease, in whom these were kept at the lowest dose tolerated in the long term. The use of induction therapy and the indication of boluses of corticosteroids to treat rejection episodes were registered.
The main outcome of the study was the development of any type of cancer after LT, including recurrence of HCC given its strong prognostic impact. Secondary outcomes were de novo malignancy (excluding HCC recurrence) and internal neoplasms (excluding non-melanoma skin cancer). For those patients experiencing more than one malignancy, only the first tumor diagnosed was computed. Tumor stage at diagnosis, oncologic therapy, and mortality were recorded.
2.3 Sample size calculation
- Prevalence of high exposure to tacrolimus in the group of cases: 26%.
- Prevalence of high exposure to tacrolimus in the group of controls: 16%.
- Statistical power: 80%.
- Alpha error: 5%.
- Insufficient data for analysis or lost in follow-up: 10%.
Under these premises, the sample size required would be n = 572, comprising 286 patients with post-LT malignancy and 286 matched controls.
2.4 Statistical analysis
Continuous variables were presented as mean and standard deviation, excepting for those with asymmetrical distribution in which median and interquartile range (IQR) were used. Categorical variables were displayed as percentages in frequency tables. Appropriate hypothesis contrast tests were used according to the type of variables involved in the analysis. Kaplan–Meier curves and log-rank test were used for survival analysis. Risk factors of post-LT malignancy were investigated using univariate and multivariate Cox regression in a two-step process. First, a model composed of demographic and clinical features was computed in the whole eligible cohort. Variables with a p < .40 in the univariate analysis entered the initial multivariate model. Non-significant co-variates were removed in a backward stepwise process. All potential interactions between covariates were tested and kept in the final model if they reached statistical significance. A second model evaluating immunosuppression-related variables on post-LT malignancy was investigated in matched cases and controls (study cohort). In this analysis, clinical predictors contained in the propensity score and immunosuppression-related variables were kept in the final model to control for possible residual bias irrespective of their p value. Clinically meaningful interactions were kept if their removal resulted in a modification of the beta coefficient of the involved variables >20%. The individual effect of CET in each type of post-LT malignancy was evaluated by univariate logistic regression due to the limited incidence of some tumors. All analyses were performed by SPSS 27.0 (IBM) and R v3.6.3 (RStudio Inc.). Every hypothesis tested was two-tailed and considered significant if p < .05.
3 RESULTS
3.1 Risk factors of cancer in the eligible cohort
The eligible cohort comprised 2495 patients (54.91 ± 9.08 years old, 22.5% women). Major etiologies of liver disease were alcoholic liver disease (52.2%), chronic hepatitis C (35.9%), and chronic hepatitis B (6.8%). The indication for LT was HCC in 1023 patients (41%). Nine hundred forty-one patients (37.7%) were active smokers at inclusion in the waiting list for LT and 1244 patients (61.1%) were past smokers or non-smokers. Data on smoking habit could not be reliably obtained in 30 patients (1.2%). After a median follow-up of 65 months after LT (IQR 49–87), including 13 922 person/years, 491 patients (19.7%) developed cancer, yielding an incidence rate of 3.5/100 person-years for all type of tumors, and 2.6/100 persons-years for de novo malignancy. The incidence of cancer remained unchanged over the study period (Figure S1). Types of malignancy, in order of frequency, were as follows: HCC recurrence (n = 118; 24%), non-melanoma skin cancer (n = 79; 16.1%), lung cancer (n = 67; 13.7%), head and neck tumors (n = 62; 12.6%), prostate cancer (n = 26; 5.3%), lymphoproliferative disorders (n = 24; 4.9%), urinary tract cancer (n = 22; 4.5%), esophageal/gastric cancer (n = 20; 4.1%), colorectal cancer (n = 17; 3.5%), pancreatic cancer (n = 10; 2%), breast cancer (n = 5; 1%), gynecological cancer (n = 4; 0.8%), Kaposi's sarcoma (n = 4; 0.8%), melanoma (n = 4; 0.8%), and sarcomas or metastases of unknown origin (n = 29; 5.9%).
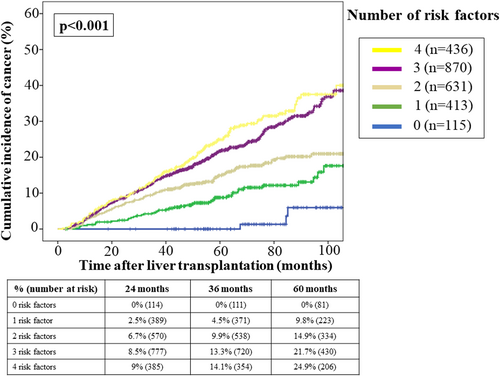
The univariate and multivariate Cox regression analyses (Table 1) identified the following clinical-independent predictors of posttransplant malignancy after controlling for baseline HCC: age at transplantation (HR = 1.06 [95% CI 1.05–1.07]; p < .001), male sex (HR = 1.50 [95% CI 1.14–1.99]; p = .004), active smoking habit at waitlist inclusion (HR = 1.96 [95% CI 1.45–2.66]; p < .001), and alcoholic liver disease (HR = 1.53 [95% CI 1.19–1.97]; p = .001). There was a significant interaction between alcoholic liver disease and hepatitis C, which was included in the final model. Age at LT increased the risk of cancer and this effect was particularly evident for patients over 50 years old (Figure S2). The accumulation of clinical predictors resulted in a progressive increase of the 5-year cumulative incidence of cancer after LT (Figure 2): 0% for patients without risk factors, 12.5% for patients with 1–2 risk factors, and 22.8% for patients with 3–4 risk factors.
| Variables | Univariate analysis |
Multivariate analysis (initial model) |
Multivariate analysis (final model) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.06 (1.05–1.07) | <.001 | 1.06 (1.04–1.07) | <.001 | 1.06 (1.04–1.07) | <.001 |
| Sex (male) | 2.06 (1.59–2.68) | <.001 | 1.56 (1.18–2.06) | .002 | 1.50 (1.14–1.99) | .004 |
| Active smokinga | 1.41 (1.19–1.69) | <.001 | 1.40 (1.16–1.69) | <.001 | 1.96 (1.45–2.66) | <.001 |
| Alcoholic liver disease | 1.59 (1.32–1.91) | <.001 | 1.24 (0.99–1.54) | .055 | 1.53 (1.19–1.97) | .001 |
| Hepatitis C | 1.09 (0.90–1.31) | .381 | 1.03 (0.83–1.28) | .770 | ||
| Hepatitis B | 0.76 (0.52–1.12) | .173 | 0.82 (0.56–1.23) | .343 | ||
| Hepatocellular carcinoma | 2.19 (1.83–2.62) | <.001 | 1.70 (1.40–2.06) | <.001 | 1.69 (1.40–2.04) | <.001 |
| Interaction alcoholic liver diseasea hepatitis C | 0.60 (0.41–0.87) | .008 | ||||
- Note: Univariate and multivariate Cox's regression analysis. Gray shading indicates not applicable.
- a Smoking habit was evaluated at inclusion in the waiting list for liver transplantation.
3.2 Prognostic impact of cancer in LT patients
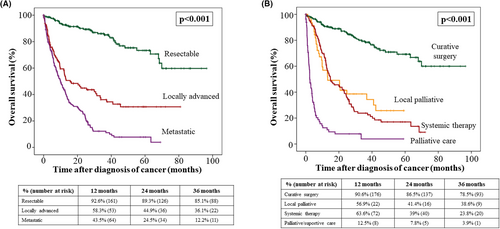
The stage of cancer at diagnosis was locally advanced in 22.8% of patients and metastatic in 34.8% of patients. Regarding the initial cancer therapy, 47% of patients received surgical resection with curative intention, 10% of patients underwent local ablation or palliative surgery, 26.1% of patients received chemotherapy, and 16.9% of patients were directly transferred for palliative care. Among 454 deaths registered in the whole cohort after a 13 922 person/years follow-up, 198 (43.6%) were attributed to post-LT malignancy. Lethality of cancer was 40.3% and the median interval between diagnosis of cancer and death was 8.7 months (IQR 3–20.1). Tumor stage at diagnosis and first-intention therapy were main determinants of survival (Figure 3). Regarding tumor stage at diagnosis, 12-month overall survival rates were 92.6% for patients with resectable tumors, 58.3% in patients with locally advanced tumors, and 43.5% for patients with metastatic disease (p < .001). Patients who underwent surgery with curative intention had 90.6% overall survival rates at 12 months, which were higher than that observed in patients receiving local ablative therapies (56.9%), systemic therapy (63.6%) or palliative care (12.5%) (p < .001). Median survival according to the type of tumor is shown in Table 2. Tumors with the worst prognosis were pancreatic cancer, gynecological tumors, melanoma, lung cancer, and HCC recurrence, with median overall survival shorter than 10 months.
| Type of malignancy | % (n) | Median survival (months) | Interquartile range (months) |
|---|---|---|---|
| Pancreatic | 2% (10) | 2.33 | 0–6.50 |
| Gynecological | 0.8% (4) | 3.71 | 0–15.98 |
| Melanoma | 0.8% (4) | 6.80 | 0–26.63 |
| Unknown origin/others | 5.9% (29) | 8.54 | 0.40–16.69 |
| Lung | 13.7% (67) | 9 | 6.97–11.03 |
| HCC recurrence | 24% (118) | 9.99 | 6.55–14.43 |
| Esophageal/gastric | 4.1% (20) | 11.92 | 6.17–17.69 |
| Colorectal | 3.5% (17) | 16.20 | 11.87–20.52 |
| Head and neck | 12.6% (62) | 19.25 | 8.79–29.71 |
| Urinary | 4.5% (22) | 21.29 | 9.32–33.26 |
| Lymphoproliferative disorders | 4.9% (24) | 26.05 | 21.40–30.71 |
| Kaposi's sarcoma | 0.8% (4) | 28.81 | 0–98.87 |
| Prostate | 5.3% (26) | 28.94 | 23.77–34.11 |
| Breast | 1% (5) | 36.37 | 0–86.88 |
| Non-melanoma skin | 16.1% (79) | 36.40 | 30–42.80 |
- Abbreviation: HCC, hepatocellular carcinoma
3.3 Immunosuppression-related risk factors of cancer
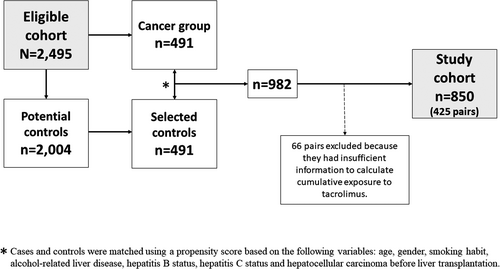
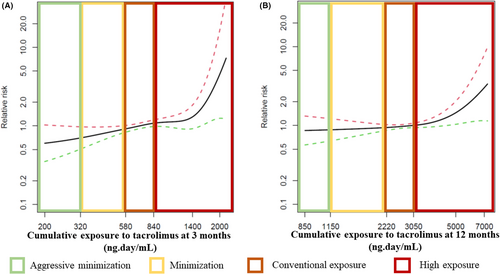
A flowchart depicting the selection of cases and controls to study the relationship between immunosuppression and post-LT malignancy is presented in Figure 4. The analysis of immunosuppression-related risk factors of cancer comprised 850 patients (425 pairs of cases and controls). Baseline features including immunosuppressive therapy of cases and controls are shown in Table 3. Cases and controls were comparable in terms of age, gender, smoking habit, etiology of liver disease, and the presence of HCC before LT. Indeed, the propensity score was identical in both groups (p = .957). The predominant immunosuppression protocol in each patient within the first 12 months after LT was similar in cases and controls (p = .509): tacrolimus alone in 32% of cases versus 30.4% of controls, tacrolimus in combination with mycophenolate in 56.5% of cases versus 60% of controls, and tacrolimus in combination with mTOR inhibitors in 11.5% of cases versus 9.6% of controls. None of the patients received azathioprine within the first 12 months after LT. Individual immunosuppressive drugs and their length of administration were also distributed homogeneously in patients with post-LT cancer and in matched controls (Table 3). The use of boluses of steroids to treat rejection episodes was also identical between groups (p = .911). Patients with post-LT malignancy had increased CET as compared with matched controls, both within the first 3 months (754 ng∙day/ml [IQR 614–920] vs. 695 ng∙day/ml [IQR 580–862]; p = .002) and within the first 12 months (2820 ng∙day/ml [IQR 2413–3334] vs. 2699 ng∙day/ml [IQR 2284–3160]; p = .009). High exposure to tacrolimus according to CET occurred in 36.3% of cases compared to 27.5% of controls at 3 months (p = .005) and in 36.5% of cases compared to 31.3% of controls at 12 months (p = .057). The smoothing splines showed that the increased risk of cancer occurred in patients within the high-exposure strata according to CET, and particularly when this parameter was evaluated at 3 months (Figure 5).
|
Cancer group (n = 425) |
Control group (n = 425) |
p | |
|---|---|---|---|
| Age | 58.09 ± 7.85 | 58.03 ± 7.17 | .904 |
| Sex, women, % (n) | 13.6% (58) | 14.1% (60) | .843 |
| Active smoking habit, % (n) | 44.8% (190) | 45% (191) | .945 |
| Alcoholic cirrhosis, % (n) | 63.8% (271) | 63.1% (268) | .831 |
| Hepatitis C, % (n) | 35.3% (150) | 33.9% (144) | .665 |
| Hepatocellular carcinoma, % (n) | 57.6% (245) | 55.1% (234) | .447 |
| Propensity score | 0.47 ± 0.24 | 0.47 ± 0.24 | .957 |
| Combination of immunosuppressive drugsa | |||
| Tacrolimus alone | 32% (136) | 30.4% (129) | .509 |
| Tacrolimus + mycophenolate | 56.5% (240) | 60% (255) | |
| Tacrolimus + mTORi inhibitors | 11.5% (49) | 9.6% (41) | |
| Conventional tacrolimus | |||
| % (n) | 44.5% (189) | 43.3% (184) | .730 |
| Number of months | 6.04 ± 4.21 | 6.50 ± 5.06 | .374 |
| Prolonged-release tacrolimus | |||
| % (n) | 82.8% (352) | 81.4% (346) | .591 |
| Number of months | 10.96 ± 2.23 | 11.12 ± 1.95 | .313 |
| Mycophenolate | |||
| % (n) | 77.2% (328) | 78.4% (333) | .680 |
| Number of months | 8.28 ± 4.34 | 8.67 ± 4.26 | .242 |
| Everolimus | |||
| % (n) | 16.5% (70) | 12.2% (52) | .078 |
| Number of months | 7.94 ± 3.65 | 8.58 ± 3.84 | .357 |
| Average trough levels | 3.88 ± 1.53 | 3.99 ± 1.70 | .741 |
| Sirolimus | |||
| % (n) | 1.2% (5) | 0.9% (4) | 1 |
| Number of months | 4 ± 5.05 | 8.25 ± 4.5 | .230 |
| Average trough levels | 10.16 ± 5.43 | 6.70 ± 3.08 | .416 |
| Basiliximab | 27.5% (117) | 28.9% (123) | .648 |
| Boluses of corticosteroids | 10.4% (44) | 10.6% (45) | .911 |
| Biopsy proven acute cellular rejection | 10.6% (45) | 10.1% (43) | .831 |
| Moderate-severe rejection (biopsy-proven) | 6.1% (26) | 4.7% (20) | .363 |
| Acute cellular rejection (biopsy-proven or treated empirically) | 13.9% (59) | 15.1% (64) | .626 |
| Cumulative exposure to tacrolimus (CET) (ng∙day/ml) | |||
| At 3 months | 754 (IQR 614–920) | 695 (IQR 580–862) | .002 |
| At 12 months | 2820 (IQR 2413–3334) | 2699 (IQR 2284–3160) | .009 |
| Exposure to tacrolimus according to CET strata at 3 months | |||
| Aggressive minimization | 0.9% (4) | 3.5% (15) | .005 |
| Minimization | 18.8% (80) | 21.6% (92) | |
| Conventional exposure | 44% (187) | 47.4% (201) | |
| High exposure | 36.3% (154) | 27.5% (117) | |
| Exposure to tacrolimus according to CET strata at 12 months | |||
| Aggressive minimization | 0.2% (1) | 1.4% (6) | .057 |
| Minimization | 16.7% (71) | 21.2% (90) | |
| Conventional exposure | 46.6% (198) | 46.1% (196) | |
| High exposure | 36.5% (155) | 31.3% (133) | |
- Abbreviations: CET, cumulative exposure to tacrolimus; mTORi, mammalian target of rapamycin inhibitors.
- a Predominant immunosuppression protocol within the first 12 months after liver transplantation.
In the multivariate Cox regression analysis, an increased CET was the only independent predictor of post-LT malignancy after controlling for clinical features, baseline HCC, and concomitant immunosuppressive drugs (Table 4). Indeed, the HR of post-LT malignancy for a 20% increase of CET within the first 3 months was 1.11 (95% CI 1.05–1.19; p = .001). Similarly, the HR of post-LT malignancy for a 20% increase of CET within the first 12 months was 1.10 (95% CI 1.03–1.17; p = .004). These results were consistent when considering exclusively de novo malignancy (CET at 3 months adjusted HR = 1.09 [95% CI 1.01–1.18]; p = .020 and CET at 12 months adjusted HR = 1.10 [95% CI 1.02–1.19]; p = .016), and internal neoplasms (CET at 3 months adjusted HR = 1.11 [95% CI 1.04–1.19]; p = .001 and CET at 12 months adjusted HR = 1.09 [95% CI 1.01–1.21]; p = .017). Noteworthy, the use of mTOR inhibitors was associated with increased risk of post-LT malignancy univariately, but it lost statistical significance in the multivariate analysis after controlling the interaction between prescription of mTOR inhibitors and baseline HCC.
| Variables | Univariate analysis |
Multivariate analysis (including CET at 3 months) |
Multivariate analysis (including CET at 12 months) |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 1.01 (0.99–1.02) | .507 | 1.01 (0.99–1.02) | .496 | 1.01 (0.99–1.02) | .501 |
| Sex (male) | 1.14 (0.86–1.50) | .357 | 0.99 (0.73–1.36) | .987 | 1.01 (0.75–1.38) | .921 |
| Active smokinga | 1.08 (0.89–1.31) | .428 | 1.05 (0.86–1.30) | .611 | 1.06 (0.87–1.31) | .552 |
| Alcoholic liver disease | 1.01 (0.83–1.23) | .933 | 1.08 (0.85–1.39) | .517 | 1.07 (0.84–1.36) | .605 |
| Hepatitis C | 1.12 (0.92–1.37) | .255 | 1.04 (0.81–1.33) | .747 | 1.02 (0.80–1.31) | .857 |
| Hepatitis B | 1.15 (0.75–1.77) | .523 | 1.19 (0.76–1.86) | .441 | 1.21 (0.77–1.89) | .410 |
| Hepatocellular carcinoma | 1.22 (1.00–1.47) | .048 | 1.07 (0.85–1.35) | .557 | 1.11 (0.88–1.40) | .381 |
| Basiliximab induction | 1.07 (0.87–1.34) | .493 | 1.20 (0.96–1.52) | .115 | 1.16 (0.92–1.45) | .207 |
| Boluses of corticosteroids | 0.99 (0.72–1.35) | .936 | 0.86 (0.62–1.19) | .374 | 0.88 (0.64–1.22) | .446 |
| Mycophenolate | 1.04 (0.83–1.31) | .723 | 1.14 (0.89–1.45) | .300 | 1.12 (0.88–1.43) | .349 |
| mTOR inhibitors | 1.71 (1.33–2.20) | <.001 | 1.48 (0.88–2.51) | .139 | 1.42 (0.85–2.41) | .182 |
| Interaction hepatocellular carcinomaa mTOR inhibitors | 1.30 (0.71–2.35) | .394 | 1.38 (0.76–2.51) | .289 | ||
| CET at 3 monthsb | 1.09 (1.03–1.15) | .005 | 1.11 (1.05–1.19) | .001 | ||
| CET at 12 monthsb | 1.06 (0.99–1.13) | .062 | 1.10 (1.03–1.17) | .004 | ||
- Note: Gray shading indicates not applicable.
- Abbreviation: CET, cumulative exposure to tacrolimus.
- a Smoking habit was evaluated at inclusion in the waiting list for liver transplantation
- b Relative risks and confidence intervals computed for a 20% increase in CET.
The effect of CET was evaluated for each type of cancer univariately (Figure S3). An increase of CET by 20% within the first 3 months was associated with significantly higher risk of colorectal cancer (OR = 2.37 [95% CI 1.20–4.69]; p = .013), lung cancer (OR = 1.31[95% CI 1.03–1.65]; p = .024), and HCC recurrence (OR = 1.26 [95% CI 1.07–1.48]; p = .006). On the other hand, an increase of CET by 20% within the first 12 months, was associated with higher risk of skin cancer (OR = 1.34 [95% CI 1.03–1.74]; p = .030) and colorectal cancer (OR = 2.06 [95% CI 1.05–4.02]; p = .034). A subgroup analysis of patients with HCC recurrence is presented in Supplementary Material.
After diagnosis of cancer, tacrolimus dose was reduced by >25% in 93 patients (21.9%), either in combination with mycophenolate (n = 40, 9.4%) or in combination with mTOR inhibitors (n = 53; 12.5%). Tacrolimus was completely withdrawn in 92 patients (21.6%), either in combination with mycophenolate (n = 9, 2.1%) or in combination with mTOR inhibitors (n = 83, 19.5%). The immunosuppression regimen remained unchanged after the diagnosis of cancer in 56.5% of patients. Modifications in the immunosuppression protocol had no influence on overall survival, neither in the entire cohort (Log Rank p = .347) nor in the HCC recurrence subgroup (Log Rank p = .193).
4 DISCUSSION
This multicenter study has shown the increased incidence of cancer in patients receiving a LT under current immunosuppression protocols, including both de novo malignancy and recurrence of HCC, together with its significant prognostic impact. The use of a quasi-cohort approach, which minimizes the risk of bias in pharmacoepidemiology,10 has allowed to demonstrate a dose-dependent pro-oncogenic effect of tacrolimus in real clinical practice irrespective of demographic features and concomitant immunosuppressive drugs. An objective and easy-to-implement parameter, termed CET,11 would allow for a more rational use of immunosuppression in order to reduce the risk of cancer after LT.
The incidence rates of cancer in LT patients are at least twice as high as those observed in age- and gender-matched general population.3-6, 12 There are some clinical characteristics inherent to the LT population which may partially explain these findings. First, chronic alcohol consumption is a major driver of chronic liver disease,13 which in turn is the leading cause of LT worldwide.14, 15 Although patients are required to withdraw alcohol consumption before LT and they are strongly advised to remain abstinent in the long term, heavy alcohol relapse rates after LT are 10%–20%.16 Alcohol consumption and smoking habit exert a synergic pro-oncogenic effect for many types of cancer17 showing increased standardized incidence rates in transplant patients (head and neck tumors, lung cancer, gastrointestinal tumors, pancreatic cancer, etc.).8 In the present study, active smoking doubled post-LT cancer rates, and history of alcoholic liver disease increased this risk by 53%, aligning with previous observations.6, 18-20 Smoking cessation before LT is mandatory in some transplant programs and this strategy may have a beneficial impact on the risk of malignancy. However, the risk would be still higher than in never-smokers, particularly for head and neck tumors, gastrointestinal cancer, respiratory tract cancer, female genitalia, and urinary tract tumors.19 These types of malignancies showed median survival shorter than two years in the present cohort. Therefore, cancer screening programs after LT should be tailored to the history of alcoholic liver disease and smoking habit, including more frequent examinations by the otolaryngologist, gynecologist, urologist, and probably using periodical upper gastrointestinal endoscopies.
Evading the immune system, as part of the immunoediting process, is one of the hallmarks of cancer.7, 21 Nascent tumors naturally select less immunogenic cells to proliferate and invade other organs and this task could be facilitated by the use of immunosuppressive drugs.22 In experimental models, calcineurin inhibitors and azathioprine promote oncogenesis by activating different pathways such as TGF-β,23 and by impairing the DNA-repair machinery,24 respectively. Conversely, the inhibitors of the mammalian target of rapamycin (mTOR) would exert antiproliferative properties.25 Therefore, modulating immunosuppression after LT could be the only modifiable factor to decrease the risk of posttransplant malignancy to the levels observed in the non-transplant matched population. Unfortunately, most clinical studies failed to identify a particular drug (or combination of drugs) able to promote the development of cancer. Indeed, studies evaluating the relationship between immunosuppression and malignancy need to overcome important sources of bias: different combinations of immunosuppressive drugs, dosing, and timing; lack of a tool to assess cumulative exposure to each drug; time-dependent and heterogeneous outcome (each type of tumor may have individual risk factors other than immunosuppression); and potential confounders including demographic features and etiology of liver disease. As a result, the optimal immunosuppression protocol to prevent cancer after LT is unknown. In the present study, the quasi-cohort approach, the propensity score matching and the use of the CET tool, which is an objective and reproducible parameter that has already demonstrated a correlation with renal impairment,11 have allowed to overcome these limitations.
Calcineurin inhibitors are the mainstay of immunosuppression in LT.26 The only randomized trial performed hitherto comparing two regimes of cyclosporine-based immunosuppression (75–125 ng/ml vs. 150–250 ng/ml) in 231 kidney transplant recipients found increased 5-years cancer rates in the high-dose group (32.2% vs. 19.8%; p = .034).27 Regarding tacrolimus, a retrospective single-center study showed increased trough concentrations in patients who developed malignancy compared with those who did not.20 In patients receiving a LT because of HCC, the use of tacrolimus trough concentrations higher than 10 ng/ml within the first month after LT was associated with tripled tumor recurrence rates.28 Nowadays such high doses of tacrolimus are not aimed in routine clinical practice and it is unclear whether current immunosuppression protocols could be further refined to decrease the risk of malignancy. In the present study, CET was an independent predictor of post-LT malignancy, including any type of cancer and de novo malignancy. Those types of cancer with a more evident relationship with CET were skin cancer, colorectal cancer, lung cancer, and HCC recurrence, although the reduced number of other types of tumors claim for caution when interpreting these results. The most pronounced increase of cancer risk was seen in the high exposure strata of CET (equivalent to >10 ng/ml within the first month and >8 ng/ml thereafter approximately (Figure 1)11), and particularly within the first 3 months after LT. This high exposure according to CET may occur in up to one third of patients receiving tacrolimus-based immunosuppression as demonstrated in the present study, and in 21.6% of patients according to a previous report.11 Monitoring CET within the first 3 months would allow to identify these patients in order to implement appropriate dose adjustments to decrease CET at 12 months. Although a moderate tacrolimus minimization may be sufficient to reduce the risk of posttransplant malignancy, patients with risk factors (older age at transplantation, smoking habit, alcoholic liver disease, and baseline HCC), should be considered for a more aggressive minimization of tacrolimus (CET <580 ng∙day/ml at 3 months and CET<2220 ng∙day/ml at months) after a careful risk/benefit evaluation.
Concomitant immunosuppression drugs administered in combination with tacrolimus could modulate the risk of cancer. Antibody induction agents could increase the risk of non-Hodgkin lymphoma, lung cancer, thyroid cancer and melanoma in kidney transplant recipients.29 Azathioprine increases the risk of skin squamous cell carcinoma.30 A potential anticancer effect has been suggested for mycophenolate including reduced risk of posttransplant lymphoproliferative disorders and prolonged time to any type malignancy.31, 32 Finally, the antiproliferative effect of mTOR inhibitors has been proven in experimental models but there are conflicting results in clinical practice.33-35 It is of note that both mycophenolate and mTOR inhibitors aim to reduce the exposure to tacrolimus in order to decrease the risk of adverse events, mainly renal impairment. The reduced cancer rates reported in the past for patients receiving combination therapies could be equally claimed by the added drug or by the reduced exposure to tacrolimus, since the latter factor was not controlled in any of the above referred studies. This is the first study which has controlled both factors in the analysis and it has demonstrated that the true anticancer effect may be explained by a reduced exposure to tacrolimus and not by the use of mycophenolate or mTOR inhibitors per se in their usual doses. The use of the CET tool in future randomized trials would allow to quantify tacrolimus exposure in experimental arms aiming at drug minimization or withdrawal, thus providing information on whether tacrolimus minimization has been clinically meaningful thorough the study.
Despite a regular follow-up in transplant clinics, tumors are not diagnosed at earlier stages in LT patients and most of them have a worse prognosis compared to immunocompetent individuals.36 In some situations, LT patients may not be eligible for certain therapies due to significant comorbidities or interaction with immunosuppressants, but it may well be also that tumors may exhibit a more aggressive biological behavior under immunosuppression. To reduce immunosuppression as much as possible after diagnosis of cancer is a common practice but its scientific support is restricted to cancers related with viral infections such as Kaposi's sarcoma. We failed to demonstrate any prognostic benefit of tacrolimus reduction or withdrawal after diagnosis of malignancy. Future studies are needed to provide clinical guidance in this scenario.
The present study has inherent limitations. Some peculiarities of our cohort, including geographic distribution and increased prevalence of HCC, claim for caution when generalizing results to other scenarios. Cancer after LT is a heterogeneous outcome and each type of tumor may have particular risk factors, including genetic predisposition and environmental expositions, which could not be controlled. The association of CET with low incidence malignancies could be unreliable due to insufficient statistical power. However, the consistent effect of CET in any type of malignancy, in de novo tumors and in internal neoplasms, reinforces the clinical utility of tacrolimus minimization. Finally, smoking cessation and recidivism of alcohol consumption after LT have not been recorded.
In conclusion, the incidence of cancer after LT is high and carries a dismal prognosis. A consensus is needed to delineate cancer screening strategies after LT, which should be tailored according to patient's age, sex, history of smoking, and alcohol consumption. Early minimization of tacrolimus using the CET tool would allow to reduce the incidence of cancer in LT patients. Concomitant immunosuppressive drugs such as mycophenolate or mTOR inhibitors may not increase the risk of cancer and could be useful as long as they allow for a safer and more effective tacrolimus minimization after LT.
ACKNOWLEDGMENTS
This study was funded by the Consejería de Salud y Familias de la Junta de Andalucía (PI-0164-2019) and by the Fondo Europeo de Desarrollo Regional (FEDER). The funding agents covered the expenses to design the webpage of cumulative exposure to tacrolimus (Web CET [imibic.org]) but they had no role in the data analysis, manuscript preparation, or in the decision to publish the study. The University of Córdoba covered the expenses for open access.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Manuel Rodríguez-Perálvarez: Chiesi (lecture fee). Jordi Colmenero: Chiesi, Novartis, and Astellas (lecture fee). Mikel Gastaca: Astellas, Novartis, and Chiesi (advisory and lecture fee). Sonia Pascual: Chiesi and Novartis (lecture fee). The other authors have no conflicts of interest to disclose.
APPENDIX
1 Chronic Immunosuppression and Cancer Spanish multicenter group.
Hospital Clínic Barcelona: Gonzalo Crespo; Jesús Rivera and Laia Escudé.
Hospital Universitario Ntra. Sra. de la Candelaria, Tenerife: Estefanía Berge, Dácil Diaz-Bethencourt and Silvia Acosta.
Hospital Universitario Cruces, Bilbao: Patricia Ruiz, Alberto Ventoso and Andrés Valdivieso.
Hospital Universitario Vall d´Hebron, Barcelona: Cristina Dopazo and Itxarone Bilbao.
Hospital General Universitario Gregorio Marañón, Madrid: Magdalena Salcedo and Mario Romero-Cristóbal.
Hospital Universitario Virgen de la Arrixaca, Murcia: María Luisa Ortiz and José Antonio Pons.
Hospital Universitari I Politècnic La Fe, Valencia: Andrea Boscà Robledo and Marina Berenguer.
Clínica Universidad de Navarra, Pamplona: Mercedes Iñarrairaegui.
Hospital Universitario Río Hortega, Valladolid: Ana Corcho and Esteban Fuentes.
Hospital Lozano Blesa, Zaragoza: Cristina Borao and Trinidad Serrano.
Marqués de Valdecilla University Hospital, Santander: Emilio Fábrega and Fernando Casafont.
Hospital General Universitario Alicante, Alicante: Patricio Mas.
Hospital Universitario Virgen de las Nieves, Granada: Flor Nogueras López and María Dolores Espinosa Aguilar.
Hospital Regional Universitario de Málaga: Susana López Ortega.
Hospital Universitario Virgen del Rocío, Sevilla: Miguel Ángel Gómez Bravo and Carmen Cepeda Franco.
Open Research
Open Research Badges
This article has earned an Open Data Badge for making publicly available the digitally-shareable data necessary to reproduce the reported results. The data is available at 10.17632/knh2t2kbrg.1; https://data.mendeley.com/datasets/knh2t2kbrg/1.
DATA AVAILABILITY STATEMENT
An anonymized raw database is available in a Mendeley repository at 10.17632/knh2t2kbrg.1.




