Allograft rejection: Be aware of tissue-resident T cells
Abstract
Skin-resident memory T cells independently mediate robust allograft rejection after alloantigen-sensitization.
Summary and Analysis
, , , et al. Skin and heart allograft rejection solely by long-lived alloreactive TRM cells in skin of severe combined immunodeficient mice. Sci Adv. 2022; 8(4):eabk0270.
A substantial number of T cells reside in tissues other than the lymphoid organs; they are memory-like, tissue-resident and rarely recirculate under homeostatic conditions. Such tissue-resident T cells are increasingly recognized as important players in the immune system. However, whether, how and under what conditions they impact the alloimmune response remains poorly understood. In a recent paper in Science Advances, Tian et al. reported that challenge of immunocompetent mice with a skin allograft dramatically expands the host skin tissue-resident T cells, where they also acquire the capacity to reject skin and heart allografts in the secondary immunodeficient mice. They also found that allograft rejection by such skin tissue-resident cells exhibits a Th17 program, which can be inhibited by suppressing the RORγt pathway or genetic deletion of IL-17, and consequently, graft survival is prolonged in the secondary hosts. Clearly, these findings provide strong evidence that the skin tissue-resident T cells are long-lived, highly dynamic and can contribute to allograft rejection.
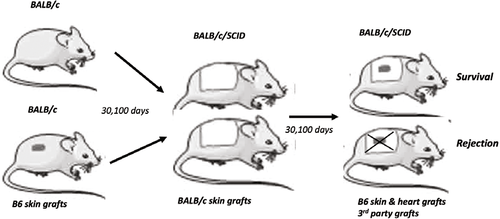
The authors initially performed a series of skin transplants involving both immunocompetent and immunodeficient mice as transplant recipients to examine features as well as roles of skin-resident T cells in allograft rejection. They showed that in unmanipulated BALB/c mice, the skin conspicuously hosts a substantial number of T cells consisting of both CD4+ and CD8+ subsets with phenotypic and transcriptional signatures of tissue-resident memory cells (TRM). Interestingly, challenge of the BALB/c mice with a fully MHC mismatched B6 skin graft (rejected around day 10) markedly expanded the TRM cells in the host skin, which peaked around day 15 after graft rejection and persisted long term in the host skin (>60 days). Those expanded TRM cells are clearly recipient-origin, highly concentrated around the initial donor skin grafting bed and show greater potential of self-renewal locally in the skin.
The authors then designed and performed a series of innovative experiments testing whether the skin TRM cells could independently mount a secondary allograft rejection response. They took advantage of the immunodeficient SCID mice (BALB/c background), and transplanted them with skin grafts from either unmanipulated BALB/c mice or BALB/c mice that had rejected the B6 skin allografts (100 days after rejection). As expected, all BALB/c skin grafts survived long term in the syngeneic SCID recipients. A secondary B6 skin allograft placed onto the healed BALB/c skin grafts from unmanipulated BALB/c mice (B6 graft on top of the BALB/c graft) also survived long term. Interestingly, the same secondary B6 skin allograft was acutely rejected by the SCID mice carrying the skin grafts from BALB/c mice that previously rejected B6 skin allografts. In this secondary allograft rejection model, the authors made several intriguing observations. The first one is that, when transplanted outside of the healed BABL/c skin grafts, the secondary B6 skin allograft could survive for a prolonged period, suggesting that rejection by the skin TRM cells is highly localized. Second, the ability to reject the secondary B6 allografts was confined to skin TRM cells around the initial grafting bed in the primary hosts, and those further away from the grafting bed (>2 cm) lost their ability to reject in the secondary SCID mice, demonstrating a strict spatial requirement for the skin TRM cells to trigger allograft rejection. Third, the skin TRM-mediated rejection is not restricted to the skin allografts, as the B6 heart allograft was also acutely rejected by skin TRM cells in the SCID recipients. Furthermore, rejection does not seem to be donor antigen-specific, since B6 alloantigen-sensitized skin TRM cells effectively rejected the third-party allografts in the SCID recipients. Finally, despite a robust expansion of CD4+ and CD8+ skin TRM cells to donor antigen stimulation, CD4+ TRM cells were sufficient to reject skin and heart allografts in the SCID hosts. In all the models, the authors confirmed that the skin TRM cells fail to recirculate, but they remain capable of entering the circulation upon donor antigen restimulation in the secondary hosts.
To explore the molecular mechanisms of graft rejection, the authors performed comprehensive RNA-seq analysis on skin TRM cells from unmanipulated and donor antigen-challenged BALB/c mice. They identified a prominent Th17-like gene signature in the donor antigen expanded skin TRM cells that was characterized by the expression of gene transcripts related to Th17 cytokines and transcription factors. The authors provide further evidence that, in the SCID hosts grafted with donor antigen-sensitized skin grafts, rejection of the secondary donor B6 skin allografts could be inhibited with chemical inhibitors targeting RORγt, a master transcription factor for Th17 cells.
Overall, this study broadens our understanding of the roles and requirements of tissue resident T cells in allograft rejection. Importantly, findings from this study may have important clinical implications, for example, in the study of graft rejection in patients with aggressive lymphodepletion therapies and those with retransplants.
Xian C. Li, MD, PhD, is professor and director of the Immunobiology and Transplant Science Center and Department of Surgery at Houston Methodist Hospital in Texas.