COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities
Abstract
Higher rates of severe COVID-19 have been reported in kidney transplant recipients (KTRs) compared to nontransplant patients. We aimed to determine if poorer outcomes were specifically related to chronic immunosuppression or underlying comorbidities. We used a 1:1 propensity score-matching method to compare survival and severe disease-free survival (defined as death and/or need for intensive care unit [ICU]) incidence in hospitalized KTRs and nontransplant control patients between February 26 and May 22, 2020. Patients were matched for risk factors of severe COVID-19: age, sex, body mass index, diabetes mellitus, preexisting cardiopathy, chronic lung disease, and basal renal function. We included 100 KTRs (median age [interquartile range (IQR)]) 64.7 years (55.3–73.1) in three French transplant centers. After a median follow-up of 13 days (7–30), transfer to ICU was required for 34 patients (34%) and death occurred in 26 patients (26%). Overall, 43 patients (43%) developed a severe disease during a median follow-up of 8.5 days (2–14). Propensity score matching to a large French cohort of 2017 patients hospitalized in 24 centers, revealed that survival was similar between KTRs and matched nontransplant patients with respective 30-day survival of 62.9% and 71% (p = .38) and severe disease-free 30-day survival of 50.6% and 47.5% (p = .91). These findings suggest that severity of COVID-19 in KTRs is related to their associated comorbidities and not to chronic immunosuppression.
Abbreviations
-
- ATG
-
- antithymocyte globulin
-
- BMI
-
- body mass index
-
- COVID-19
-
- coronavirus disease 2019
-
- CRP
-
- C-reactive protein
-
- CT
-
- chest-computed tomography
-
- eGFR
-
- estimated glomerular filtration rate
-
- FiO2
-
- Fraction of inspired oxygen
-
- HR
-
- hazard ratio
-
- ICU
-
- intensive care unit
-
- IL
-
- interleukin
-
- IQRs
-
- interquartile ranges
-
- KT
-
- kidney transplantation
-
- LDH
-
- lactic acid dehydrogenase
-
- MPA
-
- mycophenolic acid
-
- MV
-
- mechanical ventilation
-
- RT-PCR
-
- reverse transcriptase-polymerase chain reaction
1 INTRODUCTION
Since its emergence in China in December 2019 and its subsequent worldwide outbreak, coronavirus disease 2019 (COVID-19) has resulted in more than 1,300,000 deaths around the world as of November 2020. Several underlying conditions as older age (>65 years), gender (male), obesity, hypertension, cardiovascular disease, chronic lung disease, and chronic kidney disease have been associated with an increased intrinsic susceptibility of developing severe forms of disease and a higher risk of mortality.1-4
Kidney transplant recipients (KTRs) have been reported to be at high risk of developing severe forms of the COVID-19 pneumonia with a mortality rate ranging between 20% and 32%5-12 versus 1% and 14% in the general population.13-15 However, the specific impact of long-term immunosuppression on their outcome has not been elucidated yet. Indeed, apart from chronic immunosuppression that might increase the risk of severe COVID-19 diseases, most of KTRs have chronic conditions, mainly cardio-vascular disease and diabetes,16, 17 leading to higher risk of severe disease, including death.
A recent study18 compared COVID-19 outcome in 35 hospitalized solid organ recipients (including 26 KTRs) versus 100 consecutive nontransplants patients. Mortality among these two groups was comparable (23% vs. 25%, p = .8) despite a higher rate of underlying conditions in transplants patients, suggesting that transplantation itself is not a risk factor for severe COVID-19. Other studies suggest that transplant recipients could have favorable COVID-19 outcomes.19 However, no previous study compared COVID-19 outcome in transplant recipients presenting similar comorbidities than nontransplant patients to evaluate the impact of immunosuppression on disease severity in KTRs.
We aimed to report the influence of both comorbidities and long-term immunosuppression on COVID-19 severity in KTRs. Thus, we conducted a retrospective multicenter cohort study to compare survival and severe disease-free survival between KTRs and matched nontransplant patients hospitalized for COVID-19 using a propensity score matching.
2 MATERIALS AND METHODS
2.1 Patients
We included all KTRs hospitalized for COVID-19, between March 9, 2020 and May 2, 2020, in three French academic kidney transplantation (KT) centers: Necker Hospital, Paris; Foch Hospital, Suresnes and Bicêtre Hospital, Le Kremlin-Bicêtre.
Control group is issued from the cohort of a retrospective multicenter observational study, the Critical Covid-19 France (CCF) including 2878 patients hospitalized for COVID-19 in 24 medical centers in France (NCT04344327)20 (the three KT centers were not included in these centers). Immunosuppressed patients (i.e., hematologic stem cell or solid organ transplantation recipients, patients under chemotherapy or immunosuppressive treatments for systemic inflammatory diseases) and patients with missing data for one of the comorbidities needed for matching were excluded from control group.
COVID-19 was defined for all patients as the presence of a positive SARS-CoV-2 on reverse transcriptase-polymerase chain reaction (RT-PCR) testing performed on nasopharyngeal swab.
Data were collected retrospectively using hospital databases and clinical records in a similar manner for all patients (KTRs and nontransplanted patients). Each medical record was individually and manually reviewed by a medical doctor in each center. Data collection included demographic characteristics and underlying comorbidities of patients, COVID-19 infection characteristics and clinical course. For all patients, comorbidities were assessed with the same method using national recommendations. Diabetes was defined as the need for a pharmacologic anti-diabetic agent (oral or insulin therapy). Hypertension was defined as the need for at least one anti-hypertensive therapy. Cardiopathy was defined as a history of documented cardiopathy diagnosed by a cardiologist: coronary artery disease assessed by a coronarography, valvular, dilated, hypertrophic, cardiopathy, or arteriovenous fistula-associated high-output cardiac failure assessed by an echocardiography or atrial fibrillation assessed by an electrocardiogram or Holter monitoring. Chronic lung disease was defined as a history of documented lung disease on CT scan for bronchiectasis and pulmonary function tests (PFT) for COPD and asthma. Basal renal function was defined using estimated glomerular filtration rate (eGFR) according to MDRD and categorized into three categories: >60 ml/min, 30 to 60 ml/min, <30 ml/min, chronic dialysis.
For KTRs, transplantation history and characteristics including and immunosuppression regimen were assessed.
Clinical, biological and radiological COVID-19 severity criteria at admission were also collected to analyze patient's disease severity at admission in both groups. Fever was defined as a temperature ≥38°C. SpO2 was assessed using pulse oximeters oxygen saturation. Fraction of inspired oxygen (FiO2) was calculated as follow: Oxygen Flow (Liters) × 4 + 21%. Dyspnea was considered positive if NYHA score was III or IV. Moreover, disease severity at entry was evaluated using the 8-point WHO ordinal scale score.21 CT scan severity stages of pulmonary ground-glass opacities lesions were categorized as low (<30% involvement), moderate (30–50% involvement), or severe (>50% involvement).
2.2 Outcomes
Survival was defined as the time between the date of hospital admission and the date of death. Severe disease-free survival was defined as the time between the date of hospital admission and the date of intensive care unit (ICU) admission and/or the date of death. Follow-up delay was determined as the delay between hospital admission and ICU transfer and/or death or hospital discharge.
2.3 Propensity score matching
KTRs were matched to nontransplant patients by propensity score, using a 1:1 ratio, nearest neighbor method and a caliper of 0.4 with the following covariates: age, sex, body mass index (BMI), diabetes mellitus, preexisting cardiopathy (coronary artery disease, valvular cardiopathy and “other” cardiopathy including hypertrophic cardiopathy, rhythmic cardiopathy, and arteriovenous fistula-associated high-output cardiac failure), hypertension, chronic lung disease (chronic obstructive pulmonary disease, asthma, and bronchiectasis) and eGFR according to MDRD. The balance of covariate distribution between the two groups was assessed using standardized mean difference (SMD).
2.4 Statistical analysis
Continuous variables were described using medians and interquartile ranges (IQRs) and compared using the Mann-Whitney rank sum test. Categorical variables were described using proportions and compared using chi-square test or fisher's exact test. Survival and severe disease-free survival were analyzed using an univariable Cox regression and represented using Kaplan-Meier curves and compared using a two-sided log-rank test. Center effect was analyzed using a frailty modeling in which random center effect was included in the Cox model. Statistical analyses were performed using Stata and RStudio (Version 1.2.1335). All statistical tests were two-sided, and p values <.05 were considered significant.
3 RESULTS
3.1 Patient characteristics
Overall, 100 KTRs required hospitalization for COVID-19. Their basal characteristics are detailed in Table 1. Of them, 64 (64.0%) were male, median age was 64.7 years (55.3–73.1) and median body mass index (BMI) was 25.9 kg/m2 (21.9–28.2). Thirty-five patients (35.0%) had a preexisting cardiopathy, including 18 (18.0%) and 20 patients (20.0%) with coronary artery disease and cardiac arrhythmia, respectively, while 85 patients (85.0%) had hypertension and 48 (48.0%) diabetes mellitus. Eleven patients (11.0%) had chronic lung disease. Immunosuppressive induction consisted in antithymocyte globulin (ATG) in 52 patients (62.7%). At COVID-19 episode, maintenance immunosuppressive regimen was CNI based in 78 patients (83.0%) and mTORi in 8 patients (8.5%). Median basal serum creatinine was 141 μmol/l (108–197) and median estimated glomerular filtration rate (eGFR) 43.3 mL/min/1.73 m2 (29.6–59.6).
| Variable |
KTRs (N = 100) |
Controls (N = 2017) |
p-value |
|---|---|---|---|
| Age (year), median (IQR) | 64.7 (55.3–73.1) | 67.5 (55.4–79.6) | .033 |
| Men, n (%) | 64 (64) | 1167 (57.9) | .224 |
| BMI, kg/m2, median (IQR) | 25.9 (21.9–28.2) | 27.0 (24.0–31.1) | .0003 |
| Coexisting conditions | |||
| Hypertension, n (%) | 85 (85) | 1009 (50.0) | <.001 |
|
Cardiopathy, n (%) |
<.001 | ||
| None | 65 (65) | 1588 (78.7) | |
| Coronary artery disease | 18 (18) | 221 (11.0) | |
| Valvular heart disease | 1 (1) | 81 (4.1) | |
| Dilated heart disease | 3 (3) | 28 (1.4) | |
| Other cardiopathy | 13 (13) | 99 (4.9) | |
| Atrial fibrillation, n (%) | 20 (20) | 286 (14.2) | |
| Diabetes, n (%) | 48 (48) | 594 (29.4) | <.001 |
| Chronic lung disease, n (%) | .420 | ||
| None | 89 (89) | 1722 (85.4) | |
| Chronic obstructive pulmonary disease | 4 (4) | 108 (5.3) | |
| Asthma | 3 (3) | 131 (6.45) | |
| Bronchiectasis | 4 (4) | 56 (2.8) | |
| eGFRa, n (%) | <.001 | ||
| eGFR <30 mL/min/1.73 m2 | 26 (26) | 49 (2.4) | |
| eGFR 30–60 mL/min/1.73 m2 | 52 (52) | 190 (9.4) | |
| eGFR >60 mL/min/1.73 m2 | 22 (22) | 1778 (88.1) | |
| Follow-up, median (IQR) | 13 (7–30) | 7 (4–12) | <.001 |
- Abbreviations: BMI, body mass index; CNIs, calcineurin inhibitors; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
- a Determined with the MDRD equation.
For control group, 2017 patients were included before matching (Table 1). Of them, 1167 (57.9%) were male, median age was 67.46 years (55.4–79.6) and median BMI 27.0 kg/m2 (24.0–31.1). Controls had a lower rate of coexisting comorbidities: 429 patients (21.3%) had a preexisting cardiopathy, 1009 (50.0%) hypertension, 594 (29.4%) diabetes mellitus and 295 (14.6%) chronic lung disease.
3.2 COVID-19 presentation and outcome in KTRs
Table 2 shows COVID-19 presentation and management in KTRs. COVID-19 occurred after a median time of 5.1 years (3.0–12.6) after kidney transplantation.
| Variable |
KTRs (N = 100) |
n |
|---|---|---|
| Antithymoglobulin induction, n (%) | 52 (62.7) | 83 |
| Baseline serum creatinine, µmol/L, median (IQR) | 141 (108–197.3) | 100 |
| Baseline eGFRa, ml/min, median (IQR) | 43.3 (29.6–59.6) | 100 |
| Time from KT to COVID−19 (year), median (IQR) | 5.1 (2.8–12.6) | 100 |
| Immunosuppressive therapy at admission | ||
|
CNIs, n (%) |
78 (83.0) | 94 |
|
Mycophenolic acid, n (%) |
69 (73.4) | 94 |
| Azathioprine, n (%) | 7 (7.4) | 94 |
| mTOR inhibitors, n (%) | 8 (8.5) | 94 |
| Steroids, n (%) | 91 (96.8) | 94 |
| Belatacept, n (%) | 10 (10.6) | 94 |
| Presenting symptoms at admission | ||
| Fever, n (%) | 67 (72.0) | 93 |
| Diarrhea, n (%) | 32 (36.4) | 88 |
| Cough, n (%) | 55 (63.2) | 87 |
| Dyspnea, n (%) | 41 (46.1) | 89 |
| Myalgia, n (%) | 24 (30.0) | 80 |
| Anosmia/ageusia, n (%) | 10 (11.5) | 87 |
| Treatment | ||
| CNI withdrawal, n (%) | 36 (40.0) | 90 |
| Antimetabolite withdrawal, n (%) | 56 (78.9) | 71 |
| Belatacept withdrawal, n (%) | 8 (80.0) | 10 |
| Azithromycin, n (%) | 38 (45.2) | 84 |
| Hydroxychloroquine, n (%) | 11(12.9) | 85 |
| Tocilizumab, n (%) | 14 (15.9) | 88 |
| Outcome | ||
| Oxygen therapy, n (%) | 79 (84.0) | 94 |
| Hospitalization in ICU, n (%) | 34 (34) | 100 |
| Mechanical ventilation, n (%) | 29 (29) | 100 |
| Death, n (%) | 26 (26) | 100 |
| Discharged from hospital, n (%) | 62 (62) | 100 |
- Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KTRs, kidney transplant recipients; ICU, intensive care unit; KT, kidney transplantation; mTOR, mammalian target of rapamycin.
- a Determined with the MDRD equation.
The most common initial symptoms were fever (n = 67, 72.0%), cough (n = 55, 63.2%), dyspnea (n = 41, 46.1%), and diarrhea (n = 32, 36.4%). Anosmia and/or ageusia concerned 10 patients (11.5%).
Oxygen therapy was necessary for most patients (n = 79, 84.0%). Transfer to ICU was required for 34 patients (34.0%), in a median 1 day after hospitalization (0–3.75), and 29 of them underwent mechanical ventilation (MV). Median hospitalization time in ICU was 13.5 days (8.3–23). After a median follow-up of 13 days (7–32), 26 patients (26.0%) died including 17 (65.4%) managed in ICU, while 62 patients (62%) were discharged from hospital. Acute rejection episode occurred in 2/100 KTRs (2%) after COVID-19 episode.
3.3 Severe COVID-19 prevalence is not increased in hospitalized KTRs
Propensity score method allowed the matching of 83 KTRs (83.0%) to 83 controls.
Table 3 confirms that these two groups are highly comparable in terms of age, sex, and comorbidities and Figure 1 assesses covariate balance (Figure 1A) and distribution of the propensity score (Figure 1B) between matched KTRs and controls as well as unmatched KTRs and controls.
| Variables |
KTRs group (N = 83) |
Control group (N = 83) |
p-value |
|---|---|---|---|
| Age (years), median (IQR) | 67.2 (58.5–74.1) | 65.1 (56.1–79.3) | .873 |
| Men, n (%) | 52 (82.6) | 55 (82.7) | .627 |
| BMI (kg/m2), median (IQR) | 26.6 (22.7–28.7) | 26.3 (22.6–29.4) | .982 |
| Coexisting conditions | |||
| Hypertension, n (%) | 68 (81.9) | 62 (74.7) | .258 |
| Cardiopathy, n (%) | .924 | ||
| None | 54 (65.1) | 50 (60.2) | |
| Coronary artery disease | 15 (18.1) | 16 (19.2) | |
| Valvular heart disease | 1 (1.2) | 2 (2.4) | |
| Dilated heart disease | 3 (3.6) | 5 (6.0) | |
| Other cardiopathy | 10 (12.0) | 10 (12.0) | |
| Atrial fibrillation, n (%) | 19 (22.9) | 16 (19.3) | |
| Diabetes Mellitus, n(%) | 41 (49.4) | 44 (53.0) | .641 |
| Chronic lung disease, n (%) | .952 | ||
| None | 73 (88.0) | 73 (88.0) | |
| Chronic obstructive pulmonary disease | 4 (4.8) | 4 (4.8) | |
| Asthma | 3 (3.6) | 4 (4.8) | |
| Bronchiectasis | 3 (3.6) | 2 (2.4) | |
| eGFRa, n (%) | .436 | ||
| eGFR <30 mL/min/1.73 m2 | 19 (22.9) | 21 (25.3) | |
| eGFR 30–60 mL/min/1.73 m2 | 42 (50.6) | 34 (41.1) | |
| eGFR >60 mL/min/1.73 m2 | 22 (26.5) | 28 (33.7) | |
| Follow-up (days), median (IQR) | 13 (9–21) | 9 (4–13) | .001 |
- Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KTRs, kidney transplant recipients.
- a Determined with the MDRD equation.
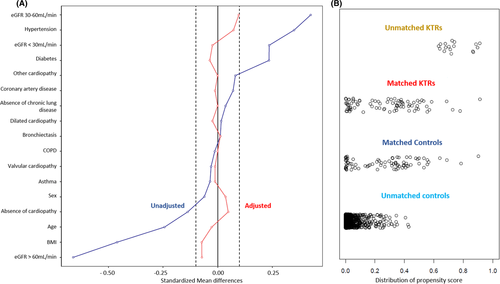
At admission, disease severity was similar in both groups (Table 4). KTRs tended to have more disease severity criteria with higher fever, CRP, troponin rates and more severe forms at CT scan. Median WHO ordinal scale score was similar in both groups (3 IQR [3–4] and 3 IQR [3–4], respectively, in KTRs and non-KTRs, p = .11).
| Variables |
KTRs group (N = 83) |
Control group (N = 83) |
p-value |
|---|---|---|---|
| Presenting symptoms at admission | |||
| Fever, n (%) | 49 (75.4) | 27 (33.4) | <.0001 |
| Dyspnea, n (%) | 33 (51.6) | 40 38 (52.8) | .887 |
| Oxygen therapy at admission, n (%) | 34 (55.7) | 34 (43.6) | .174 |
| FiO2 (%), median (IQR) | 25 (21–33) | 21 (21–29) | .132 |
| 8-points WHO ordinal scale score, median (IQR) | 3 (3–4) | 3 (3–4) | .11 |
| Laboratory findings at admission, median (IQR) | |||
| C-reactive protein (mg/dL) | 91 (45.5–166) | 64.4 (24.3–113.5) | .0215 |
| D-dimer (µg/L) | 723 (428–1307) | 630 (323–1201) | .3120 |
| LDH (UI/L) | 289.5 (229–400) | 288.5 (227–356) | .7139 |
| Troponin (µg/L) | 23 (12.6–44) | 10.3 (0.07–34) | .005 |
| Abnormalities on chest CT, n (%) | |||
| Low (<30%) | 8 (19.5) | 23 (42.3) | .012 |
| Moderate (30–50%) | 14 (13.5) | 21 (38.9) | |
| Severe (>50%) | 18 (46.3) | 10 (16.1) |
- Abbreviations: CT, computed tomography; FiO2, fraction of inspired oxygen; IQR, interquartile range.
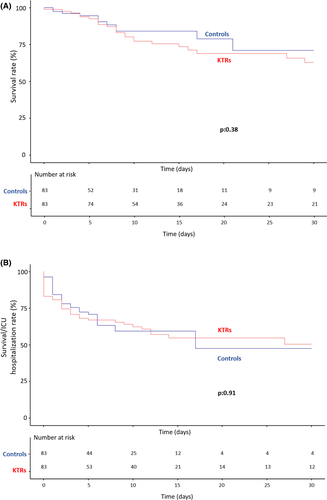
When adjusted to preexisting conditions, survival and severe disease-free survival were similar between KTRs and matched nontransplant patients with respective 30-day survival of 62.9% and 71.0%, p = .38. (Figure 2A) and respective 30-day severe disease-free survival of 50.6% and 47.5%, p = .91, (Figure 2). Overall survival HR was 1.375 (95% CI [0.667–2.832], p = .388 and severe disease-free survival (HR) was 1.03 (95% CI [0.632–1.673], p = .911) and for KTRs group.
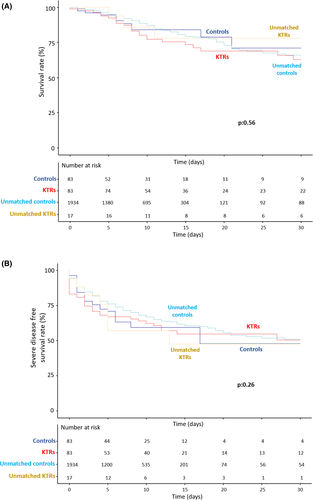
These results remain unchanged when unmatched patients (17 patients in KTRs group and 1934 in control group) were added to the analysis (Figure 3).
Results for severe disease-free survival remained equivalent after including the Center effect in the Cox model (HR = 1.03; 95% CI [0.632–1.673]; p = .911). However, the low number of deaths per center did not allow us to adjust for Center in the Cox Model for overall survival. Both overall and disease-free survivals were similar between the three transplant centers (p = .319 and p = .995, respectively).
Of note, KTRs follow-up was significantly longer than non-KTRs despite similar severity outcomes. This was due to a longer length of hospital stay in KTRs (13 [9–21] days vs. 9 [4–13] days in non-KTRs, p = .001) despite a similar discharge rate (58 [69.9%] vs. 51 [63%], p = .348). Indeed, because they were considered as vulnerable to infection, KTRs were usually hospitalized longer in transplant centers comparing to non-KTRs.
4 DISCUSSION
In our multicenter study of KTRs hospitalized for COVID-19, 43% of patients developed a severe disease and 26% of patients died during a median follow-up of 13 days (7–30), comparable to that of previous reports.5-12 However, when matched to control patients with similar chronic conditions and similar disease severity at admission, kidney transplantation was not associated with a higher rate of severe disease, that is, in-hospital mortality or hospitalization in ICU.
KTRs have been shown to be particularly vulnerable to COVID-19, presenting a high risk of developing severe forms of disease with a mortality rate between 20 and 32%5-11 versus 1 to 14% in the general population. Underlying comorbidities as well as chronic immunosuppression have been hypothesized to be responsible for progression to severe disease. In a large international study (TANGO International Transplant Consortium) reporting COVID-19 presentation and outcome in KTRs,11 the only intrinsic risk factor for severe COVID-19 was age >60 years. There was no significant difference in outcomes between patients with less than 1 year since transplantation compared to those with longer time since transplantation, nor between patients receiving depleting agents as induction therapy compared to non-depleting agents, suggesting that the degree of immunosuppression does not have a major impact on disease severity.
To date, the specific relationship between COVID-19 outcome and immunosuppressive state has not yet been elucidated. An effective antiviral response requires the activation of both innate and adaptive immunity and production of proinflammatory cytokines to control viral replication and resolve the infection.22 Long-term immunosuppression may then predispose patients to severe forms of infectious disease, and increased mortality due to an ineffective immune response, particularly CD4+ and CD8+ T cell responses. Conversely, COVID-19 has been associated with an immune dysregulation characterized by the overproduction of proinflammatory cytokines, particularly interleukin IL-6, IL-1, and TNF-α23, 24 and chemokines, leading to a “cytokine storm” responsible for tissue injury and progression to acute respiratory distress syndrome. Immunosuppression could then be theoretically protective against the hyperinflammation responses observed in COVID-19.
In our study, KTRs did not show poorer survival or severe disease-free survival than matched nontransplant patients with similar chronic conditions suggesting that poor outcomes in this population could be related to chronic conditions rather than chronic immunosuppression. Our results confirm those of Chaudhry's et al.18 that reported a similar rate of mortality, need for ICU care, and mechanical ventilation support in COVID-19 hospitalized solid transplant recipients compared to nontransplant controls. Altogether, these results suggest that transplant status by itself does not confer an increased predictive risk of mortality or severe disease.
As generally described in KTRs,16, 17, 25-28 previous KTRs COVID-19 studies showed a higher prevalence of hypertension (71.5 to 94%), diabetes mellitus (15 to 69%) and cardio-vascular disease (15 to 22.2%)5-10 compared to nontransplant patients reported in international cohorts.13-15 This might explain the high mortality rate reported in transplant populations. In line with this observation, Pereira et al. reported that patients hypertension was significantly associated with severe COVID-19.7 Conversely, comorbidities were not associated with mortality in the TANGO International Transplant Consortium study.11
Since the beginning of COVID-19 outbreak, many institutions have been concerned about potential increased susceptibility of KTRs to COVID-19. As a consequence, a major reduction in solid organ transplantation procedures was observed, mainly driven by KT.29 Our findings suggest that KT is not associated in itself with an increased vulnerability to COVID-19. Interestingly, mortality was not increased in patients experiencing COVID-19 during the first-year post transplantation (4 deaths among 17 patients). This highlights the importance of resumption of KT activity to avoid the increased morbi-mortality for patients on waiting list. However, one should remind that these patients, both on dialysis and KTRs after transplantation, have several underlying comorbidities predisposing to severe COVID-19. A large recent study found a COVID-19 mortality risk 1.28 (1.02–1.60) times higher in KTRs comparing to patients receiving dialysis. However, these patients was not matched on associated comorbidities30 Further studies are required to evaluate the risk of severe COVID-19 in patients receiving a kidney transplant compared to those on the waiting list and dialysis.
Our study has several limitations. It is a retrospective study, with a relatively short follow-up. However, most severe diseases occur in a short delay after diagnosis. In addition, we evaluated only hospitalized patients for moderate or severe COVID-19, which might be a bias of selection that does not allow us to study mild COVID-19 forms in KTRs. Another limitation is that we could only evaluate the center effect for disease-free survival (and not overall survival) due to the low number of deaths per center. However, the multicenter nature of our study remains a major strength. In addition, we observed these similar outcomes between KTRs and non-KTRs after immunosuppressive therapy reduction in KTRs. Thus, further studies are needed to compare COVID-19 outcomes in KTRs treated by full doses versus reduced doses of immunosuppressive therapy during COVID-19 episode.
5 CONCLUSION
In conclusion, KTRs present a high risk of developing severe COVID-19. However, after adjusting for major comorbidity, survival and severe disease-free survival were similar to nontransplant patients. Thus, long-term immunosuppression does not seem to increase the risk of developing a severe form of the COVID-19.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
Dataset available from the corresponding author at [email protected].




