Dissecting CMV latency and reactivation: BETing on the epigenetic regulators
Abstract
Suppression of bromodomain and extraterminal proteins regulates human cytomegalovirus latency and reactivation.
, , , et al. Bromodomain proteins regulate human cytomegalovirus latency and reactivation allowing epigenetic therapeutic intervention. Proc Natl Acad Sci USA. 2021; 118:e2023025118.
Summary and Analysis
Cytomegalovirus (CMV) infects up to 90% of the world’s population and usually becomes latent in myeloid cells following primary infections, a carrier state where the virus persists lifelong in infected individuals. In transplant patients, CMV reactivation from latency is a significant concern, since it occurs frequently and can lead to serious morbidities including graft rejection, and sometimes even patient mortality. From a clinical standpoint, therapies that directly target latently infected cells in donors and/or recipients prior to transplantation (thus reducing or eliminating the viral reservoir and viral loads before initiation of immunosuppression) would reduce the incidence of CMV reactivation post transplantation, and hopefully lead to better clinical outcomes.
In a recent study published in PNAS, Groves et al investigated the roles of a set of epigenetic modifiers in the regulation of CMV latency and reactivation. The rationale behind this effort is that the CMV latency is actively maintained by epigenetic repression of viral gene transcription. The authors examined a list of chemical inhibitors known to modulate chromatin structures epigenetically and found that treatment of the latently infected cells with histone deacetylase inhibitors (HDACis), which produces histone hyperacetylation, led to expression of the viral immediate early (IE) genes, full reactivation of CMV and production of infectious viral particles that propagate further infections. Interestingly, inhibition of the bromodomain and extraterminal domain (BET) proteins, especially the BET protein BRD4, which recognize acetylated histone residuals (i.e., chromatin readers), resulted in the expression of immunodominant viral proteins by latently infected cells, without inducing viral DNA replication and full CMV reactivation. As a result, latently infected cells treated with BET inhibitors (BETis) became visible to the endogenous cytotoxic T cells and were readily eliminated by T cells, which led to a marked reduction of the latent viral reservoir in vitro and in vivo.
In their initial studies, the authors constructed a monocytic reporter cell line latently infected with CMV, in which reactivation of the CMV is faithfully captured by the expression of the yellow fluorescence protein eYFP. They then used this reporter system to screen a list of epigenetic inhibitors. They found that among all the chemical compounds screened, HDACi and BETi showed the highest potency in eYFP expression and therefore CMV reactivation, an observation that was further confirmed using latently infected primary human monocytes. Coculturing experiments involving latently infected monocytes and human foreskin fibroblasts revealed that treatment with HDACi resulted in the production of infectious virions as well as formation of infectious plaques, which contrasted sharply to those treated with the BETi, where infectious viral particles and plaques were not observed. The authors then performed extensive RNA-seq analyses comparing latently infected monocytes treated with HDACi versus BETi, and observed striking differences in their transcriptional profiles. While HDACi treatment led to the expression of genes encoding the viral IE proteins, late proteins and DNA replication machineries (full activation cascade), BETi treatment lacked the capacity to induce the expression of genes that encode DNA replication machineries, despite the induction of viral IE and late genes, which encode the viral immunodominant proteins.
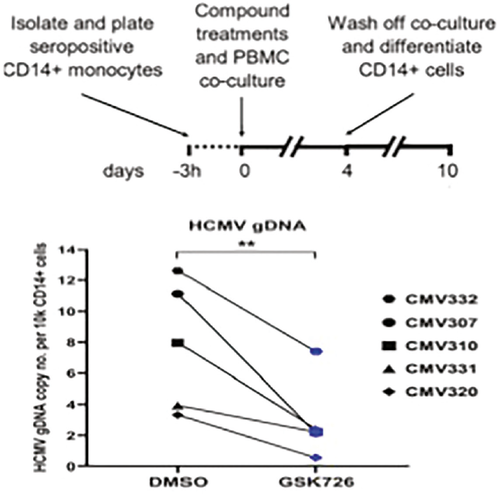
The authors further pursued the mechanisms by which BETi selectively inhibits viral replications while allowing the latently infected cells to express immunodominant proteins to be killed by T cells. They focused on the BET family member BRD4, and, by using a new BRD4-specific inhibitor, GSK726, they showed that BRD4 formed a repressive molecular complex that sequestered positive transcription elongation factor b (p-TEFb), preventing it from engaging the promoter of CMV major IE genes. Thus, BRD4 inhibition relieves p-TEFb from sequestration, resulting in the expression of immunodominant proteins by latently infected cells. Additionally, BRD4 inhibition also disrupted the activation of super enhancers that drive the transcription of genes encoding the viral DNA replication pathways.
Overall, this study demonstrates that CMV latency and reactivation are controlled by very different epigenetic mechanisms. It also suggests that the BET protein inhibitors could be safely used to pre-emptively purge the latent viral reservoir before transplantation. This notion awaits further testing in transplant patients.
Xian C. Li, MD, PhD is professor and director at the Immunobiology and Transplant Science Center and Department of Surgery at Houston Methodist Hospital in Texas.