Impact of COVID-19 in solid organ transplant recipients
Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exploded onto the world stage in early 2020. The impact on solid organ transplantation (SOT) has been profound affecting potential donors, candidates, and recipients. Importantly, decreased donations and the pressure of limited resources placed on health care by the pandemic also disrupted transplant systems. We address the impact of COVID-19 on organ transplantation globally and review current understanding of the epidemiology, outcomes, diagnosis, and treatment of COVID-19 in SOT recipients.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- ARDS
-
- acute respiratory distress syndrome
-
- BAL
-
- bronchoalveolar lavage
-
- CP
-
- convalescent plasma
-
- COVID-19
-
- coronavirus disease 2019
-
- IL-6
-
- interleukin-6
-
- PCR
-
- polymerase chain reaction
-
- RR
-
- risk ratio
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- SOT
-
- solid organ transplantation
-
- UK
-
- United Kingdom
-
- UNOS
-
- United Network of Organ Sharing
1 SARS-COV-2 INFECTION, IMMUNITY, AND PATHOGENESIS
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). While infection with SARS-CoV-2 is frequently asymptomatic or mild, clinical manifestations can also be serious. Risk factors for serious disease and mortality in the general population include older age, obesity, and comorbidities affecting the lung or the heart.1, 2 Disease severity may be influenced by the viral inoculum, dissemination, pre-existing immunity toward other coronaviruses, individual immunocompetence, and other host factors.3-9
In the general population, infection is followed by the induction of innate inflammatory responses and of SARS-CoV-2-specific humoral and cellular immunity including CD4 and CD8 T cells.3-6, 10 The magnitude of specific immunity may be directly related to viral load, dissemination to the lower respiratory tract, and/or disease severity.6, 10 In addition, SARS-CoV-2-specific T cells in patients with severe disease have a restricted functionality and higher expression levels of co-inhibitory receptors, which also extended to T cells in general.6, 11 Together this suggests that adaptive immunity is readily induced and contributes to viral control in the majority of infected individuals. However, in some patients, a cytokine storm is triggered that mediates severe lung inflammation and widespread systemic pathology.12 High levels of specific adaptive immunity and the hyperinflammatory syndrome observed in severe disease emphasize the need for contraction processes to counteract excess immunopathology in the lungs.
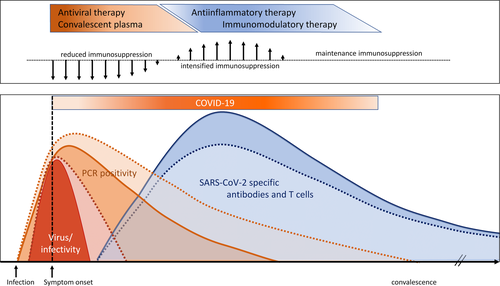
In solid organ transplant (SOT) recipients, the extent of immunosuppression correlates with the severity of diverse infectious diseases,13 which led to the initial prediction that SOT recipients may be more susceptible to severe COVID-19. Indeed, the risk of mortality in transplant recipients seems higher than in the general population.14-17 However, this has not been universally noted. Early after infection, strong immunosuppression may adversely affect sufficient induction of specific immunity, which may account for insufficient control of viral load and frequently observed prolonged detection of viral RNA after disease onset.18-21 In contrast, in later periods of the disease, immunosuppressive drugs may be beneficial in suppressing proinflammatory processes and supporting functional inactivation and contraction of cellular immunity. Thus, modulation of immunosuppression may be harmful or beneficial depending on the clinical stage of the infection in SOT. The time course for viral replication, infectivity, and induction of adaptive immunity in immunocompetent patients and potential alterations in SOT recipients, and implications for therapeutic management is outlined in Figure 1.
2 THE IMPACT OF COVID-19 ON TRANSPLANTATION
COVID-19 had immediate impact on transplant activity as the infection became more widespread throughout the world. Initial reports from the Italian epicenter revealed a 25% decline in deceased donation nationally with a more pronounced decline in northern Italy where the rates of COVID-19 were highest.22 During the height of the first wave of the pandemic in Spain, there was nearly an eightfold decrease in transplant activity.23 France, the Netherlands, and the United Kingdom (UK) also experienced substantial declines with lower transplant rates driven by 50–90% decrease in deceased donation during the peak COVID-19 months.24-26 Review of data from the United States’ United Network of Organ Sharing (UNOS) comparing monthly transplants in January and February 2020 with those performed in April 2020 demonstrated a 35.9% decrease in organs transplanted.27
Several themes emerged from all reports. The impact on specific programs exposed notable regional variation, reflecting in part local COVID-19 rates,28-31 but also individualized approaches to resource allocation and prioritization.30 The impact on organ transplantation also varied with respect to organ type with preferential deferral of kidney transplant candidates who were stable on renal replacement therapy and/or had lower immunologic barriers to transplantation.23, 26, 27, 31, 32 However, the majority of reports also noted a decline in transplantation in all organ types.32 Living donor programs were generally curtailed or suspended in many sites.25, 32, 33
Reasons for the decline in donations were diverse and explained by changes at multiple levels in the transplant process, although the impact of individual policies remains uncertain at this time. An overall decline was driven by a decrease in available ICU beds for maintaining donors due to use for treatment of critically ill COVID-19 patients.22 The demographics of the available donors shifted with a 5% decline in trauma death donors, 35% increase in donor death by substance abuse, and a decreased willingness to use donors with circulatory deaths in whom post-operative transplant recovery would be anticipated to be prolonged.22, 34 Donor screening practices varied but in many cases, donors with potential SARS-CoV-2 exposure or presentations consistent with COVID-19, regardless of testing results, were excluded.23 Moreover, in many locations, instead of greater geographic sharing of organs, there was a preference for using local organs where regional COVID-19 rates were known and the ability to protect the procurement teams might have been greater.33 Given restricted hospital access and concerns for SARS-CoV-2 exposures, the authorization process for deceased donation involved more telephonic and virtual communication.34 Live donation also declined, in part due to concerns about exposing healthy living donors to a greater risk of contracting SARS-CoV-2.25, 35 Notably, restricted air travel may also have affected prompt organ transport, with potential impact on cold ischemic times.36
Other factors impacting transplantation included limitations of resources (beds, surgical suites, ventilators, blood products, and renal replacement therapies) and personnel due to local COVID-19 demands.32, 37 Surveys also revealed a reluctance to bring stable transplant candidates into hospitals where they might be at greater risk of being exposed to SARS-CoV-2.32
The impact on outcomes of the decline in organ donation and transplantation is difficult to assess at this time. Although recent reports from UNOS suggest that overall waitlist deaths may not have significantly increased in 2020,27 an increase in waitlist hospitalizations and deaths were observed at least in some areas in the first wave of the pandemic.27, 38, 39 In the US, waitlist deaths were more numerous in areas with the highest rates of COVID-19; a study from New York revealed a mortality of 34% of waitlist patients as compared to 16% of kidney transplant recipients.39 Whether candidates died of COVID-19 or of the indirect effects of limited on-site care has not been elucidated. It is also unknown how many individuals died without being added to the waitlist; however, the reports of curtailed evaluations at some centers suggest the possibility that the pandemic may have indirectly contributed to deaths in individuals who never had access to transplant evaluation.37, 40
Despite the ongoing community spread of SARS-CoV-2, there is evidence that transplant activity has been resumed in many locations.28 Whether rising rates of SARS-CoV-2 infections will again impact donation and transplantation remains unclear. Guidance for transplant management including suspension and resumption of transplantation has been provided by national and international transplant societies/organizations (Table 1) and there are also published guidance documents; however, there has been heterogeneity of actual practices and these principles may guide activity during the second and third waves of the pandemic.32, 37, 41-46 General recommendations for full resumption of transplantation activity 41, 44 as well as recommendations on donation practices have been updated during the pandemic to improve the safety of both the donor organ and procurement teams 47-50 (Table 1). The increasing use of telemedicine for outpatient management at all phases of care has been described internationally during the pandemic and has been increasingly adopted.32, 51
| Society | Decision to transplant | Deceased donation | Living Donation | Transplant Candidates |
|---|---|---|---|---|
| American Society of Transplantation52 |
Balance local COVID−19 epidemiology, resource availability (including of health care staff) with need for transplant. Temporary suspension of elective living donor transplantation or non-urgent deceased donor transplants may be considered based on local concerns. |
Epidemiologic screen and ≥1 respiratory tract NAT (BAL for lungs) within 72 hours of donation. |
Exclude living donors with COVID−19 (symptoms and/or NAT positive). Epidemiologic and NAT screening (respiratory tract only) within 72 hours of donation. Pre donation self- quarantine 14 days. |
Defer transplantation for active SARS-CoV−2 infection. NAT negative at time of transplant; disease-free interval not specified. |
| American Society of Transplant Surgeons53 |
Consider curtailing transplantation services when 20–25% of hospital resources are committed to COVID−19 care or there is a very rapid increase in COVID−19 population numbers. Assumes availability of appropriate PPE and measures for HCW screening, social distancing and COVID−19 free areas in transplant center. |
Epidemiology screen including local donor hospital environment/exposures by NAT (BAL recommended). Chest CT findings of ground glass should be assessed in view of COVID−19 risk. Local team organ recovery preferred Strict adherence to use of PPE for all phases of recovery (including travel) |
Exclude living donors who are NAT positive. NAT screening 2 days prior to donation. Negative CXR Asymptomatic donors should self-isolate for a minimum of 7 days, preferably 7–14 days. Special informed consent form of COVID−19 risk appreciation. |
Transplant surgery/immunosuppression is not advised in symptomatic or asymptomatic infected individuals with COVID−19 |
| The Transplantation Society54 | All transplant-related teams should develop plans for: HCW absences due to illness, identification of remote workforce, messaging for patients with contacts in case of illness, risk mitigation, screening of sick patients. |
Epidemiologic and NAT screening of all donors. Defer use of donors with COVID−19 unless 14 days since symptom onset and ideally two negative NAT for SARS-CoV−2. |
Epidemiologic and NAT screening of all donors. Defer use of donors with COVID−19 unless 14 days since symptom onset and ideally two negative NAT for SARS-CoV−2. |
Discussion of risk benefit of transplantation with candidate during the ongoing pandemic. Defer transplantation of infected patients until 10–14 days since symptom onset, resolution of symptoms and two negative NAT tests separated by 24 hours. |
| International Society of Heart and Lung Transplantation55 |
Decisions regarding transplantation made locally based on rate of SARS-CoV−2 infection in the community and availability of health care resources, unless otherwise directed by regional or national authorities. Regularly re-evaluate policy and consider individual patient risk benefit as well as local resource issues. Transplant cessation not recommended unless dictated by local circumstances. |
Defer donors with active infection. Consider donors with former COVID−19 if two negative NAT 24–48 hours apart if complete clinical recovery and at least 28 days from symptom onset. |
Not applicable |
Defer transplantation for NAT positive candidates and those with consistent symptoms (regardless of NAT). If history of symptomatic COVID−19, defer transplantation until two negative NAT tests separated by 24–48 hours following full resolution of illness. If history of asymptomatic COVID−19, 14 days must have elapsed since diagnosis and must have two negative NAT tests 24–48 hours apart. |
- Abbreviations: NAT, Nucleic acid test; BAL, Bronchoalveolar lavage; HCW, health care worker; PPE, personal protective equipment.
3 EPIDEMIOLOGY AND OUTCOMES
As the COVID-19 pandemic developed, the impact on transplant recipients began to emerge with reports initially from China, then Italy and Spain.56-70 The prevalence of infection in SOT during the first wave of the pandemic varied geographically. In New York City, 22 (5.5%) of approximately 400 heart transplant recipients followed at a single center acquired COVID-19.71 Similarly, 5% (66/1216) kidney transplant recipients in a French surveillance program were identified with COVID-19.72 A study from 12 kidney transplant centers following 9845 patients reported 144 (1.5%) kidney transplant recipients hospitalized with COVID-19 over a 9-week period.73 The largest and most comprehensive evaluation to date interrogated the UK transplant registry over a 4 months period from February to May 2020.74 Positive testing for SARS-CoV-2 was identified in 3.8% (197/5184) of waitlisted patients and 1.3% (597/46789) of transplant recipients.
The average age of SOT recipients with COVID-19 ranged from 50 to 71 years and presented an average of 3–6 years posttransplant.17, 71, 75-80 According to several reports, Blacks and Hispanics were disproportionately affected with US centers reporting 39–100% of SOT admissions with COVID-19 involving Black patients and one center noting that 15% of COVID-19 infected patients were Hispanic.81-83 These differences were seen globally with 40% Hispanic and 25% Black in cases from the TANGO collaborative of centers in Spain, Italy, and the United States.73 More extensive investigation is underway to address the underpinning causes of these differences which may be related not only to local penetrance of SARS-CoV-2 and prevention measures but also to significant concerns of systemic bias related to socioeconomic status and race.
The presentation of SOT recipients with COVID-19 appears similar to the general population. Fever (61–83%), cough (45–75%), and diarrhea (22–57%) were the most common symptoms reported.14-16, 71, 75, 79, 80, 83-88 Abnormalities in chest imaging occurred frequently; cohorts of SOT recipients in New York City reported abnormal chest radiographs in 96%–100%.83, 84 As testing availability increased and understanding of the spectrum of symptoms improved, the proportion of SOT recipients with abnormal initial chest radiographs decreased to 50–75%.75, 89 In the limited number of patients with initial chest CT, all were abnormal with half (4/8) showing infiltrates in more than 50% of the lung.89
Hospitalization, morbidity, and mortality from COVID-19 ranged broadly across populations and countries. Reported hospitalization rates ranged from 32 to 78% in most studies that included outpatients.14-17 However, reporting bias certainly occurred, especially in large cohorts with voluntary reporting of cases and limited testing availability early in the pandemic for non-hospitalized patients. In recent international cohorts, 78–89% of identified patients were hospitalized which is higher than in the general public, although this also may reflect reporting or testing bias of differential health care utilization for transplant patients.17, 90 Once hospitalized, rates of transition to the intensive care unit ranged from 8.6% in the Netherlands to 18%–34% in other cohorts internationally.15, 17, 33, 79, 84, 91, 92 Intubation and non-invasive ventilation ranged broadly from 8 to 60% in smaller cohorts 78, 79, 83, 93; however, the largest cohorts reported 30%–39% non-invasive ventilation or intubation.17, 84, 92 Pre-existing comorbidities associated with disease, morbidity, and mortality in the general population have been frequently reported in SOT recipients with COVID-19, potentially impacting the high rates of hospitalization and severe disease. At least one comorbidity was recorded in 18 of 26 (69%) heart transplant recipients from Italy and in 443 of 482 (92%) SOT recipients in a large multi-national cohort.17, 94 Hypertension (9%–94%), diabetes mellitus (41%–69%), and chronic kidney disease (37–89%) were most common.17, 72, 83, 84, 86, 92, 95
Across all organ types, acute kidney injury (AKI) was reported in 20%–70% of hospitalized patients.73, 80, 85, 86, 89, 96 In comparison to non-transplant patients admitted for COVID-19, statistically significant increases in AKI during hospitalization were reported in SOT recipients (20% vs. 5%) with a trend to statistical significance in another SOT cohort being compared to critically ill non-transplant patients (37% vs. 27%).86, 96 Unusual complications observed in kidney and heart transplant recipients included encephalopathy, renal infarction, and the appearance of donor-specific antibodies.97-101 Incidence of superinfection occurred at a higher rate in SOT recipients compared to controls (50% vs. 15.5%).102
Mortality ranged from 9 to 46% with most in the 18–30% range depending on the cohort and circumstances.14, 15, 17, 33, 72, 73, 75, 77-79, 83-85, 88, 89, 91, 94, 95, 103, 104 All-cause mortality during the study period of the UK registry reached 26% for SOT recipients and 10% for those on the waitlist, although local utilization decisions related to resource availability may have impacted these numbers.74 The largest cohort to date including 482 SOT recipients from more than 50 transplant centers reported 20.5% mortality, and two compilations of cases found 18%–19% mortality overall.17, 87, 88 In a UK database analyzing 10926 COVID-19 related deaths, SOT recipients had a hazard ratio of 3.53 [95% CI 2.77–4.49] for death as compared to the general population.105 Mortality rates may be biased by hospitalization. In a small study among 35 SOT recipients that was restricted to hospitalized patients, morbidity and mortality was similarly high compared to hospitalized non-transplant patients (48% vs. 40%).86 Intubation portended poor outcome with 40%–100% of ventilated patients dying in small cohorts.71, 92 Interestingly, according to a series of 26 pediatric SOT recipients, children did not suffer significant morbidity, with none requiring oxygen support and all recovering within 7 days, mirroring the less severe course described in immunocompetent pediatric patients.106 Many studies have addressed risk factors for mortality with older age,17, 73, 74, 77, 86, 89, 95, 105 underlying cardiovascular or lung disease,17, 77, 95 increased inflammatory markers73, 77, 89 and lymphopenia17, 73 most commonly associated with increased mortality. Additional risk factors included obesity17, 95 and pre-existing frailty.91 More recently, the presence of SARS-CoV-2 viral RNAemia was reported as increased risk for both disease severity and mortality in kidney transplant recipients, while viral load from swabs of the upper respiratory tract was not related to disease severity.20 Differences in the intensity of immunosuppression did not appear to affect mortality 17, 77 aside from a report in heart transplant recipients where discontinuation of immunosuppression was associated with mortality.94
Persistence of symptoms including fatigue and dyspnea for more than 60 days has been reported in a non-transplant population from northern Italy;107 however, data on long-term patient and graft outcomes among transplant recipients are currently lacking.
4 SARS-COV-2 TESTING IN TRANSPLANT RECIPIENTS
Direct SARS-CoV-2 assays and information on infectivity in the setting of organ transplantation are essential to identify infected patients, to discontinue isolation and to screen potential donors, candidates and recipients. Specimen sources include nasopharyngeal, nasal or throat swabs, and bronchoalveolar lavage (BAL) with polymerase chain reaction (PCR) platforms or antigen testing employed. With early testing platforms, negative initial testing did not eliminate the possibility of infection. In one cohort, as many as 8% of SOT recipients with initial negative SARS-CoV-2 PCR had subsequent positive testing.84
Evidence from the general population including viral culture assays suggests that PCR positivity from nasopharyngeal swabs declines within 3 weeks, whereas infectivity already decreases within 8 days after symptom onset, respectively.108-110 Longer PCR positivity may apply for BAL or sputum samples. Interestingly, prolonged duration of positive PCRs from nasopharyngeal swabs was reported fairly early in the pandemic in SOT recipients. A positive PCR was discovered in a heart transplant recipient 35 days after onset of symptoms, and in a kidney transplant recipient 63 days after onset despite positive serologic response on day 47.18, 19 Other cases in kidney and lung recipients have confirmed prolonged PCR positivity at more than 30 days post-symptom onset,21 with up to 25% of cases in a French cohort.20 Additional data regarding prolonged PCR positivity and potential transmission of replication-competent SARS-CoV-2 from SOT recipients are needed to address issues around infection prevention and isolation practices, as correlation between PCR positivity and infectivity in SOT recipients remains uncertain. Rapid antigen tests with acceptable performance characteristics are now becoming available; this may improve screening time in some settings.111
Indirect assays such as SARS-CoV-2-specific serology and cellular immunity have been evaluated in limited circumstances in SOT recipients. Serologic response to SARS-CoV-2 has been reported in cases and series of kidney and lung recipients.21, 112 In a small study among seven patients admitted to the hospital, all developed IgG against the nucleocapsid protein of SARS-CoV-2 between 5 and 27 days after the onset of symptoms.21 In 116 samples from 35 kidney transplant recipients either IgM or IgG against SARS-CoV-2 recombinant nucleocapsid and spike antigens were positive in all survivors and samples more than 14 days after symptom onset, and sustained through day 59.20 Data from the general population suggest that SARS-CoV-2-specific CD4 and CD8 T cells are induced shortly after infection, contributing to viral control,6, 10 which also was observed in a case report of a renal-pancreas recipient 113 and a small series of kidney transplant recipients.114 However, no data are available on the stability of cellular immunity in the long term. As new data continue to emerge, understanding the role of serology and specific T cells in defining prior infection or in predicting outcome, recovery, and protection from reinfection will be essential.
5 TREATMENT STRATEGIES INCLUDING IMMUNOSUPPRESSION-RELATED MODIFICATIONS
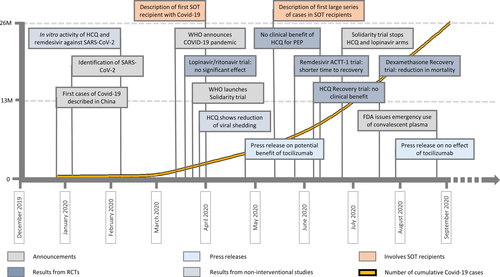
Given the high mortality associated with COVID-19 in hospitalized patients, and in particular for SOT recipients, several antiviral or immunomodulatory drugs have been given as compassionate use for therapy of COVID-19 since the beginning of the pandemic (Figure 2).
5.1 Antiviral therapy
Antiviral drugs for SARS-CoV-2 were initially chosen based on observational data obtained during the SARS-CoV-1 and MERS-CoV outbreaks,115, 116 or in vitro activity against SARS-CoV-2.117, 118 The first series of cases of SOT recipients infected with SARS-CoV-2 showed that a significant percentage of patients were treated with hydroxychloroquine (ranging from 25% to 90%) and/or lopinavir/ritonavir (3%-50%).17, 75, 84, 89 None of these drugs have shown efficacy in clinical trials and are currently not recommended.119, 120
The most promising antiviral drug tested for COVID-19 is remdesivir.121 Remdesivir is an inhibitor of the viral RNA-dependent RNA polymerase with in vitro activity against SARS-CoV-2. EC50 of remdesivir in in vitro models against MERS-CoV and SARS-CoV-2 was 0.09 µM and 0.77 µM, respectively.117, 122 A double-blind placebo controlled trial including more than 1000 patients given 10 days of remdesivir treatment showed a significant reduction of time to recovery from 15 days in the placebo group to 10 days in the remdesivir group (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49).123 However, reduction of mortality was not statistically significant. The beneficial effect of remdesivir was predominantly seen in patients needing oxygen but was not apparent in those on mechanical ventilation or on ECMO. Of note, no significant toxicity has been observed in trials using remdesivir, or in case reports specifically in SOT recipients.66 Drug-drug interaction with immunosuppression has not been described and is not anticipated. More recently, the Solidarity trial, a large international WHO-sponsored trial compared 2700 patients receiving remdesivir with the local standard of care with preprint preliminary results available124 and noted no effect of remdesivir on mortality (10.9% vs. 11.1% with standard of care). A meta-analysis of all existent interventional data on remdesivir was included in the Solidarity publication and showed a risk ratio (RR) of death with remdesivir of 0.91 (95% CI 0.79–1.05, p = .20), being 0.80 (95% CI 0.63–1.01) in patients without mechanical ventilation. Thus it appears that remdesivir should be given early after the infection onset to reduce viral load and avoid the development of the cytokine storm phase in patients who may have risk factors for a worse outcome, a category that includes SOT recipients.121
Administration of convalescent plasma (CP) of infected patients has been approved for emergency use in the US based on observational data that includes an acceptable safety profile.125 Although the first randomized clinical trial was underpowered and failed to show clear clinical benefit across all patients, it appears that CP is superior in reducing viral load and time to clinical improvement when administered early in the disease course rather than after the onset of life-threatening disease.126 A more recent trial compared CP with standard of care in non-transplant patients (n = 464); no reduction in progression to severe disease or mortality was noted with CP,127 perhaps due to the presence of similar neutralizing antibody titers in both study arms. Thus, it seems that sufficient antibody titers may be essential to confer clinical efficacy. Data on the use of CP in SOT recipients are limited to case reports.128, 129 Apart from antibody treatment with CP, several highly active monoclonal antibodies against SARS-CoV-2 are currently under evaluation.130, 131 An anti-spike neutralizing antibody named LY-CoV555 has shown a reduction in SARS-CoV-2 viral loads in outpatients with COVID-19 in a phase II trial.132
5.2 Immunomodulatory therapy
The unique clinical course of COVID-19, with initial viral clearance followed by the development of a second clinical phase characterized by the release of inflammatory cytokines and coagulation factors, prompted the introduction of anti-inflammatory and immunomodulatory drugs for reducing the deleterious effects of the immune reaction to SARS-CoV-2. Given that interleukin-6 (IL-6) was elevated in patients with COVID-19, several studies assessed whether inhibiting IL-6 by blocking the IL-6 receptor with tocilizumab could have beneficial effects. In an uncontrolled study including 20 non-transplant patients with mild to moderate COVID-19 in China, a reduction in inflammatory parameters (including CRP) and clinical symptoms (fever, dyspnea) was observed after administration of tocilizumab; none of the patients died.133, 134 However, none of three recent randomized controlled trials having compared tocilizumab with standard of care and/or placebo in the general population has shown a reduction of mortality.135-137 In SOT recipients, a Spanish cohort of kidney transplant recipients receiving tocilizumab for COVID-19 reported an overall mortality of 32%. However, tocilizumab was given to patients with more severe disease, so that the effect of tocilizumab on mortality cannot be assessed.138 A case-control study involving 117 SOT recipients from New York showed that tocilizumab was not associated with a reduction of mortality.139
Steroids have been evaluated for treatment of COVID-19. The Recovery trial including more than 11000 patients in several arms, compared the efficacy of dexamethasone 6 mg once daily with the standard of care alone for treatment of COVID-19.140 Patients on dexamethasone had an overall 17% decrease in mortality (rate ratio, 0.83; 95% CI, 0.75–0.93). This effect was particularly seen in patients who received oxygen (rate ratio, 0.82; 95% CI, 0.72–0.94) and those on mechanical ventilation (rate ratio, 0.64; 95% CI, 0.51–0.81), but not in patients not receiving oxygen (rate ratio, 1.19; 95% CI, 0.91–1.55).140 A recent meta-analysis of seven randomized controlled trials confirmed the beneficial effect of steroids on reducing COVID-19 mortality (OR, 0.66; 95% CI, 0.53–0.82).141 These data indicate that steroids should be used in all patients with COVID-19 who need oxygen and/or mechanical ventilation. In SOT recipients, increasing the dose of prednisone or adding dexamethasone as part of the modulation of immunosuppressive therapy may be recommended in case of advanced disease.
Clinical trials are currently testing other immunomodulatory drugs such as Janus kinase inhibitors (baricitinib),142 IL-1 blockers (anakinra),143 and anti-C5 inhibitors (eculizumab) among many others.144
5.3 Management of immunosuppression and risk of rejection
Modification of the immunosuppressive regimen is part of the therapeutic prescription in SOT recipients who develop a viral infection. Transplant physicians usually suspend antimetabolites and/or reduce calcineurin inhibitors dosing in case of severe viral infection, such as CMV disease or influenza, in an attempt to restore antiviral immunity and consequently increase viral clearance.145 However, in patients with COVID-19 modulation of immunosuppression is a more pressing challenge, as most of the severe manifestations of COVID-19 are consequence of the imbalanced host response consisting of low expression of interferons and high expression of pro-inflammatory cytokines.146 Theoretically, maintenance of immunosuppression with inhibition of T cell immunity may have beneficial effects on reducing this inflammatory response.147 However, the potential benefit of immunosuppression in patients with COVID-19 is counterbalanced by the high number of comorbidities present in SOT recipients.75, 84 Experience in cohorts of SOT recipients showed that calcineurin inhibitors were held in 18%-29% of patients and antimetabolites were held in 66%-88% of patients during the clinical course of COVID-19.17, 84, 95 It has been hypothesized that belatacept, by blocking the costimulatory signal, may prevent a severe clinical course of COVID-19; however, reports of both mild and severe cases of COVID-19 in SOT patients receiving belatacept have been published.63, 148 Despite the lack of strong evidence on optimal immunosuppression management in SOT recipients,149, 150 a stepwise reduction of immunosuppression according to the severity of the clinical presentation may be appropriate. In asymptomatic patients and patients not requiring hospitalization, modification of immunosuppression may be deferred. In patients needing low-flow oxygen, a dose reduction of the metabolite and/or reduction of calcineurin inhibitors or mTOR inhibitor levels may be necessary, especially in patients receiving other immunomodulatory drugs. In more severe cases, including those requiring ICU admission with mechanical ventilation and/or ECMO, some centers have applied a more significant immunosuppression reduction strategy, with temporary discontinuation of all immunosuppressive drugs, except steroids. This strategy needs to be balanced with a potential increased risk for the development of acute rejection and/or graft loss, particularly in life-saving transplants. However, other experts propose continuation of calcineurin inhibitors (particularly cyclosporine) during advanced disease to control the inflammatory phase.149
Another matter of concern is a potential increase in acute rejection rates due to administration of less potent immunosuppressive regimens for transplant recipients transplanted during the pandemic. Data from the Scientific Registry of Transplant Recipients showed a reduction in the use of ATG induction after March 2020 as compared to previous months, despite the fact that ATG was associated with a reduction in acute rejection rates and had no effect on mortality.151 In addition, suboptimal posttransplant follow-up with concerns in drug compliance during lockdown may additionally result in increased rejection risk.152 However, few studies have assessed the rates of acute rejection associated with COVID-19 itself or due to modulation of immunosuppression during infection. While a significant rate of AKI has been reported in kidney transplant recipients, the actual incidence of acute rejection has not been systematically reported, mostly due to the absence of allograft biopsies performed.153 In a multicenter cohort involving 482 patients, only seven episodes of rejection were observed (six cellular and one humoral rejection).17 Other studies in kidney and heart recipients with COVID-19 did not report diagnosis of rejections, despite reduction or withholding of immunosuppression in significant proportions of patients.15, 94 Increased doses of steroids administered during COVID-19 may partially explain the observed low rates of acute rejection. In any case, the complex management of immunosuppression during the course of infection should be discussed in a multidisciplinary approach by transplant physicians, ICU doctors, and transplant infectious diseases specialists.
6 CONSIDERATIONS IN THE PERI-TRANSPLANT PERIOD
SARS-CoV-2 infections in the early period after transplantation appears to have a higher morbidity and mortality as compared to infections in long-term transplant recipients, which may be directly related to the intense immunosuppressive drug regimens including induction therapies. Among 36 patients, 2 of 10 patients who died were early transplant recipients with T cell depleting agents received within the previous 5 weeks.83 Similarly, two of five recent liver transplant patients died after nosocomial infection diagnosed 9 and 36 days after transplantation.154 Finally, among three kidney and one liver transplant recipients who contracted SARS-CoV-2 infection from an asymptomatic surgeon between 7 and 10 days after transplantation, one kidney recipient died after rapid clinical deterioration.155 Peri-transplant infection may also adversely affect graft outcome as suggested by a kidney transplant recipient with SARS-CoV-2 infection 24 days after transplantation who developed acute respiratory distress syndrome (ARDS) and AKI with induction of donor specific antibodies.101 Based on the recognition of this higher risk, multiple transplant organizations have released recommendations regarding protecting newly transplanted patients from acquiring SARS-CoV-2.41, 45 Potential reasons for COVID-19 in the peri-transplant period include asymptomatic infection of the recipient at or around the time of transplantation, donor-derived infections, community acquired infections by family members or social contacts, or nosocomial transmission by health care workers and/or patients in health care facilities.154-156 Screening of recipients and donors to exclude infection at the time of surgery seems mandatory as any type of surgery in a SARS-CoV-2 infected patient has been associated with significant postoperative pulmonary complications and high mortality. A European study involving 1128 patients with confirmed SARS-CoV-2 infection within 7 days before or 30 days after surgery were found to have a 30-day mortality of 23.8%, which increased to 38.0% among the 52.2% of patients with pulmonary complications.157 Thus, apart from significant comorbidities among transplant recipients and intense immunosuppression in the early transplant period, transplant surgery itself might impact the outcome of recipients with asymptomatic or donor-derived infection. Although proven donor-derived infections have not yet been reported, this may be more likely to occur in lung transplant recipients due to a high burden of viable virus in the lung allograft. Given detection of viral RNA in other organs such as the gastrointestinal tract,158, 159 liver 160 or kidney,161 transmission could also occur. The lack of donor-derived infections should not be considered as a low likelihood for transmission, but success of prevention policies including anamnesis to identify high-risk contacts and donor screening.41, 45, 162 Continued vigilance and testing will not only protect potential recipients but also health care and transplant procurement teams and prevent viral transmission between institutions during procurement. Transplantation of infected candidates and utilization of organs from donors with COVID-19 are currently only recommended after resolution of clinical symptoms and negative PCR testing. Initial reports of transplant recipients with resolved SARS-CoV-2 infection have shown favorable outcome.163 Case reports of inadvertent transplantation of asymptomatic SARS-CoV-2 positive donors without transmission to the recipient 164 may indicate a potential use of PCR positive donors for life-saving procedure, especially when more is known about the correlation between PCR positivity and infectivity.108-110 After transplantation, strict adherence to careful infection prevention strategies and physical distancing are important preventive measures to prevent SARS-CoV-2 acquisition.41, 45, 162 In SOT recipients who have contracted SARS-CoV-2, longer periods of PCR positivity 18-21 may require a longer duration of isolation and testing to reduce the risk for transmission.
7 TRANSPLANTATION AS TREATMENT FOR SEVERE COVID-19
Given concerns for irreversible organ damage especially to the lungs from COVID-19, there has been increasing interest in transplantation for COVID-19 induced end-stage lung disease. First cases of lung transplantation in patients with COVID-19 have been reported in China and in Austria. In China, five lung transplantations were reported in PCR negative patients; one patient died 1 day after surgery.165, 166 An Austrian team reported successful lung transplantation of a 44-year-old woman.167 Her PCR was still positive but non-infectivity was confirmed by negative culture. Two additional cases who had developed end-stage pulmonary fibrosis have been transplanted in the United States.168 Careful definition of clinical indications 169 and long-term results are needed to more safely delineate when to consider transplantation as a treatment option for end-stage lung disease.
8 CONCLUSIONS
SARS-CoV-2 and the resulting COVID-19 pandemic have had a profound impact on the world and SOT in particular. Our current understanding has benefitted from the immense productivity and collaboration of scientists and clinicians around the globe, specifically in describing the epidemiology and impact of the virus on transplantation. As data develop regarding the pathogenesis of the virus and its immunologic impact, emerging therapies will require investigation specifically in SOT recipients, a population in the whom the balance between viral control, immune activation, and preservation of graft function requires careful navigation. Global efforts in the development and clinical evaluation of SARS-CoV-2 vaccines have proceeded at an unprecedented pace. Several types of vaccines including attenuated or inactivated whole viruses, protein or peptide vaccines, viral vectors or viral nucleic acids are being explored with promising safety and immunogenicity profiles, and large phase 3 studies are currently being performed worldwide.170, 171 Apart from reluctance toward the use of attenuated whole viruses after transplantation, all vaccine types should hold promise for application in SOT recipients. Nevertheless, data on safety and immunogenicity are currently lacking for SOT, and the potential benefits of a SARS-CoV-2 vaccine in effectively reducing the burden of disease will need to be specifically evaluated in the transplant population. In the meantime, passive immunization with convalescent plasma with high neutralizing titers or with monoclonal antibodies may serve as a temporary intervention, especially early in infection. Challenges remain during the ongoing pandemic not only for transplant infrastructure related to resource availability but also from knowledge gaps in potential donor transmission and candidate optimization.
ACKNOWLEDGMENTS
The authors thank Allan Kirk and Sandy Feng for the opportunity to provide this review.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
L.D.-I., E.B., M.S., and O.M. contributed equally to the development and writing of this manuscript. All authors approved the final version of the manuscript.




