Association between Mycoplasma and Ureaplasma airway positivity, ammonia levels, and outcomes post–lung transplantation: A prospective surveillance study
Abstract
Hyperammonemia syndrome (HS) is a rare complication with high mortality described after lung transplantation. Its pathophysiology is still unclear, but previous studies, including murine models, have linked the identification of Mycoplasmataceae in airway specimens with HS occurrence. This study explores the association between Mycoplasmataceae polymerase chain reaction (PCR) positivity, ammonia levels, HS, and mortality post–lung transplant. Adults who underwent lung transplantation between July 2017 and August 2019 had prospective surveillance testing for Mycoplasma and Ureaplasma using PCR on post-operative bronchoscopy samples. One hundred and fifty-nine patients underwent lung transplantation during the study period. Mean age was 54 (±13) years; baseline diseases were predominantly pulmonary fibrosis (37.7%) and chronic obstructive pulmonary disease (35.8%). Mycoplasma and/or Ureaplasma airway positivity was found in 42 (26.4%) of tested patients, represented mostly by M. salivarium (26/43; 60.4%), U. parvum (7/43; 16.2%), and U. urealyticum (5/43; 11.6%). Median peak ammonia levels were higher in those with Ureaplasma colonization compared to uncolonized patients (p = .04), however, only three patients developed HS. Recipient airway Ureaplasma positivity was independently associated with younger (aOR 0.94, 95% CI 0.88–0.99, p = .04) and female donors (aOR 4.29; 95% CI 1.01–18.2, p = .05).
Abbreviations
-
- CSA-IRD
-
- Canadian Standards Association increased-risk donor
-
- HS
-
- hyperammonemia syndrome
-
- IQR
-
- medians and interquartile ranges
-
- NAAT
-
- nucleic acid amplification testing
-
- NML
-
- National Microbiology Laboratory
-
- OPO
-
- organ procurement organization
-
- PCR
-
- polymerase chain reaction
-
- SDs
-
- standard deviations
-
- SOFA
-
- Sequential Organ Failure Assessment
1 INTRODUCTION
Hyperammonemia syndrome (HS) is a rare complication of immunocompromised hosts described in both adults and children following hematological malignancy, stem cell or solid organ transplantation, particularly in lung transplants.1-6 It was firstly described in lung transplant patients by Lichtenstein and Kotloff in 1993.7 This syndrome is characterized by elevated serum ammonia levels associated with worsening sensorium (i.e., lethargy, confusion, and agitation), seizures, and normal liver enzymes. Cerebral edema may develop, leading to death in some cases.8-10 Previous studies have demonstrated a relationship between Ureaplasma urealyticum, Ureaplasma parvum, and Mycoplasma hominis identification, mostly but not limited to airway specimens, and the development of HS.4-6, 8, 11-13 An immunocompromised murine model also demonstrated a relationship specifically between Ureaplasma urealyticum infection and HS.14
The occurrence of HS in lung transplant patients is rare, estimated between 1 and 4%, but mortality rates range from 69% to 75%. The syndrome typically presents within 3 weeks posttransplant, with a median time of onset between days 6 and 9.15-17 There is still no consensus on ammonia thresholds to guide clinical interventions, but most reported HS cases have ammonia levels twice the upper limit of normal,3 with ammonia peaks ranging from 193 μmol/L to 1000 μmol/L.5, 17, 18 Hyperammonemia pathogenesis is thought to be related to the overgrowth of urea-splitting organisms leading to increased urea hydrolysis and subsequent elevation of serum ammonia.4
The reasons for overgrowth and predilection of Mycoplasmataceae in transplanted lungs are still unknown, however, it has been hypothesized by many authors to be donor derived.12, 19 Others believe that local immunosuppression and the use of broad spectrum antimicrobials peri-transplant might play a role in their proliferation.14, 20 A recent prospective study identified Ureaplasma positivity in 14.2% of lung-transplant donor-recipient matched pairs, and associated Ureaplasma positivity with donors who had multiple sexual partners and aspiration events prior to brain death.13
Previous studies have tested bronchoalveolar samples for Mycoplasma and Ureaplasma using nucleic acid amplification testing (NAAT) and Mycoplasma culture in cases of suspected HS,4 but universal surveillance post–lung transplantation is not standardized and in vivo relationships between airway mollicute identification and the occurrence of HS are not well understood.
Treatment is generally multi-faceted and varies by center. It generally includes antimicrobial therapy but may also include reduced protein intake, nitrogen scavenger agents, and renal replacement therapy. Some authors have extrapolated data from cirrhotic patients and have used rifaximin and lactulose, aiming to reduce ammonia-producing colonic bacteria and promote ammonia excretion from the gastrointestinal tract, respectively.15, 18
Even though HS has been associated with Ureaplasma spp and M. hominis airway positivity, there are few prospective studies correlating airway positivity and ammonia levels in asymptomatic patients. This relatively large study aims to describe airway positivity among post–lung transplant patients using institutional Mycoplasma and Ureaplasma active surveillance data, and correlate these data with donor characteristics, ammonia levels, hyperammonemia syndrome, and mortality.
2 MATERIAL AND METHODS
Adult patients (≥18 years) who underwent lung or heart-lung transplantation from July 2017 to August 2019 underwent prospective surveillance for hyperammonemia and mollicute positivity posttransplant. Our surveillance protocol includes testing for Mycoplasmataceae on the first bronchoalveolar lavage fluid obtained between days 1 and 3 posttransplant, as well as daily serum ammonia testing for 7 days post-transplant (normal range 20–50 μmol/L) (Figure 1). The testing protocol includes Mycoplasmataceae family 16S rRNA gene detection using PCR, with final species identification performed on positive samples by sequencing at the National Microbiology Laboratory (NML), Winnipeg, Manitoba. Multiple samples from individual patients were pooled for testing.
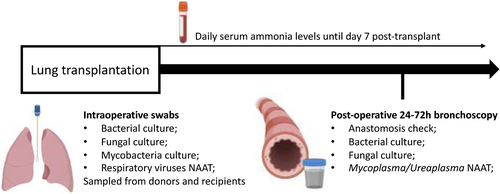
Baseline variables including recipient gender, age at time of transplant, baseline pulmonary disease, Acute Physiology and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores at the time of transplant were collected. Antimicrobial use was also collected during the first 14 days posttransplant.
Compliance to the protocol was calculated if both PCR testing and daily ammonia serum level (for 7 days posttransplant) were collected. Hyperammonemia syndrome was defined as any new neurologic abnormality (agitation, decreased level of consciousness, seizures, or cerebral edema), without an alternative diagnosis, with serum ammonia levels above 50 μmol/L and normal liver enzymes. Our criteria were revised using previously published criteria21, 22 to include ruling out any urea cycle or metabolism disorders.
Donor data were retrieved from local organ procurement organization (OPO) records. Donor variables of interest included gender, age, date of transplant, province of procurement, race, cause of death, increased risk donor status, any active and frequent use of tobacco and or marijuana preceding organ procurement, any use of intravenous drugs previous to organ procurement, and high-risk sexual behaviors (as per 2012 Canadian Standards Association increased-risk donor [CSA-IRD] criteria). Also, the use of antimicrobials in the 72 h prior to organ procurement was retrieved.
Demographic variables were reported as numbers and percentages for categorical variables and means with standard deviations (SD) or medians and inter-quartile ranges (IQR), as appropriate for continuous variables. Median peak ammonia levels by mollicute PCR positivity and outcome categories were compared using the Independent Samples Median Test. Univariate analyses were performed using the Chi-square or Fisher's Exact tests to assess for correlations between recipient/donor risk factors and mollicute identification, incidence of hyperammonemia syndrome and 30-day mortality. Multivariable logistic regression was subsequently performed to assess for associations between donor variables and PCR-positivity status. Clinically relevant variables (e.g., donor age and sex) were included in the model regardless of significance on univariate analysis. Variables identified as being significant (p ≤ .10) on univariate screen were also included. Separate models were constructed to identify associations with Mycoplasma and/or Ureaplasma positivity and Ureaplasma positivity alone. C-statistic values were reported as measures of goodness of fit. All p-values were two tailed with statistically significant defined by a p < .05. All statistical analyses were performed using IBM SPSS Statistics, Version 26.0 (IBM Corp).
3 RESULTS
3.1 Study population and baseline diseases
One hundred and fifty-nine adults underwent lung transplantation during the study period. Most patients were male (n = 106, 66.6%) and mean age was 54.0 years (±13.2). Most common baseline diseases pretransplant included pulmonary fibrosis (n = 60, 37.7%), chronic obstructive pulmonary disease (n = 57, 35.8%), and cystic fibrosis (n = 21, 13.2%). Baseline APACHE II and SOFA scores were 19.7 (±5.8) and 7.9 (±3.1) at the time of ICU admission posttransplant, respectively (Table 1).
|
Total n = 159 (100%) |
PCR positive n = 42 (29%) |
PCR negative n = 105 (71%) |
p-value | |
|---|---|---|---|---|
| Male gender, n (%) | 106 (66.6) | 33 (79) | 62 (59) | .025 |
| Age (years), mean (SD) | 54.2 (13.20) | 53.5 (13.0) | 53.7 (13.7) | .92 |
| Type of transplant, n (%) | ||||
| Bilateral sequential lung transplant | 154 (96.8) | 42 (100) | 100 (95.2) | .97 |
| Heart-lung transplant | 3 (1.8) | 0 (0) | 3 (2.8) | |
| Single-lung transplant | 2 (1.2) | 0 (0) | 2 (1.9) | |
| Baseline disease, n (%) | ||||
| Pulmonary fibrosis | 60 (37.7) | 23 (55) | 34 (32) | .15 |
| Chronic pulmonary obstructive disease | 57 (35.8) | 10 (24) | 42 (40) | |
| Cystic fibrosis | 21 (13.2) | 4 (10) | 15 (14) | |
| Pulmonary hypertension | 15 (9.4) | 4 (10) | 10 (10) | |
| Alpha-1 antitrypsin deficiency | 6 (3.7) | 1 (2.3) | 4 (3.8) | |
| APACHE II, at ICU admission, mean (SD) | 19.7 (5.8) | 21.6 (6.8) | 19.0 (5.4) | .018 |
| SOFA, at ICU admission, mean (SD) | 7.9 (3.1) | 8.2 (3.7) | 7.8 (2.8) | .53 |
- Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; PCR, polymerase chain reaction; SOFA, sequential organ failure score.
3.2 Protocol compliance and microbiologic findings
Compliance to the protocol was 92.4% (147/159) for Mycoplasma/Ureaplasma PCR in the first post-operative bronchoscopy specimen. After excluding five early deaths (within 7 days of transplant) 95.4% (147/154) of patients had at least one serum ammonia level collected and 67.5% (104/154) had complete 7-day ammonia testing posttransplant. Forty-two (26.4%) patients had Ureaplasma or Mycoplasma airway PCR positivity. M. salivarium was the most common isolated species (n = 26, 59.0%), followed by U. parvum (n = 7, 15.9%), U. urealyticum (n = 5, 11.3%), M. hominis (n = 3, 6.8%), and M. pneumoniae (n = 2, 4.5%). There was one patient with Mycoplasma non-pneumoniae identified (2.2%), but further sub-speciation was not possible. Two patients (4.7%) had more than one mollicute species identified (Table 2).
| Total of isolates (%) | |
|---|---|
| Mycoplasma salivarium | 26 (59.0) |
| Ureaplasma parvum | 7 (15.9) |
| Ureaplasma urealyticum | 5 (11.3) |
| Mycoplasma hominis | 3 (6.8) |
| Mycoplasma pneumoniae | 2 (4.5) |
| Mycoplasma non-pneumoniae (not further speciated) | 1 (2.2) |
| Co-identification of species | 2/42 (4.7) |
3.3 Ammonia levels posttransplant
Median peak ammonia levels were 45 μmol/L (IQR 36–58) in PCR-negative patients, 52 μmol/L (IQR 39–60) in those who tested positive for Mycoplasma spp., and 61 μmol/L (IQR 46–75) in those who tested positive for Ureaplasma spp (Figure 2). Median peak ammonia levels varied significantly among the groups (p = .03); and were specifically higher in those with Ureaplasma-positivity compared with PCR-negative patients (p = .04), but not when compared with Mycoplasma-positive patients (p = .46). Those who tested positive for Mycoplasma did not have significantly higher ammonia levels when compared with PCR-negative recipients (p = .20). Median day of ammonia peak posttransplant also varied among groups (p = .03); 4 days (IQR 2–6) in Mycoplasma-positive recipients, 4 days (IQR 1–5) in Ureaplasma-positive recipients, and 2 days (IQR 2–4) in PCR-negative recipients (Figure 3). Pairwise comparisons only demonstrated a difference in median day of ammonia peak between PCR-negative recipients and those who tested positive for Mycoplasma (p = .02). The median peak ammonia level in patients with HS (n = 3) was 79 μmol/L (IQR 67.5–110.5) compared to 47.5 μmol/L (IQR 36–59.5) in patients without HS (p = .19) (Figure 4).

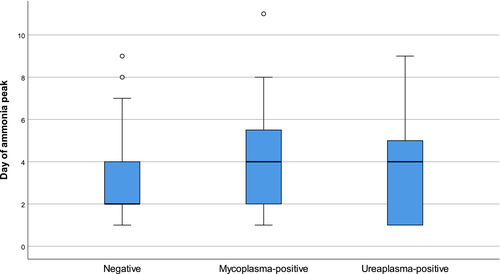
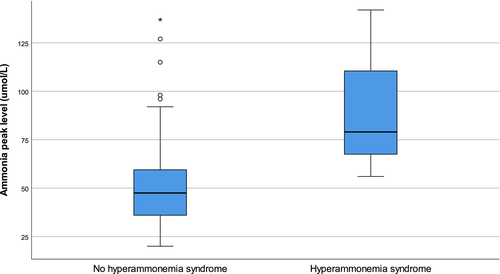
3.4 Donor characteristics
Donors were mostly male (n = 84, 52.8%) and Caucasian (n = 120, 75.4%), and the mean age was 37.7 years (±14.8). Increased risk sexual behavior (n = 60, 37.7%) and increased risk donor status (n = 77, 48.4%) were common. The most common cause of death was hemorrhagic cerebrovascular accident (n = 46, 28.9%), followed by opioid overdose (n = 18, 11.3%) and traumatic brain injury (n = 14, 8.8%).
3.5 Characteristics and outcomes in patients with mollicute PCR-positive airway samples
Recipients who tested positive for Ureaplasma or Mycoplasma had younger donors, with increased risk sexual behavior and increased frequency of marijuana use (Table 3). Ureaplasma/Mycoplasma-positive recipients were mostly male and had higher APACHE II scores, but not higher SOFA scores (Table 1).
|
Total n = 159 |
PCR positive n = 42 (29%) |
PCR negative n = 105 (71%) |
p-value | |
|---|---|---|---|---|
| Age, years, mean (SD) | 36 (14.8) | 32.9 (13.9) | 40.3 (14.7) | .006 |
| Male gender, n (%) | 84 (52.8) | 26 (62) | 48 (46) | .076 |
| Donor classification, n (%) | ||||
| NDD | 135 (84.9) | 35 (83) | 90 (86) | .54 |
| DCD | 22 (13.8) | 7 (17) | 13 (12) | |
| MAID | 2 (1.2) | 0 (0) | 2 (2) | |
| Donor ethnicity, n (%) | ||||
| Caucasian | 119 (74.8) | 31 (74) | 80 (76) | .89 |
| Indigenous | 22 (13.8) | 6 (14) | 14 (13) | |
| Asian | 15 (9.4) | 5 (12) | 10 (10) | |
| Others | 3 (1.8) | 1 (2) | 2 (2) | |
| Group characteristics, n (%) | ||||
| IRD | 77 (48.4) | 25 (60) | 44 (42) | .053 |
| Tobacco use | 95 (59.7) | 29 (69) | 57 (54) | .1 |
| Marijuana use | 70 (44.0) | 25 (60) | 37 (32) | .007 |
| IDU | 28 (17.6) | 7 (17) | 17 (16) | .94 |
| High-risk sexual behaviora | 37 (23.2) | 16 (38) | 17 (16) | .014 |
| Steroid use pretransplant | 84 (52.8) | 19 (42) | 60 (57) | .19 |
- Abbreviations: DCD, donation after cardiac death; IRD, increased risk donor, IDU, injection drug use; MAID, medical assistance in dying; NDD, neurologic determination of death; PCR, polymerase chain reaction; SD, standard deviation.
- a Missing data (22 patients).
The only donor characteristic associated with Mycoplasma and/or Ureaplasma positivity on multivariable logistic regression was marijuana use (aOR 2.30, 95% CI 1.03–5.14, p = .04) (C-statistic 0.70). Multivariable analysis for Ureaplasma positivity alone demonstrated independent associations with younger donor age (aOR 0.94, 95% CI 0.88–0.99, p = .04) and female donors (aOR 4.29; 95% CI 1.01–18.2, p = .05) (C-statistic 0.84) (Tables 4 and 5).
| Variable | aOR (95%CI) | p-value |
|---|---|---|
| Age | 0.97 (0.94–1.00) | .062 |
| Female gender | 0.53 (0.25–1.16) | .11 |
| Increased risk donor | 1.09 (0.40–3.00) | .87 |
| Marijuana use | 2.30 (1.03–5.14) | .042 |
| High-risk sexual behavior | 1.02 (0.55–1.91) | .95 |
- Abbreviations: 95% CI, 95% confidence interval; aOR, adjusted odds ratio.
- a 12 patients did not have Mycoplasma/Ureaplasma PCR testing.
| Variable | aOR (95%CI) | p-value |
|---|---|---|
| Age | 0.94 (0.88–0.99) | .046 |
| Female gender | 4.30 (1.01–18.26) | .048 |
| Increased risk donor | 2.12 (0.39–11.60) | .39 |
| Marijuana use | 5.67 (0.99–32.5) | .052 |
| High-risk sexual behavior | 0.59 (0.20–1.78) | .35 |
- Abbreviations: 95% CI, 95% confidence interval; aOR, adjusted odds ratio.
- a 12 patients did not have Mycoplasma/Ureaplasma PCR testing.
Of 159 donors, 125 (78.6%) were treated with antimicrobials prior to organ procurement, however, only one received antimicrobial therapy active against Mycoplasma/Ureaplasma spp.
Hyperammonemia syndrome was identified in nine recipients (1.8%), all of whom underwent bilateral sequential lung transplant, one of whom died. All cases had elevated ammonia levels and neurologic symptoms (including confusion, agitation, decreased level of consciousness, or diffuse cerebral edema) with no other etiology. The median day of HS diagnosis was 5.0 days (±2.8) posttransplant and median ammonia level at time of diagnosis was 69.0 (±9.0) μmol/L. Of these three patients, one was positive for Ureaplasma urealyticum and two patients had both Ureaplasma parvum and Mycoplasma hominis identified. All three cases were negative for urea cycle disorders. In addition, there was no history of metabolic diseases in any of the donors. Survivors were treated with active antimicrobial therapy (one with doxycycline, the other with doxycycline and ciprofloxacin), dietary modifications, nitrogen scavenger agents, and renal replacement therapy. On univariate analysis, Ureaplasma infection was associated with HS (p < .05). Further analyses were not conducted due to inadequate statistical power.
With regard to specific treatments for Mycoplasmataceae airway positivity, 24 recipients (15.1%) received active antibacterial therapy, of which 16 (10.0%) received antimicrobials specifically for hyperammonemia. Of those treated, 14 (58.3%) were treated with doxycycline, 8 (33.0%) with ciprofloxacin, 1 (4.2%) with levofloxacin and 1 (4.2%) with minocycline. The mean duration of therapy was 8 (±6) days. The use of active antimicrobials was not associated with decreased 30-day mortality (p = 1.00) in our analysis. It is important to mention that, as a send-out test, most clinicians did not have Mycoplasmataceae PCR results at the time of HS diagnosis. As such, antimicrobial therapy would have been started empirically.
The median ICU length of stay in this cohort was 6.09 (3.9–14.03) days, and median hospital length of stay was 27 (17–47) days. There were no differences in ICU or hospital lengths between Mycoplasma-positive or Ureaplasma-positive versus PCR-negative recipients (p-values .42 and .18, respectively). The overall 30-day mortality rate was 4.4% (7/159) in this cohort.
4 DISCUSSION
In summary, hyperammonemia syndrome was rare in our cohort of lung transplant recipients, but it was diagnosed early and treated aggressively. We report high Mycoplasma and Ureaplasma positivity rates (28.5%) immediately post–lung transplant and a significant association between Ureaplasma-positivity and elevated ammonia levels. This association, however, cannot be considered causative at this time.
We believe the higher ammonia levels seen in recipients with Ureaplasma-positive airway samples may be explained by the different metabolic pathways used by these bacteria; Mycoplasma species are able to metabolize carbohydrates and amino acids to produce energy alternatively to urea, while Ureaplasma species are non-fermentative mollicutes—relying strictly on urea for their growth as evidence by in vitro models.23 However, the occurrence of hyperammonemia syndrome has also been linked to host factors. Rueda et al hypothesize that glutamine synthetase deficiency might increase the likelihood of ammonia accumulation, justifying why low protein intake may be a therapeutic option.2 Another potential hypothesis is that the lungs are known for being highly metabolic post–lung transplantation and could be overwhelmed by specific bacterial co-signaling, leading to elevated ammonia production.21 Indeed, ammonia has been previously reported as a marker for inflammatory lung diseases and hyperammonemia syndrome has been well described in lung and hematopoietic stem cell transplants with mollicute-positive airway samples.24, 25
It has been hypothesized that Mycoplasmataceae infection posttransplant is donor derived. Wright et al. described three donor bronchoalveolar lavage samples with M. hominis identified by PCR in two of three matched recipient samples, one of which developed HS.19 Similarly, Fernandez et al. systematically tested recipients and donors for mollicutes colonization. None of the recipients in Fernandez's study had a positive bronchoalveolar lavage fluid for Ureaplasma or Mycoplasma, whereas four donors (14%) had positive samples for Mollicutes testing. Fernandez also demonstrated an association with younger donor age and high-risk sexual activity,13 as we have reported in this study. We were unable to microbiologically confirm whether PCR positivity was donor derived as we only tested recipients posttransplant, however, we believe this is most likely. In particular, increased donor sexual risk behavior can facilitate donor airway positivity with these genitourinary pathogens and subsequent recipient transmission. The association with female gender is novel but may relate to the high prevalence of Mollicutes genitourinary colonization (specifically Ureaplasma spp.) in childbearing age women.26
Subgroup analysis did not show different positivity rates for Mycoplasma and/or Ureaplasma spp among various pulmonary diseases. Regarding the prevalence by species, Mycoplasma salivarium was the most identified species, which has not been widely reported in the literature. We suspect this species is not the same in terms of pathobiology compared to other mollicute species, given its nature as an upper airway and oral cavity contaminant. In support of this, most cases of hyperammonemia syndrome reported in the literature have been associated with M. hominis or Ureaplasma sp. positivity, not with M. salivarium. The high prevalence of M. salivarium may be the result of our screening method, 16S rRNA PCR, which has a high sensitivity for detection.
Unfortunately, our surveillance testing requires sending specimens to a reference laboratory as we do not have in-house testing available, which results in delayed turnaround times (TAT). In our cohort, the mean TAT of the 159 sent specimens was 14.9 (±7.7) days. Development of on-site, rapid, real-time PCR testing for Ureaplasma spp and Mycoplasma hominis may be justified in large transplant centers to ensure early diagnosis and therapy, and avoidance of empiric therapies.
Despite the previous association between Ureaplasma infection and high ammonia levels in vivo,4, 11, 14, 24, 25 the role of antimicrobial therapy in these cases is still unclear but has been associated with favorable outcomes.13 As Mollicutes lack a cell wall, empiric therapy with ß-lactams (usual empiric choices for post-operative prophylaxis, including at our site) is ineffective. Previous case reports have used tetracyclines, fluoroquinolones, and azithromycin as empiric options, however, independent associations with outcomes are lacking. In our center, we use doxycycline empirically, given gyrA and gyrB fluoroquinolones-resistance mutations are increasingly common and M. hominis is highly resistant to macrolides.27-29
This study highlights the potential value of prospective surveillance of serum ammonia and Mollicutes airway positivity posttransplant as we believe that prompt (and directed) initiation of therapy can lead to better outcomes. Ideally, point-of-care testing for Ureaplasma would be available in all large transplant centers to facilitate prompt diagnosis of hyperammonemia. Although not supported by high-quality data, we suggest treating hyperammonemia syndrome not only with antimicrobials, but also with dietary modifications, nitrogen scavenger agents and renal replacement therapy. The length of treatment is not established but varies between 10 and 21 days in previous reports with good outcomes.6, 11, 12, 25
Despite our findings, our study has several limitations. First, it is a single-center study and thus may not be generalizable. Second, compliance with the surveillance protocol was not 100%, leading to missing data (12 patients [7.6%] did not have any Mycoplasma nor Ureaplasma testing, and ammonia levels were not systematically tested daily for 7 days in 55 [35.6%] patients). Third, given that both mortality and hyperammonemia syndrome were rare conditions posttransplant, the study was underpowered to examine these outcomes. Fourth, given that we defined HS as any elevated serum ammonia level associated with neurologic dysfunction not attributable to another cause, we risked misclassifying patients as previously reported cases of HS are usually associated with marked elevated ammonia levels. However, given that hyperammonemia can develop very rapidly, we believe this definition best identifies patients early, avoiding late diagnosis and poor outcomes. With only three cases of HS, misclassification could not be substantial. Last, both donor and recipient testing would be required to determine if recipient airway positivity is truly donor derived, however, only post-operative recipient testing was possible in our population.
In conclusion, we have demonstrated that patients positive for Ureaplasma from airway specimens post–lung transplant have higher ammonia levels and may be at risk for hyperammonemia syndrome. In fact, all three patients with hyperammonemia syndrome in this cohort were positive for Ureaplasma spp (one with Ureaplasma urealyticum and two with positive samples for Ureaplasma parvum in the context of Mycoplasma hominis co-colonization). Ureaplasma identification was independently associated with younger, female donors. Based on these data, surveillance and/or pre-emptive therapy for Ureaplasma in selected groups may improve outcomes post–lung transplantation.
ACKNOWLEDGMENTS
We would like to acknowledge the Provincial Laboratory for Public Health (ProvLab) in Alberta and the National Microbiology Laboratory (Winnipeg, MB), for performing PCR surveillance testing. Also, TRACER program for retrieving supplementary data about critical care scores and Alberta Health Services to allow us to access its database to develop this study.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




